One stone two birds: electrochemical and colorimetric dual-mode biosensor based on copper peroxide/covalent organic framework nanocomposite for ultrasensentive norovirus detection
Guoo Ning,Qunmei Dun,Hun Ling,Huifng Liu,Min Zhou,Chunln Chen,Chong Zhng,Hui Zho,,Cnpeng Li,
a School of Chemical Science and Technology, Yunnan University, Kunming 650091, China
b Laboratory for Conservation and Utilization of Bio-resource, Yunnan University, Kunming 650091, China
c Kunming Customs Technology Center, Kunming 650228, China
Keywords: Norovirus Specif ic peptides
ABSTRACT Norovirus (NoV) is regarded as one of the most common causes of foodborne diarrhea in the world.It is urgent to identify the pathogenic microorganism of the diarrhea in short time.In this work,we developed an electrochemical and colorimetric dual-mode detection for NoV based on the excellent dual catalytic properties of copper peroxide/COF-NH2 nanocomposite (CuO2@COF-NH2).For the colorimetric detection,NoV can be directly detected by the naked eye based on CuO2@COF-NH2 as a laccase-like nonazyme using “peptide-NoV-antibody” recognition mode.The colorimetric assay displayed a wide and quality linear detection range from 1 copy/mL to 5 000 copies/mL of NoV with a low limit of detection (LOD) of 0.125 copy/mL.For the electrochemical detection of NoV,CuO2@COF-NH2 showed an oxidation peak of copper ion from Cu+ to Cu2+ using“peptide-NoV-antibody” recognition mode.The electrochemical assay showed a linear detection range was 1-5 000 copies/mL with a LOD of 0.152 copy/mL.It’s worthy to note that this assay does not need other electrical signal molecule,which provide the stable and sensitive electrochemial detection for NoV.The electrochemical and colorimetric dual-mode detection was used to detect NoV in foods and faceal samples,which has the potential for improving food safety and diagnosing of NoV-infected diarrhea.1 These authors contributed equally to this work.*Corresponding authors.
1.Introduction
Norovirus (NoV) is a pathogen of infectious disease that causes gastroenteritis,or inflammation and diarrhea of the gastrointestinal tract.Among the human NoV (HuNoV),the most common types are GII and GI HuNoV is highly infectious and has multiple transmission routes,can be transmitted through people,food,and water sources[1].The highest risk of NoV infection group are children and the elders,which has the highest incidence rate of hospitalization and morbidity[2].Hence,the detection of HuNoV is very necessary.For the HuNoV detection,various methods including immunoelectron microscopy (IEM)[3],real-time polymerase chain reaction (RT-PCR)assay[4],and enzyme-linked immunosorbent assay (ELISA) have been developed.However,there are some disadvantages,such as time-consuming,complex process of pretreatment,requirement of expensive equipment,or need of professional operation,which limits their application in diagnosis and analysis.Moreover,NoV detection in low virus load samples,such as in water or foods (for example oyster,strawberry,and lettuce)[5]is of significance for efficiently preventing foodborne diarrhea.Therefore,it is necessary to develop a detection method with low cost,simple operation,short time,and high sensitivity to provide protection for people’s health.
Hydrogel materials show many excellent properties,including high water content,biocompatibility,and a porous structure.In addition,hydrogel also has the advantages of continuous porosity,large surface area,and low density,which has been widely used in many fields[6],including biomedical aspects,sensors,and therapy.Recently,Wu et al.[7]reported a dopamine coated double noble metal hydrogel by a simple and effective methods.Thanks to the excellent conductivity of both precious metal and dopamine,precious metal hydrogel has the potential application in electrocatalytic area.Therefore,in this work,similar precious metal hydrogel with good conductivity were used as a material modified on the electrode of electrochemical sensors.In recent years,various nanomaterials have attracted substantial research attention,such as metal organic framework (MOF)[8-12],Fe3O4[13],and covalent organic framework (COF) and so on.Among various nanomaterials,COF is a kind of material that connects organic building units together through covalent bonds to form a porous framework with periodic structure.Meanwhile,COF has high crystallinity,adjustable pore size,large surface area,and unique molecular structure.Which has been widely used in adsorption,energy storage,catalytic degradation of pollutants,and sensing.Such as electrochemical sensors,fluorescent sensors,colorimetric sensor,and photoelectrochemical sensors[14].Lohse et al.[15]synthesized COF containing amino group for the adsorption of lactic acid.Here,we believe that the amino group on the surface of COF can be used as linkage group that coordinate with metal nanoparticles,which may be used in the construction of biosensors.
Laccase is a polyphenol oxidase containing four copper ions,which belongs to copper blue oxidase and exists in the form of monomer glycoprotein[16].It has been reported that some Cu-containing nanomaterial shows laccase-like activity.Interestingly,copper peroxide (CuO2) is composed of copper ions and peroxides,which can reverse release hydrogen peroxide in acidic environment[17].Although copper peroxide has laccase-like activity and can catalyze the color development of phenolic substrates,CuO2NPs tend to aggregate and decrease the catalytic activity of catalyst.To avoid the aggregation of CuO2NPs,in this study,CuO2NPs was anchored on the surface of COF-NH2through coordination between CuO2NPs and -NH2.Meanwhile,it is well known that COF is a porous material,which was often used in adsorption field[18].Therefore,in a catalytic reaction,more substrates could be enriched by COF,which may benefit to the improvement of the catalytic activity.
Compared with antibodies,affinity peptides have the advantages of easy synthesis,easy modification,high affinity,good stability,and low molecular weight.Therefore,the use of peptides as a recognition molecule not only can reduce the cost of the sensor,but also can facilitate the processing and stability of the biosensor[19].In recent years,affinity peptides have been used in biosensors as specific recognition molecule.For the affinity peptide of NoV,a high affinity peptide has been identified by evolutionary phage display has specific recognition elements and can captures NoV capsid protein[20].More recently,we have developed an electrochemical biosensor for NoV using COF/pillararene heterosupramolecular nanocomposites (COF/PA)as a probe,where COF/PA can enrich the electrochemical signal molecule methylene blue (MB) and amplify the signal of biosensor[21].However,MB is toxic and may be degraded in the presence of light,high temperatures,the presence of oxidants,or coexistence with precious metal catalysts during storage.Meanwhile,compared with the noble metal nanoparticles,non-noble metal nanopartiles can decrease the cost of the biosensor.Therefore,it is of significance to construct a cheap and stable electrochemical biosensor using a high-stability and low-cost nanomaterial.Interestingly,the as-mentioned CuO2@COF-NH2may meet such demand.Here,as a simple and cheap nanocomposite,CuO2@COF-NH2can be directly used as a signal molecule,showing an oxidation peak of copper ion from Cu+to Cu2+instead of the traditional electrical signal molecule MB.Furthermore,as a Cu NPs-containing nanocomposite,CuO2@COF-NH2may play laccase-like activity and catalyze the oxidation of substrates to generate coloured product,which is expected to be used not only as direct electrochemical signal but also as the colorimetric signal for biosensor.
In this work,a high affinity peptide for NoV was used as secondary recognition molecule instead of antibody,then CuO2@COF-NH2was combined with affinity peptide as capture probe and signal probe.Based on its electrochemical properties and laccase-like activity of CuO2@COF-NH2,a dual-mode“peptide-target-antibody” sandwich assay for NoV detection was designed,where the electrochemical and colorimetric signals could be achieved.The dual-mode assay was successfully used to sensitively detect NoV in the real samples (Fig.1).
