Functionalized selenium nanoparticles ameliorated acetaminophen-induced hepatotoxicity through synergistically triggering PKCδ/Nrf2 signaling pathway and inhibiting CYP 2E1
Si Zou,Yeto Gong,Xiujie Li,Ynbin Wu,Jinzhong Wu,Jinguo Wu,,K-Hing Wong
a College of Pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou 350122, China
b Research Institute for Future Food, The Hong Kong Polytechnic University, Hong Kong 999077, China
c Department of Food Science and Nutrition, The Hong Kong Polytechnic University, Hong Kong 999077, China
Keywords: PTR-SeNPs (polysaccharide-protein complex functionalized selenium nanoparticles)Acetaminophen-induced hepatotoxicity Nuclear factor erythroid 2-related factor 2 Cytochrome P450 enzyme 2E1 Mitochondria
ABSTRACT Selenium nanoparticles (SeNPs) have been demonstrated potential for use in diseases associated with oxidative stress.Functionalized SeNPs with lower toxicity and higher biocompatibility could bring better therapeutic activity and clinical application value.Herein,this work was conducted to investigate the protective effect of Pleurotus tuber-regium polysaccharide-protein complex funtionnalized SeNPs(PTR-SeNPs) against acetaminophen (APAP)-induced oxidative injure in HepG2 cells and C57BL/6J mouse liver.Further elucidation of the underlying molecular mechanism,in particular their modulation of Nrf2 signaling pathway was also performed.The results showed that PTR-SeNPs could significantly ameliorate APAP-induced oxidative injury as evidenced by a range of biochemical analysis,histopathological examination and immunoblotting study.PTR-SeNPs could hosphorylate and activate PKCδ,depress Keap1,and increase nuclear accumulation of Nrf2,resulting in upregulation of GCLC,GCLM,HO-1 and NQO-1 expression.Besides,PTR-SeNPs suppressed the biotransformation of APAP to generate intracellular ROS through CYP 2E1 inhibition,restoring the mitochondrial morphology.Furthermore,the protective effect of PTR-SeNPs against APAP induced hepatotoxicity was weakened as Nrf2 was depleted in vivo,indicating the pivotal role of Nrf2 signaling pathway in PTR-SeNPs mediated hepatoprotective eff icacy.Being a potential hepatic protectant,PTR-SeNPs could serve as a new source of selenium supplement for health-promoting and biomedical applications.1 These authors contribute equally to this work.*Corresponding authors.
1.Introduction
Acetaminophen (APAP) taken at therapeutic dose is generally c onsidered to be safe and effective.APAP is rapidly excreted after metabolized into nontoxic water soluble compounds,but a small fraction (approximately 5%-9%) of which is metabolized by the cytochrome P450 enzymes (CYPs),mainly CYP 2E1 into a highly reactive intermediate metabolite,namelyN-acetyl-p-benzoquinone imine (NAPQI),which is further detoxified by co njugation with glutathione (GSH)[1].In case of APAP overdose,excessive NAPQI is produced together with extensive GSH depletion and overproduction of reactive oxygen species (ROS).The excessive ROS results in endogenous oxidative stress,ultimately causing cytotoxicity,cellular dysfunction even necrosis[2-3].N-acetyl cysteine (NAC),an antioxidant as well as a precursor for cellular GSH synthesis,is the primary antidote in clinical use for attenuating APAP-induced hepatotoxicity[4].However,due to side effects and narrow therapeutic time window,there is pressing need to develop new drugs superior to NAC[5].
Based on the hepatotoxicity-inducing mechanism of APAP,suppression of CYP 2E1 activity and e licitation of Phase II antioxidant enzyme expression represent as a promising detox strategy through effectively reducing the oxidative stress in hepatocytes[6].Usually,oxidative stress,which is regarded as an imbalance,results from altered gene expression[7].Nuclear factor erythroid 2-related factor 2 (Nrf2),an important nuclear transcription factor,regulates the constitutive and inducible expression of many intracellular detoxifying and antioxidant genes that account for cytoprotective processes,including GSH synthesis;antioxidative stress system activation;and conjugation,transportation and excretion of toxic metabolites[8].Normally,Nrf2 is sequestered in the cytoplasm by a Kelch-like ECHassociated protein 1 (Keap1) under physiologic unstressed conditions.However,when stimulated by exogenous and endogenous molecules,such as ROS,Keap1 inhibitor or Keap1-Nrf2 protein-protein interaction inhibitors,Nrf2 dissociates from Keap1 and transfers into nucleus,further interacts with an antioxidant response element(ARE),thereby transcriptionally activating an array of cytoprotective and antioxidant genes,such as glutathione peroxidases (GSH-Px),glutamate cysteine ligase (GCL),heme oxygenase-1 (HO-1),and NADPH quinone reductase-1 (NQO1)[9].Protein kinase C (PKC),especially the PKCδ isoform,facilitate dissociation from Keap1 and nucleus translocation,thus regulating the activation of the Nrf2/ARE pathway[10].Mounting evidence has confirmed that the activation of PKCδ/Nrf2 signaling pathway contributed significantly to the prevention of oxidative stress-induced cell damage[11].Thus,the PKCδ/Nrf2 signaling pathway might serve as a potential target for the development of hepatoprotectants against APAP[12].