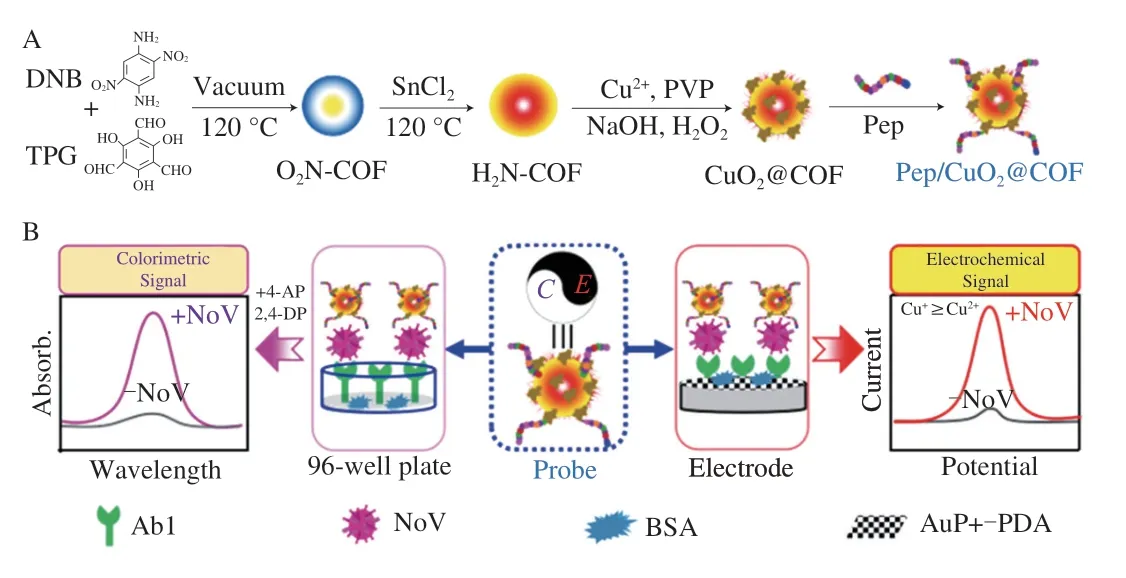
Fig.1 Schematic illustration of the electrochemical and colorimetric dualmode biosensor for ultrasensentive norovirus detection.(A) The preparation of bifunctional probe;(B) Principle of the electrochemical and colorimetric dualsignal biosensor for ultrasensentive norovirus detection.
2.Materials and methods
2.1 Materials and apparatus
Copricchloride dihydrate(CuCl2·2 H2O),1,3,5-triformylphloroglucinol (TPG),3,3’-dinitrobenzidine (DNB),mesitylene,1,4-dioxane,sodium hydroxide,aqueous acetic acid,acetone,anisole,hydrogen peroxide,polyvinylpyrrolidone (PVP)(Mw=10 000),dopamine,NaCl,KCl,2-morpholinoethanesulfonic acid (MES),acetic acid alacial,sodium acetate,sodium phosphate monobasic dihydrate (NaH2PO4·2H2O),sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O),2,4-dichlorophenol(2,4-DP),potassium hexacyanoferrate(III) [K3Fe(CN)6],potassium hexacyanoferrate(II) trihydrate [K4Fe(CN)6·3H2O],4-aminoantipyrine(4-AP),HAuCl4,H2PtCl6,NaBH4,glucose,Tris-HCl Buffer and ethanol were all purchased from Aladdin Industrial Corporation(Shanghai,China).The 96-well polystyrene plate was obtained from Jiete (Guangzhou,China).The other reagents were all of analytical grade and used without further purification.All solutions and buffers were prepared with Milli-Q water.The concentration of all PBS buffer,acetic acid buffer and MES buffer were 0.1 mol/L,50 mmol/L and 50 mmol/L,respectively.
The transmission electron microscope (TEM) images,elemental mapping and energy dispersive X-ray spectroscopy (EDX) analyses were obtained on a JEM-2100F transmission electron microscope operated at 200 kV (JEM 2100,Japan);Fourier transform infrared spectrometer (FTIR) spectra were accomplished by Thermo Fisher Scientific Nicolet IS10 FTIR Impact 410 spectrophotometer(Waltham,USA);A Bruker D8-advance powder diffractometer(XRD,Germany) with Cu Kα radiation was used to carry out X-ray powder diffraction (XRD) spectra;An ESCA LAb 250 X-ray photoelectron spectrometer (Thermo-VG Scientific,USA) using a monochromatic Al Kα radiation excitation source was used to measure X-ray photoelectron spectra (XPS);The ultraviolet-visible (UV-Vis) absorption spectra was gained by Shimadzu UV-2880A UV-Vis spectrophotometer (Shanghai,China),respectively;differential pulse voltammetry (DPV),cyclic voltammetry (CV),and electrochemical impedance spectroscopy(EIS) were conducted on a CHI660E electrochemical workstation from Shanghai Chen Hua Instrument (Shanghai,China);The glass carbon electrodes as work electrode,Ag/AgCl electrode as reference electrode,Pt electrode as the compared electrode,Affinity 176 Peptide for NoV (QHKMHKPHKNTKGGGGSC)[20]was synthesized by GL Bio 177 Chem (Shanghai,China) with > 95% purity;RT-PCR system (Thermo Fisher Scientific,MA,USA) was used for qPCR experiment.
2.2 Preparation of CuO2@COF-NH2 composite
COF-NO2was synthesized according to a previous work[15].In brief,0.23 mmol TPG and 0.31 mmol DNB were pured into a 25 mL Schott Duran glass bottle with a Teflon sealed PBT cap which contained 8 mL of dry anisole and 2 mL of 12 mol/L AcOH.The mixed solution was heated at 120 °C for 96 h.A reddish brown precipitate was obtained by filtration and washed with 100 mL acetone.Then the obtained COF product was resuspended in 5 mL anisole in a 25 mL Schott Duran glass bottle and heated at 120 °C for 24 h.The COF-NO2powder was obtained by filtration and washed with 100 mL acetone three times.For the synthesis of COF-NH2,3 g of SnCl2·2H2O and 150 mg of COF-NO2powder were added to a round bottom flask containing 5 mL dry THF and refluxed at 120 °C for 3 h.The product was cooled down to room temperature,and brown powder was obtained by filtration and resuspended in 10 mL HCl.Then the product was washed with 70 mL HCl 10 times,70 mL DW three times,and 100 mL acetone once.The brown product was resuspended in 5 mL anisole which was placed in a 25 mL Schott Duran glass bottle and heated at 120 °C for 24 h.Finally,the COF-NH2was collected by filtration and washed with acetone three times.
The synthesis of CuO2@COF-NH2composite was synthesized according to a previous work with minor modifications[17].Briefly,a certain amount (0.5-5 mg) COF-NH2was added to a CuCl2·2H2O aqueous solution (5 mL,0.01 mol/L) contained PVP (0.5 g),the mixture was stirred at room temperature for 1 h,and then NaOH(5 mL,0.02 mol/L) and H2O2(100 μL) were added with stirring at at room temperature for 30 min.The CuO2@COF-NH2composite was collected by filtration and washed with DW three times.
2.3 Synthesis of polydopamine-capped AuPt hydrogel
The polydopamine-capped AuPt hydrogel (AuPt-PDA) was synthesized according a previous work[7].Firstly,HAuCl4(10 mol/L)and H2PtCl6(10 mol/L) were dissolved in 33 mL 10 mmol/L Tris-HCl buffer pH 8.0 containing 2.5 mg dopamine,then 2 mL 0.05 mol/L fresh prepared NaBH4solution quickly poured into above mixture solution at 60 °C by sitrring for 60 s.Then mixture solution was stirred at 60 °C for 2 h and AuPt-PDA hydrogel was collected by centrifugation and washing with DW three times.
2.4 Preparation of probe (Pep/CuO2@COF-NH2)
The CuO2@COF-NH2was used as a probe.For the preparation of Pep/CuO2@COF-NH2probe,first,100 μL 1 mg/mL peptide solution was added to a 1 mL 1 mg/mL CuO2@COF-NH2dispersion and stirred at 4 °C for 12 h,then the suspension was washed with DW three times.The precipitate was dried by freeze drying,the Pep/CuO2@COF-NH2was re-dispersed with a 50 mmol/L PBS buffer pH 7.0 and stored at 4 °C for future using.
2.5 Electrochemical and colorimetric detection procedure
For the electrochemical assay,in a typical procedure,glassy carbon electrode (GCE) was polished through 0.3 and 0.05 μm aluminum oxide (Al2O3) powder and ultrasonicated with DW and ethanol for cleaning surfaces.Next,10 μL 1 mg/mL AuPt-PDA hydrogel was dropped on GCE and dried in nitrogen flow.Then,10 μL 1 mg/mL antibody of NoV (Ab1) was dropped on AuPt-PDA/GCE and dried in nitrogen flow to obtain Ab1/AuPt-PDA/GCE,then the electrode was rinsed with PBS to remove the unbound antibody.Next,10 μL 1% BSA was added to Ab1/AuPt-PDA/GCE to block nonspecific sites and obtain BSA/Ab1/AuPt-PDA/GCE,and washed with PBS thrice.Then,a series of 10 μL different concentrations of NoV were added to Ab1/AuPt-PDA/GCE to obtain Ag/Ab1/AuPt-PDA/GCE,then incubated at 37 °C for 1 h and rinsed with PBS to remove the unbound antigen.10 μL 1 mg/mL Pep/CuO2@COF-NH2was added to Ag/Ab1/AuPt-PDA/GCE to obtain Pep/CuO2@COF-NH2/Ag/Ab1/AuPt-PDA/GCE,incubating at 37 °C for 1 h and rinsed with PBS to remove the unbound probe.Finally,the modified electrodes was placed in an acetic acid buffer pH 4.0 to obtain electrical signal by DPV.
For the colorimetric detection of NoV.First,50 μL 10 μg/mL Ab1 was added to the 96-well plate and placed overnight at 4 °C.Next,PBS buffer containing 0.05% Tween-20 (PBST) was used to wash 96-well plate and rinsed the unbound antibody with three times.Afterwards,50 μL 1% BSA was added to 96-well plate to block nonspecific sites and wished with PBST thrice.Then,a series of 50 μL different concentrations of NoV were added to 96-well plate,and was incubated at 37 °C for 1 h and rinsed with PBST to remove the unbound antigen for three times.Next,50 µL 1.5 mg/mL Pep/CuO2@COF-NH2was added to 96-well plate,incubating at 37 °C for 1.5 h and rinsed with PBST to remove the unbound probe for three times.Finally,50 µL 1 mg/mL 2,4-DP,50 µL 1 mg/mL 4-AP solution and 100 µL MES buffer pH 6.8 were added to 96-well plate,then was reacted at room temperature for 2 h and the absorbance value of the solution was recorded at 510 nm.
2.6 Optimization of experimental conditions
As experimental conditions can affect the performance of sensors,to obtain the sensor with the optimal performance,the conditions were screened.First,for the electrochemical sensor,the optimal ratio of composite (CuO2:COF-NH2),the optimal pH,incubation time,and probe concentration were screened.At the same time,for the colorimetric assay,the optimal ratio of composite (CuO2:COF-NH2),the optimal pH,and probe concentration were screened.
2.7 Determination of kinetic parameters of CuO2@COF-NH2
In order to evaluate catalytic performance of CuO2@COF-NH2,the kinetic parameters,michaelis constant (Km) and maximum speed values (vmax) values of CuO2@COF-NH2was measured using 2,4-DP at different concentrations (10,20,40,60,80 and 100 μg/mL)and 4-AP (150 μg/mL) in MES (50 mmol/L) at pH 6.8 and 25 °C.Time-dependent absorbance at 510 nm was measured at 25 °C[22].TheKmandvmaxvalues are calculated using the Lineweaver-Burk plot derived from the Michaelis-Menten equation.
Where,thev0was the initial reaction rate and [S] was the concentration of the substrate.From the intercept,the maximum reaction velocityvmaxwas obtained,and the Michaelis constantKmvalue was calculated from the value of the slope[23].
2.8 Statistical analysis
All results were presented as the mean ± standard deviations (SD).
3.Results and discussion
3.1 Characterization of AuPt-PDA hydrogel
The AuPt-PDA hydrogel was characterized using TEM and microscopy electron microscopies (SEM).As shown in Fig.2A,from the TEM,the AuPt-PDA hydrogel mainly consisted of interconnected nanowires with an average diameter of about 20 nm.The thickness of the PDA layer outside the AuPt-PDA hydrogel was~5 nm.It could be clearly seen from Fig.2B,the SEM image of the AuPt-PDA hydrogel exhibited 3D porous nanowire network structure,which was consistent with the result in a previous report[7],indicating the successful preparation of the material.The XRD pattern of AuPt-PDA was shown in Fig.2C,three diffraction peaks at 39o,45o,and 67owere observed and assigned to the (111),(200),and (220) crystal planes of AuPt alloy,respectively,which were consistent well with the result in a previous work[7].The FTIR spectra was used to further investigate the coating of PDA on AuPt alloys.As shown in Fig.2D,the peaks at 1 515 and 1 605 cm-1of the AuPt-PDA hydrogel appeared,which belong to indole or indoline structures of PDA[7],also indicating the successful preparation of the material.
Furthermore,the XPS images of AuPt-PDA hydrogel exhibited the presence of Au,Pt,C,N,and O elements (Fig.2E) in AuPt-PDA hydrogel.The EDX was used to determine elements of AuPt-PDA hydrogel,the result showed that coexistence of Au,Pt,C,and O elements in AuPt-PDA hydrogel (Fig.2F),which were agree with XPS results.In addition,element mapping analysis showed that Au,Pt,C,N,and O elements coexisted (Fig.3) in the AuPt-PDA hydrogel composite.It is worthy to note that the Au and Pt elements were uniformly distributed with C,N,and O elements,which would be advantageous to improvement of catalytic activity.The above results indicated the successful preparation of AuPt-PDA hydrogel.

Fig.3 Elemental mapping of AuPt-PDA hydrogel.(A) TEM of AuPt-PDA hydrogel;(B) C;(C) N;(D) O;(E) Pt;(F) Au.
3.2 Characterization of CuO2@COF-NH2 composite
The XRD pattern of COF-NO2was shown in Fig.4A,the peaks at 3.5o,6.0o,9.4o,and 26owere assigned to the (100),(110),(210),and (001) crystal planes of COF-NO2,respectively[15],which were in agreement with the reported theoretical prediction of an AA stacking arrangement in a reported work[24].Subsequently,SnCl2·2H2O was used as a reducing agent to further synthesize COF-NH2by reducing-NO2to -NH2.The pattern of the COF-NH2showed a diffraction peaks consistent with COF-NO2in Fig.4A.Furthermore,FTIR spectroscopy was used to verify the formation of amino group in COF-NH2.As shown in Fig.4B,the strong peaks at 1 511 and 1 336 cm-1appeared,which belonged to the asymmetrical and symmetrical telescopic vibration peaks of nitro group in COF-NO2,respectively.After being reduced by SnCl2·2H2O,the stretching vibrations of the nitro group of COF-NO2sharply reduced,and the peaks at 1 291 cm-1appeared,which belonged to the C-N telescopic vibration peak of primary amine,indicating the successful synthesis of COF-NH2[15].The morphology of COF-NH2was characterized using TEM,it can be clearly seen from Fig.4D that COF-NH2presented a circular structure as a whole and exhibited a polycrystalline structure with random crystal orientation[15].The above results showed the successful synthesis of COF-NH2.The TEM image of CuO2was performed(Fig.4E),it showed that CuO2was a cotton-like structure.When CuO2was loaded on COF-NH2,CuO2seemed to be easily dispersed and the cotton-like structure of CuO2did not seem to be obvious than before loading (Fig.4F).We speculate that the copper ions were anchored to COF-NH2through coordination with amino group,so that CuO2NPs were uniformly formed on COF-NH2in subsequent synthesis,which may be benefit to improving the catalytic activity of CuO2.
Subsequently,the valence state of copper in CuO2@COF-NH2was characterized by XPS.In a high-resolution XPS spectrum(Fig.4C),two main peaks at 934.02 and 954.00 eV observed and belonged to the Cu 2p3/2 and Cu 2p1/2 electrons of Cu2+,respectively.Together with satellite peaks at 943.58 and 962.17 eV,the result indicated that the valence state of Cu in CuO2@COF-NH2was +2[17].Furthermore,element mapping analysis of CuO2@COF-NH2showed that Cu,O,C,and N elements coexisted were evenly distributed on CuO2@COF-NH2(Fig.5).All of the above results indicated the successful preparation of CuO2@COF-NH2composite.
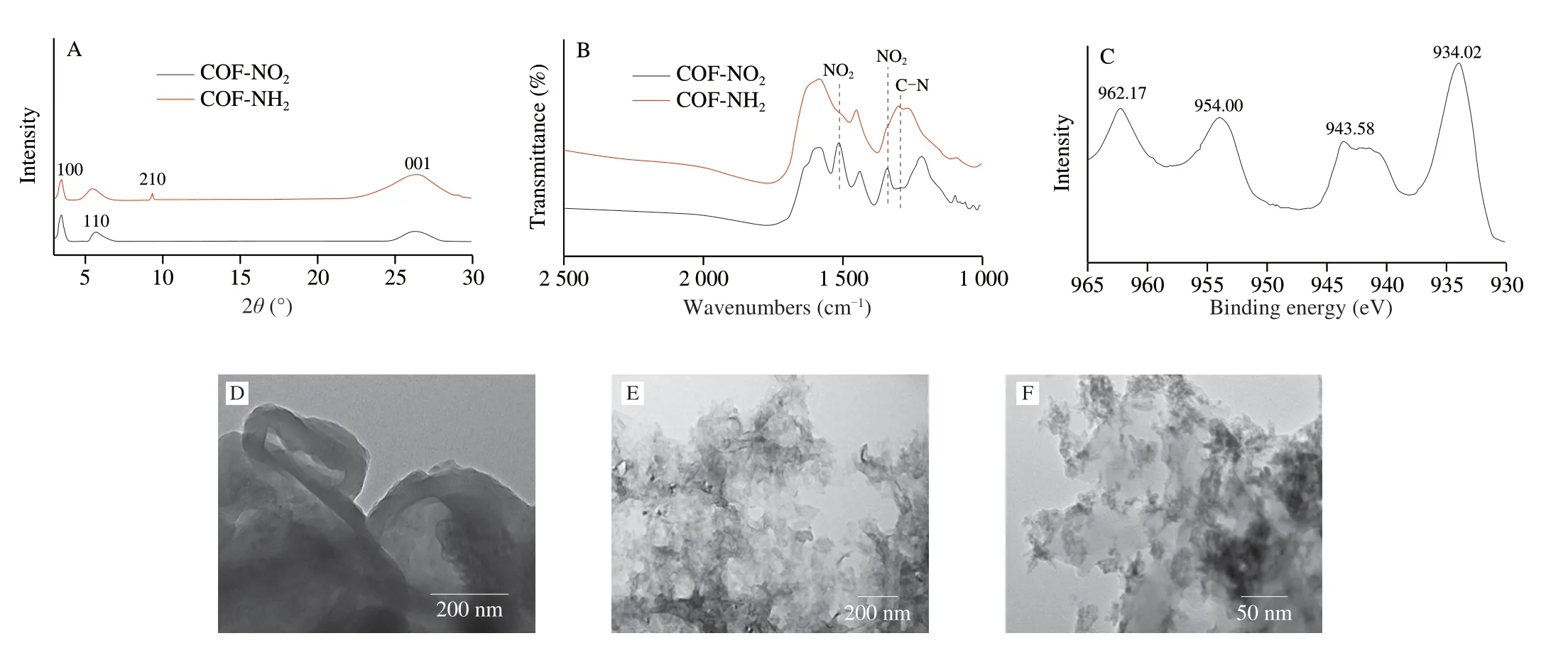
Fig.4 Characterization of COF-NO2,COF-NH2,CuO2,and CuO2@COF-NH2.(A) XRD survey spectra of COF-NO2 and COF-NH2;(B) FTIR spectra of COF-NO2 and COF-NH2;(C) High resolution Cu 2p survey XPS spectra of CuO2@COF-NH2;(D) TEM image of COF-NH2;(E) TEM image of CuO2;(F) TEM image of CuO2@COF-NH2.
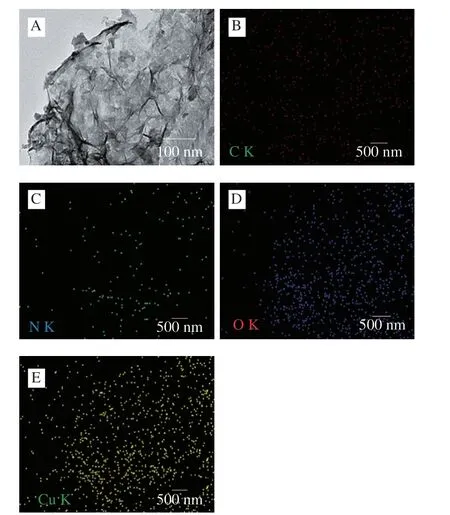
Fig.5 Elemental mapping of CuO2@COF-NH2.(A) TEM of CuO2@COF-NH2;(B) C;(C) N;(D) O;(E) Cu.
3.3 Catalytic activity and electrochemical properties of CuO2@COF-NH2
The laccase-like activity of CuO2@COF-NH2was measured according to a previous method.Here,CuO2@COF-NH2catalyze oxidation of 2,4-DP and 4-AP to form a pink solution,showing an absorption peak at 510 nm[25].As shown in Fig.6A,the same quality of COF-NH2,CuO2and CuO2@COF-NH2were added to the buffer solution containing 2,4-DP and 4-AP,then the absorbance of the product solution was recorded at 510 nm (pink).Both solutions changed from colorless to obvious pink in the presence of CuO2or CuO2@COF-NH2.However,the absorption value at 510 nm of solution catalyzed by CuO2@COF-NH2was higher than that of CuO2.Furthermore,the catalytic properties of CuO2@COF-NH2and laccase were compared by measuring theKmand the enzymatic reaction reaches thevmaxof catalysts (Fig.7).Compared with natural laccase,copper peroxide exhibits lowerKmvalue and highervmaxvalue,indicating that copper peroxide has better laccase-like activity(Table 1).The excellent catalytic performance of CuO2@COF-NH2was due to the following reason: i) the more uniform distribution of CuO2on COF-NH2which can efficiently improve the catalytic performance;ii) more substrate molecules may be enriched with the benzene ring-containing porous COF through non-covalent interactions (e.g.,and hydrophobic interactions) and efficiently increased contact between substrate and catalyst,and therefore improved the catalytic performance;iii) more active sites may be exposed after CuO2being loaded on COF-NH2and further increase catalytic capability of catalyst[26].

Table 1 Catalytic kinetics of CuO2@COF-NH2 and laccase towards the reaction between 4-AP and 2,4-DP at room temperature.
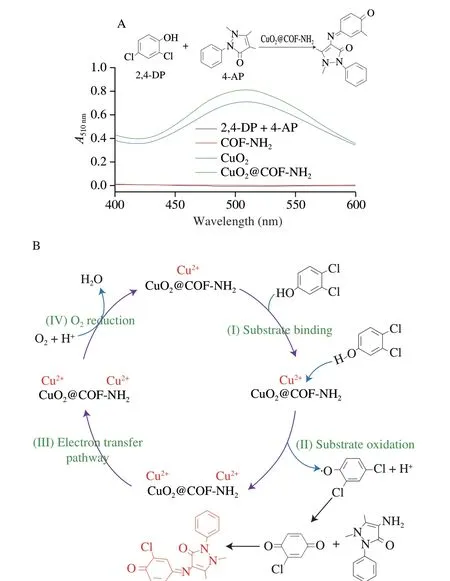
Fig.6 (A) Determination of laccase-like activity of CuO2@COF-NH2;(B) Speculation on laccase-like catalytic mechanism of CuO2@COF-NH2.
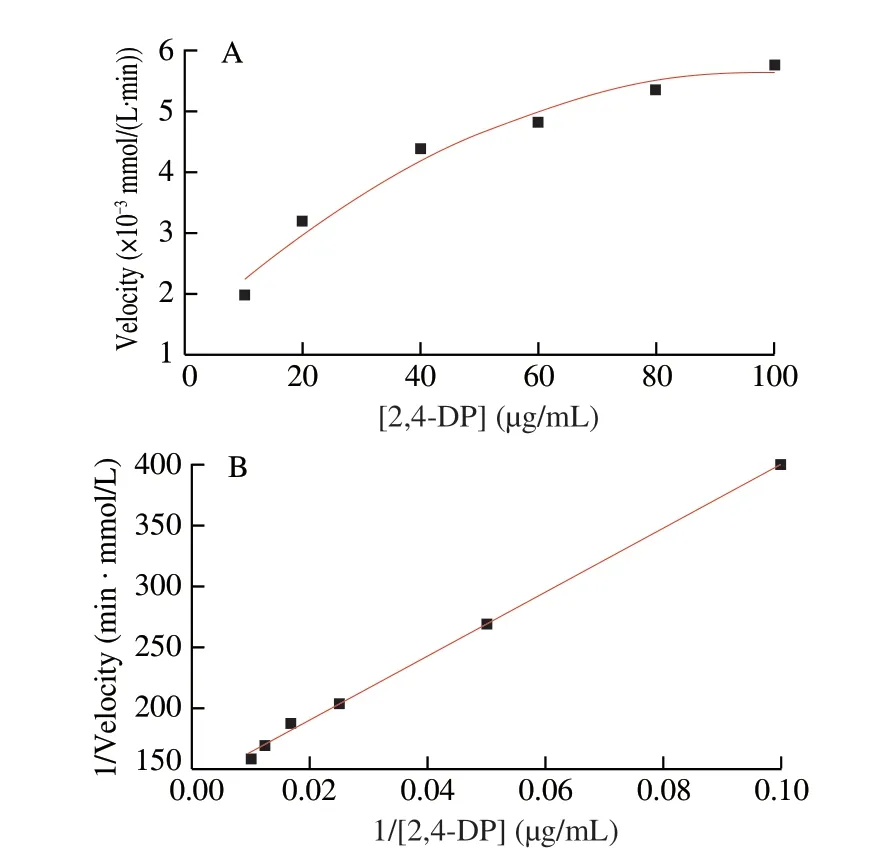
Fig.7 (A) Michaelis Menten and (B) Lineweaver-Burk plot of CuO2@COF-NH2.
Based on the above-mentioned outstanding laccase-like performance,CuO2@COF-NH2was employed as a nanoprobe for visual detection of NoV in a “Pep-NoV-antibody” sandwich ELISA assay.Natural laccase is a multi-copper containing oxidoreductases enzyme which can catalyze the oxidation of phenols,aromatic compounds or aliphatic amines with the reduction of molecular oxygen to water[16].Laccases catalyze four-electron substrate oxidations,in the laccase-catalyzed oxidation process,Cu1is the primary electron acceptor.Then electrons are transferred via a His-Cys-His tripeptide to a tri-nuclear cluster,which includes Cu2and Cu3atoms,next electrons reduce O2to H2O[27].According to the catalytic process of natural laccase,the laccase-like catalytic mechanism of CuO2@COF-NH2was speculated as follows: The laccase-like catalyzed oxidation process of CuO2@COF-NH2,includes four steps[28],(I) substrate binding,(II) substrate oxidation,(III)electron transfer and (IV) O2reduction to H2O.First,phenolic substrate 2,4-DP was absorbed to Cu2+of CuO2@COF-NH2nanozyme(Fig.6B).Then,phenolic substrates were oxidized to phenolic hydroxyl radicals,which can be observed from electron paramagnetic resonance (EPR) experiment (Fig.8).Meanwhile,Cu2+was reduced to Cu+.Subsequently,the reduced Cu+was further oxidized to Cu2+by O2which was regarded as the main acceptor site of single electron oxidation.And the electron was transferred from Cu+to Cu2+through space interaction (covalent bond and H-bond).Afterwards,Cu2+converted to Cu+after obtaining electrons.Owing to be short of protection of chemical group,Cu+was oxidized to Cu2+by O2in the reaction system,at the same time,O2was reduced to H2O by 4 electron transfer.Finally the CuO2@COF-NH2nanozyme were regenerated.
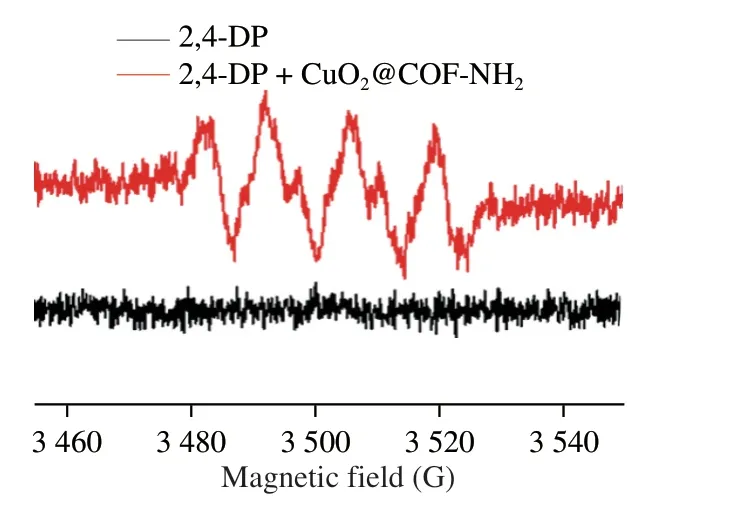
Fig.8 EPR spectra of the substrate (2,4-DP) catalyzed by CuO2@COF-NH2.
For the electrochemical detection,as shown in Fig.9,CuO2and CuO2@COF-NH2showed a strong oxidation peak at -0.05 and-0.13 V,respectively,which was attributed to the electron transfer from Cu+to Cu2+[29].It’s worth noting that,when CuO2was loaded on COF-NH2,the potential of oxidation peak of CuO2@COF-NH2shifted to a negative value possibly due to the π-π between benzene ring of COF-NH2and five-membered nitrogen heterocyclic ring of PVP on the surface of CuO2.Furthermore,combined with biological macromolecules through Cu-S.It can be employed as signal probe and capture probe in electrochemical biosensors.CV and EIS were used to evaluate the electrochemical properties of modified electrode materials.First,CV of (a) GCE and AuPt-PDA modified (b) GCE were measured in 5 mmol/L [Fe(CN)6]3-/4-and 0.2 mol/L KCl.As shown in Fig.10A,GCE showed a relatively low current,while AuPt-PDA modified GCE showed an increased current response,which caused by the excellent conductivity of AuPt-PDA,and can be used as a substrate material of electrodes.The current response reduced after the AuPt-PDA-modified GCE incubated with (c) Ab1,suggesting Ab1 was successfully fixed on the electrode.After incubation with(d) BSA,the current further decreased,indicating the non-specific site was successfully blocked.Next,the peak current response further decreased to a lower value after incubation with target (e) NoV,suggesting NoV was successfully bound on the electrode.Finally,after incubation with the probe (f) Pep/CuO2@COF-NH2,the peak current response was minimized.Moreover,the CV results are consistent well with those of EIS measurements (Fig.10B),further suggested that the electrode was successfully assembled step by step.
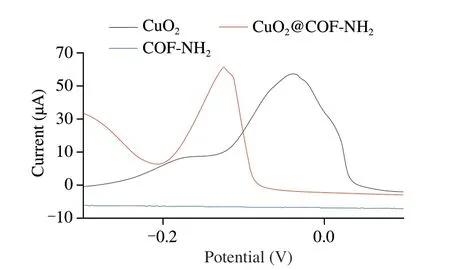
Fig.9 DPV for CuO2,CuO2@COF-NH2,and COF-NH2.

Fig.10 Assembly of modified electrodes characterized by (A) CV and (B) EIS in 0.1 mol/L PBS containing 5.0 mmol/L [Fe(C)6]3-/4- and 0.2 mol/L KCl: a,GCE;b,AuPt-PDA/GCE;c,Ab1/AuPt-PDA/GCE;d,BSA/Ab1/AuPt-PDA/GCE;e,NoV/BSA/Ab1/AuPt-PDA/GCE;f,Pep/CuO2@COF-NH2/NoV/BSA/Ab1/AuPt-PDA/GCE;(C) DPV curves for different concentrations of NoV in acetic acid buffer with pH 4.0;(D) Calibration plot for lg CNoV (copies/mL) vs.DPV response in the range of 1-5 000 copies/mL.Error bar=standard deviation (SD),n =3.
3.4 Optimization of experimental conditions
For the electrochemical assay,as shown in Fig.11A,with the increase of ratio of CuO2:COF-NH2(m/m),the current response increased gradually,when the ratio of CuO2and COF-NH2(m/m)was 5:1,the current response reached the maximum value,therefore 5:1 was selected as the optimal ratio of the composite.The current decreased when increasing the pH 3.5-6.0,when pH was 4.0,DPV current reach a higher value,so pH 4.0 was used as the optimal pH in the subsequent experiment (Fig.11B).In addition,the DPV current was increased when increasing the incubation time of Pep/CuO2@COF-NH2from 30 min to 80 min but was decreased more than 60 min(Fig.11C),and 60 min was selected as the optimal incubation time.Finally,the current was increased when increasing the probe concentration and when reached to 2 mg/mL (10 µL),the current decreased slightly (Fig.11D),therefore 2.0 mg/mL was regarded as the optimal probe concentration.
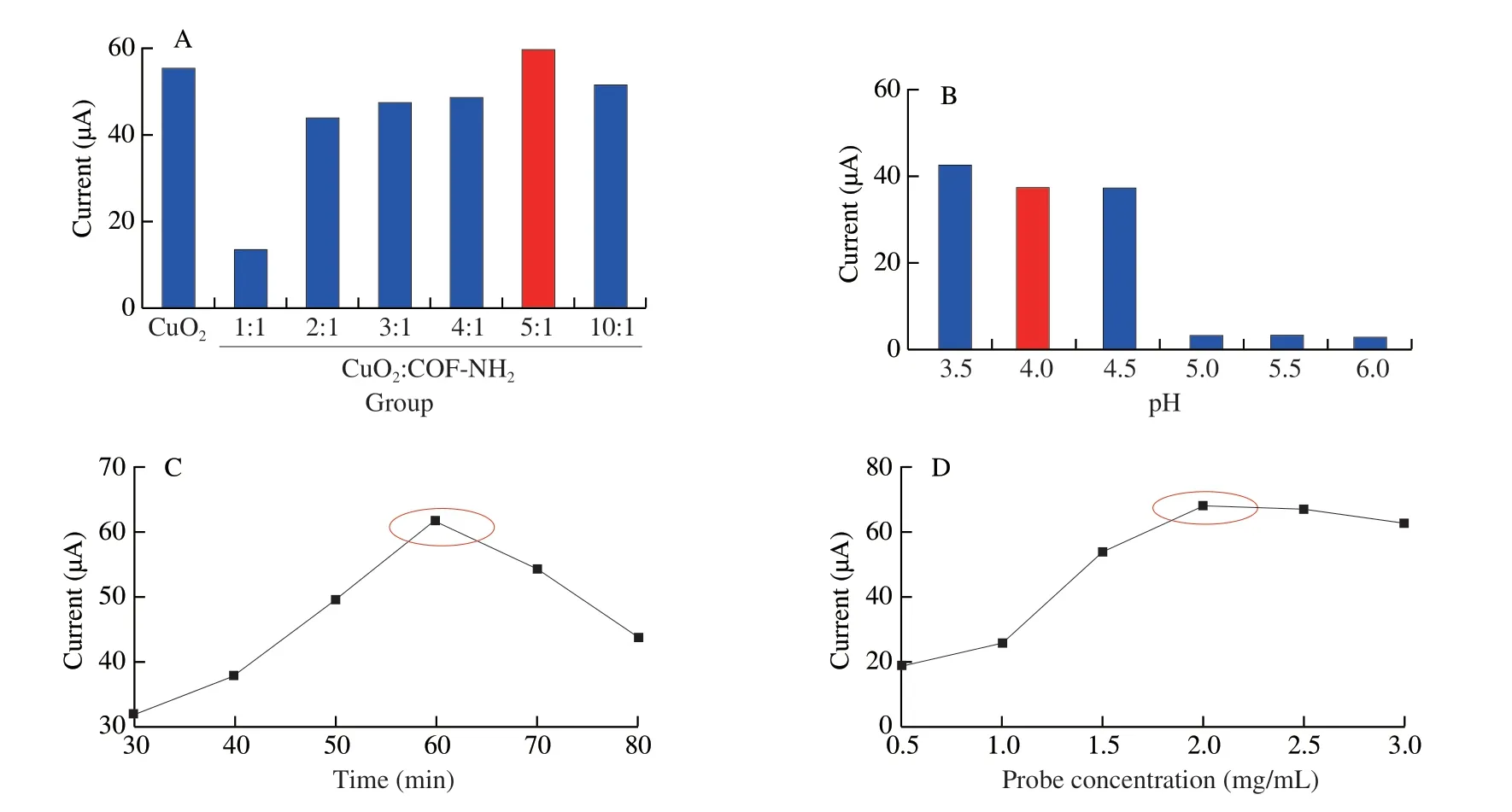
Fig.11 Optimization of the electrochemical experimental conditions.(A) Ratio of composite (CuO2:COF-NH2);(B) pH value;(C) incubation time of Pep/CuO2@COF-NH2 bioconjugates;(D) concentration of biological probe.
On the other hand,for the colorimetric assay,the optimal ratio of composite,the optimum pH,and probe concentration were screened.First,the absorbance increase with the increase from pH 3.5 to pH 6.8 while decreased from pH 6.8 to pH 7.0,therefore pH 6.8 was used as the optimal pH (Fig.12A).Next,CuO2@COF-NH2composite had the highest catalytic activity when the ratio of CuO2and COF-NH2of CuO2@COF-NH2composite was 5:1,consequently,therefore 5:1 was selected as the optimal proportion of composite (Fig.12B),it is interesting that although net weight of CuO2NPs in CuO2@COF-NH2was less than in CuO2,the CuO2@COF-NH2showed more excellent catalytic ability towards substrate oxidation.Finally,the absorbance was maximum when probe concentration was 1.5 mg/mL (Fig.12C),the optimal probe concentration was 1.5 mg/mL.
3.5 Dual-mode detection of NoV
Under the optimum conditions,the constructed biosensor was employed to detect NoV.For the electrochemical assay,as shown in Fig.10C,the response current increased with the increase of NoV concentration from 1 copy to 5 000 copies.The linear relationship between the logarithm of NoV concentration and the response current wasI(μA)=10.90 lgCNov(copies/mL)+14.64 (R2=0.996 8) with a LOD of 0.152 copy/mL (Fig.10D),the LOD was defined by the equation of LOD=3 × Sb/k,where Sbis the standard deviation of the control groups,andkis the slope of the calibration curve.To the best of our knowledge,our biosensor showed a lower LOD among the reported assays (Table 2),which can be applied in the detection of low concentration NoV.
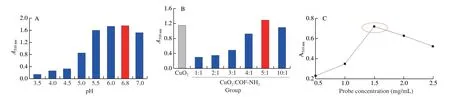
Fig.12 Optimization of the colorimetric experimental conditions.(A) pH value;(B) Ratio of composite (CuO2:COF-NH2);(C) concentration of biological probe.
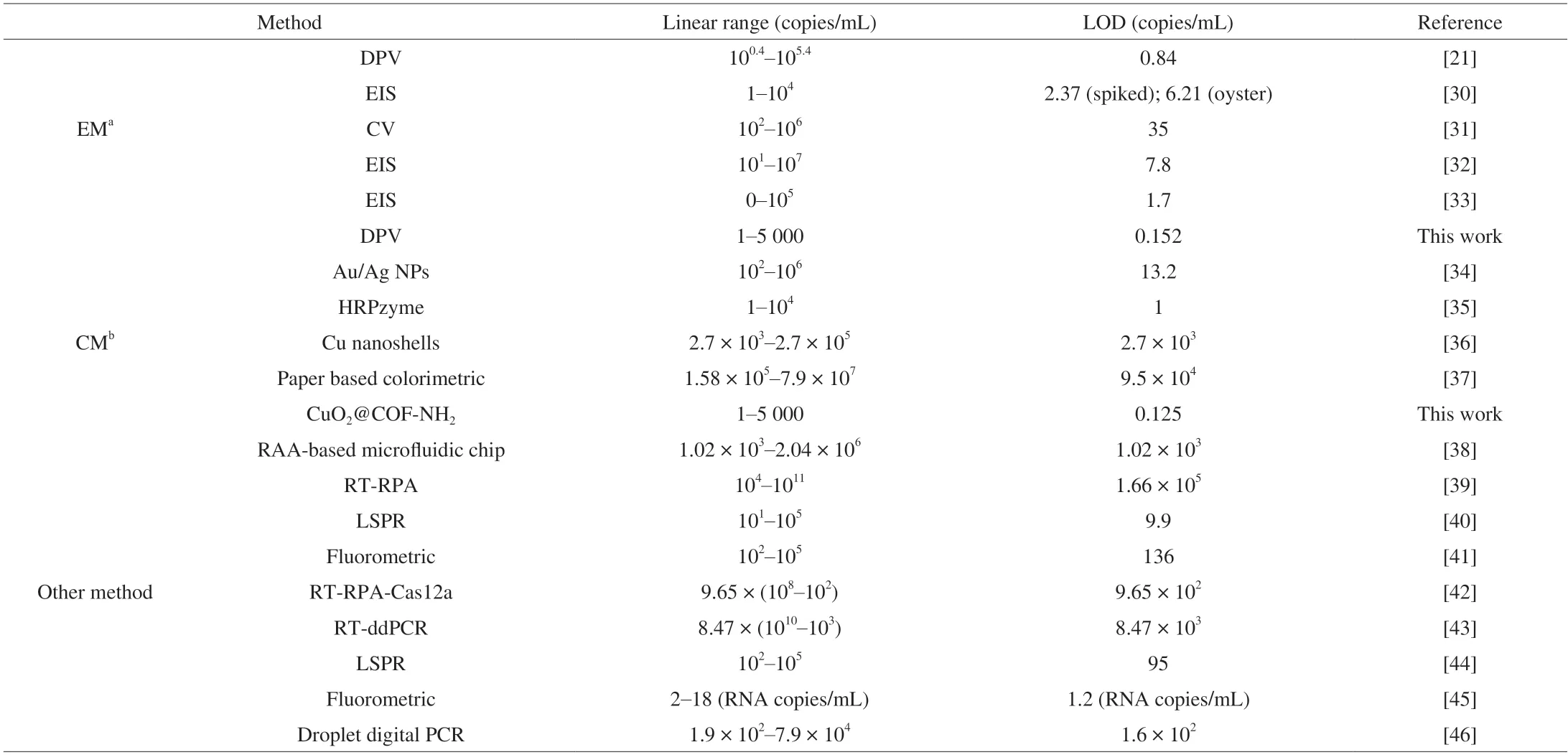
Table 2 Comparison of various immunosensors for detection of NoV.

Fig.13 (A) Absorbance curves for different concentrations of NoV in acetic acid buffer with pH 4.0;(B) Calibration plot for lg CNoV (copies/mL) vs.absorbance response in the range of 1-5 000 copies/mL.Error bar=standard deviation (SD),n=3.(C) Selectivity,and (D) repeatability of the electrochemical biosensor.
For colorimetric assay,as shown in Fig.13A,the absorbance at 510 nm increased with the increase of NoV concentration ranging 1-5 000 copies/mL.The linear relationship between the logarithm of NoV concentration and the absorbance value (510 nm) isA=0.168 7 lgCNov(copies/mL)+0.298 0 (R2=0.998 1) with a LOD of 0.125 copy/mL (Fig.13B),which is lower LOD among the reported assays (Table 2).Meanwhile,in the absence of NoV,the absorbance value at 510 nm was extremely low and the solution was almost colorless,however,in the presence of NoV,the color of solution changed to pink,which can identify NoV via observing the color depth of solution by naked eye.Thus,electrochemical and colorimetric dual-mode detection of NoV based on CuO2@COF-NH2nanocomposites were successfully constructed for ultrasensitive detection of NoV.Simultaneously,the constructed detection for NoV was compared with previous ones.For the IEM method,it requires a high viral load of fecal sample,which has low sensitivity and specificity.It is worthy to note that our assay showed a much lower LOD than previously reported assays (Table 2),which may be suitable to the detection of low NoV load samples.ELISA is also often used for the detection of NoV,while the use of natural enzyme(horse radish peroxidase,HRP) labeled antibodies is necessary,which may enhance the cost.In our method,nanozyme was used instead of HRP,which may lower the cost.For the RT-PCR assay,the extraction of viral nucleic acid and isothermal amplification processes are needed and therefore time-consuming.However,our method can directly detect virus particles without nucleic acid extraction,which can shorten the detection time efficiently.In this work,we constructed an electrochemical and colorimetric dual-mode assay for NoV detection.The electrochemical signal of the biosensor is caused by oxidation peak of copper ion from Cu+to Cu2+of CuO2@COF-NH2,which is different from the signal of MB reported in our previous work[21]and may be benefit to construction of a stable biosensor.Furthermore,specific peptide for NoV was bound to CuO2@COF-NH2through Cu-S bond to prepare probe,providing the cheaper linker than Au NPs in a previous work for fixation of recognition molecule.Simultaneously,due to excellent laccase-like activity of CuO2NPs which can catalyse the oxidation of phenolic substrates to generate color product,NoV can be directly detected by the naked eye.Therefore,CuO2@COF-NH2is not only used to fix aptamer of NoV but also used as electrochemical and colorimetric probe for construction of dual-mode biosensor of NoV.
3.6 Selectivity, reproducibility, and stability of biosensors
Firstly,the selectivity of electrochemical sensor was evaluated in presence of NoV (1 000 copies/mL) or interferents (1 mg/mL).Such as Na+,K+,glucose (Glu),AA,rotavirus (RV),enterovirus (EV),Escherichia coli,BSA,and lysozyme (LZ).As shown in Fig.13C,in the presence of individual interference or their mixture,electrochemical sensor didn’t show obvious current,however,in the presence of NoV,a strong current observed.The above results indicated that the biosensor constructed had a good selectivity for NoV.Secondly,the reproducibility of the immunosensor was evaluated by measuring 5 individual modified electrodes.It can be clearly seen that 5 electrodes showed similar current response,indicating that the sensor had a good reproducibility (Fig.13D).For colorimetric assay,the selectivity of sensor was evaluated in presence of NoV (1 000 copies/mL) or interferents (1 mg/mL).Similar to the electrochemical assay,the colorimetric assay had a good selectivity for NoV (Fig.14A).In addition,the reproducibility of the immunosensor was studied by measuring absorbance of 5 parallel wells.As shown in Fig.14B,5 parallel wells showed similar cabsorbance value at 510 nm,suggesting good reproducibility.Finally,the stability of the sensor was investigated by measuring absorbance value at 510 nm followed by storage at 4 °C.It can be clearly seen from Fig.14C that the absorbance of the constructed assay reached 93.81% of the initial absorbance value,showing the good stability of sensor.
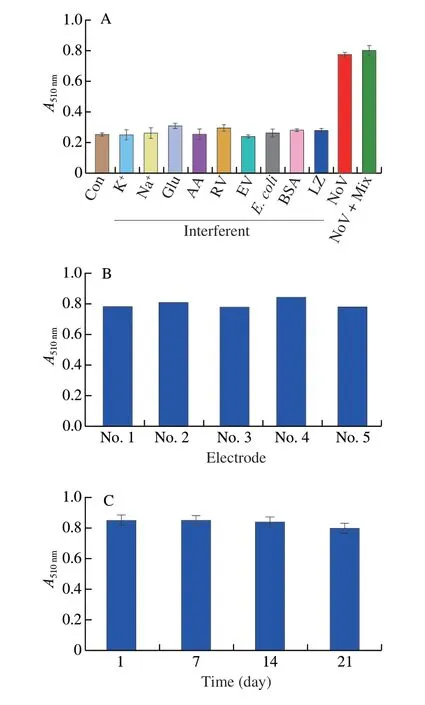
Fig.14 (A) Selectivity,(B) repeatability,and (C) stability of the colorimetric biosensor.
3.7 NoV determination in real sample
To evaluate the practicability of the sensor,the constructed sensor was used to detect NoV concentration in oyster,strawberry,and lettuce using a standard addition assay.Foodborne infection of NoV are commonly caused by oyster,strawberry,and lettuce[5],accurate detection of NoV in these samples is beneficial to human health.Firstly,1 g of oyster,strawberry,or lettuce was vortexed in 10 mL PBS buffer for 1 min to obtain oyster,strawberry,and lettuce samples,respectively.Then,various concentrations of NoV (10,102,103copies/mL) were added to oyster,strawberry,and lettuce samples.The corresponding results were shown in Table 3,the recoveries of the electrochemical sensor were 97.5%-104.7%,96.2%-103.8%,and 96.9%-104.3% for oyster,strawberry,and lettuce samples with three replicates,respectively.Meanwhile,the recoveries of the colorimetric sensor were 96.7%-104.8%,97.2%-104.1%,and 96.1%-103.7%for oyster,strawberry,and lettuce samples with three replicates,respectively.These results suggested that the sensor showed the potential to detect NoV in real samples.
Furthermore,the immunosensor was also further used to detect known NoV diluent in patients’ feces which had been quantified by RT-PCR assay in Supplementary Information.Above all,1 g of human feces was dispersed in 10 mL PBS to obtain a 10% feces sample for future use.Next,a total of 30 samples,including two GII.2,two GII.3,two GII.6,and 24 GII.4 of NoV were tested (Table S1).As shown in Fig.15,compared with the RT-qPCR results,the detection results of the colorimetric sensor constructed for the above 30 NoV samples showed that detection rate of GII.4 NoV was 100%,while that of GII.2,GII.3 and GII.6 samples was 50%.The results of 30 NoV samples detected by electrochemical sensor showed that detection rate of GII.4 NoV was also 100%,and that of GII.2,GII.3 and GII.6 samples was 50%,50% and 100%,respectively.Among the types of NoV,GII is the major type contributors to NoV infection in the world (70%-80%)[47],therefore the successfully detection of GII type NoV is of great importance.The above results showed that the assay constructed in this work had a good effect on the detection of NoV,which is expected to be used for the actual detection of NoV in complex matrix.
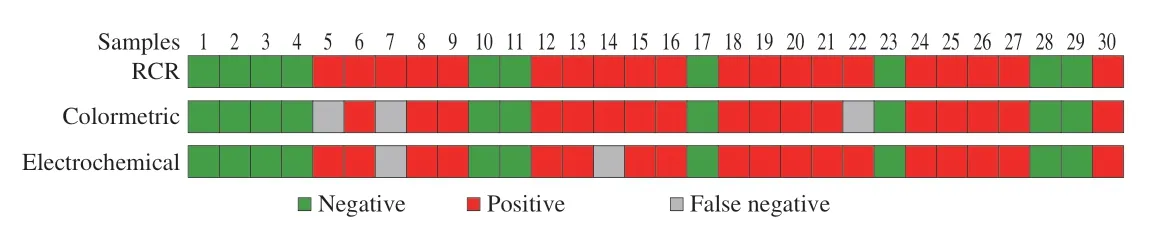
Fig.15 Detection results of NoV using RT-PCR and constructed electrochemical and colorimetric biosensors.
4.Conclusions
In summary,we constructed an electrochemical and colorimetric dual-mode immunosensor for NoV detection based on CuO2@COF-NH2nanocomposites.CuO2@COF-NH2nanomaterials show an oxidation peak of copper ion from Cu+to Cu2+in electrochemical detection,which can be used directly for electrochemical detection instead of electrical signal molecules such as MB.On the other hand,it can be used as a capture probe to couple with biomolecules.More interestingly,the CuO2@COF-NH2nanomaterials show laccase-like activity to realize naked-eye colorimetric detection of NoV.Both electrochemical and colorimetric immunosensor showed a wide linear range with a lower LOD for NoV detection.Meanwhile,our assay showed excellent selectivity,reproducibility,and good stability.The assay was successfully used to detect NoV in real samples.It is worthy to note that our assay does not need process of nucleic acid or protein extraction,which can shorten the pretreatment time efficiently.Meanwhile,the NoV can be determined at a low concentration by naked eye,which is benefit to the case of NoV identification under simple laboratory conditions,such as in the wild.Thus,this work provided a novel dual-mode strategy for the accurate detection of GII type of NoV,which has the potential application in the NoV detection in foods or the diagnosis of NoV-infcetion diseases.For the further application,in the biosensor for NoV,the long-time stability of CuO2@COF-NH2is needed,which is in progress in our laboratory.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was financially supported by National Key Research and Development Program of China (2022YFC2601604),Major science and technology project of Yunnan Province(202202AE090085),the National Natural Science Foundation of China (32 160597;32160236),Science and technology talent and platform plan of Yunnan,Key Scientific and Technology Project of Yunnan (202203AC100010),Spring City Plan: the High-level Talent Promotion and Training Project of Kunming (2022SCP001),and the second phase of “Double-First Class” program construction of Yunnan University,grants from State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan,Yunnan University (2021KF005),Key Scientific and Technology Project of Yunnan (202002AE320005),Program for Excellent Young Talents of Yunnan University,and the Program for Donglu Scholars of Yunnan University.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250079.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