Selenium (Se),functions as an essential component of selenoproteins,such as GSH-Px,and exerts cytoprotective property from oxidative damage[13].Se supplementation has positive health benefits,but Se deficiency or overdose may cause undesirable consequences[14].Recently,Se nanoparticles (SeNPs) have been garnering additional attention due to their unique physicochemical properties,promising biomedical functions as well as low toxicity,thus they are regarded as a new candidate for Se supplementation[15].A previous study showed that SeNPs could improve liver function and possess a protective activity against APAP-induced hepatotoxicity in male Sprague-Dawley rats by increasing hepatic GSH contents and glutathione reductase activity[16].Besides,SeNPs have been reported to protect brain and kidney against acetaminophen toxicity via ameliorating the oxidative stress in male albino rats[17].Moreover,SeNPs were found to exhibit protective effect against cyclophosphamide-induced hepatotoxicity and genotoxicity in Swiss albino mice[18].Nevertheless,SeNPs are poor in stability and easy to aggregate and precipitate,greatly hindering their further development and application[19].Functionalization of the SeNPs,which has emerged as a hot research issue,helps in enhancing the chemotherapeutic activity with lower toxicity and higher biocompatibility.
Pleurotustuber-regium(Fr.) Sing.(PTR),an edible basidiomycete,has been utilized as a functional food to enhance physique.Direct feeding with PTR was found to protect against the CCl4induced oxidative damage of the hepato-renal system in albino rats possibly through its antioxidant defense mechanism[20-21].And short-term oral treatment with hot aqueous extract of PTR could significantly reduce the elevated aspartate aminotransferase (AST),alanine aminotransferase (ALT) and alkaline phosphatase (ALP) in both APAP and CCl4models of liver toxicity[22].A previous study showed that polysaccharides from the sclerotium of PTR possessed strong antioxidant properties,including scavenging effect on free radicals as well as inhibitory effect on mice liver lipid peroxidation,liver mitochondria swelling,and red blood cell hemolysis,indicating their great potential to act as effective antioxidant for alleviating oxidative stress[23].By using the polysaccharide-protein complex extracted from PTR sclerotium as capping agent,we have successfully prepared a highly stable and safe SeNPs (entitled “PTR-SeNPs”) with a wide spectrum of beneficial effects such as antibacterial,antifungal,anticancer and bone formation[24-28].
Based on the remarkable hepatoprotective effect and antioxidant activity of SeNPs and PTR,in this study,bothinvitroandinvivoprotective effects of PTR-SeNPs against APAP-induced hepatotoxicity were investigated.Besides,further elucidation of the underlying molecular mechanism,in particular their modulation of Nrf2 signaling pathway and CYP 2E1 inhibition was also conducted.Findings of this study would provide important insights into the clinical application of SeNPs on oxidative stress-associated diseases.
2.Materials and methods
2.1 Materials and chemicals
Polysaccharide-protein-complex was extracted from mushroom sclerotia ofP.tuber-regium(PTR).3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT),tert-Butylhydroquinone(t-BHQ),phosphate buffer saline (PBS),dimethyl sulfoxide (DMSO),sodium selenite (Na2SeO3),vitamin C were purchased from from Sigma Aldrich (Saint Louis,Missouri,USA).Minimum essential medium (MEM),penicillin-streptomycinxture,0.25% trypsin-EDTA,fetal bovine serum (FBS) were purchased from Gibco-BRL Life Technologies (Grand Island,NJ).APAP and NAC were obtained from Yuanye Bio-technology Co.,Ltd.(Shanghai,China).ALT,AST,ALP,lactate dehydrogenase (LDH),malondialdehyde (MDA),catalase (CAT),superoxide dismutase (SOD),GSH-Px and GSH assay kits were purchased from Jiancheng Institute of Biotechnology(Nanjing,China).Nuclear and Cytoplasmic Protein Extraction kit was obtained from KeyGEN Biotechnology Development Co.,Ltd.(Nanjing,China).Anti-PKCδ,Anti-p-PKCδ (Tyr311 site),anti-Keap1,Anti-GCLC (GCL catalytic subunit),anti-GCLM (GCL modifier subunit),CYP 2E1,anti NQO-1,HO-1 antibodies were purchased from Abcam (Cambridge,MA,USA).Antibody directed against Nrf2 was obtained from Cell Signaling Technology (Beverly,MA,USA).Anti-Lamin B1 monoclonal antibody was purchased from Proteintech (Chicago,IL,USA).Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse IgGs were gained from Millipore(Billerica,MA,USA).All other chemicals were also obtained from commercial sources with the highest purity available.
2.2 Cells and mice
The hepatocellular carcinoma HepG2 cell line was obtained from American Type Culture Collection (ATCC,Rockville,MD).The cells were cultured in MEM supplemented with 10% FBS,streptomycin(100 µg/mL),and penicillin (100 U/mL) at 37 °C in a humidified atmosphere of 95% air and 5% CO2.C57BL/6J wild-type (WT)mice and Nrf2 knock out (KO) mice (8-week old,20-25 g) were provided by Cyagen Biosciences INC (Suzhou,China) and housed in a specific pathogen-free environment with a light/dark cycle(12 h/12 h),temperature ((24.0 ± 1) °C);and humidity ((65.0 ± 5)%)control.All animal experimental procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals,with the approval of the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (FJTCM IACUC: 2020072).
2.3 Preparation and characterization of PTR-SeNPs
Standardized PTR-SeNPs was prepared using our patented nanotechnology (US patent No.: 9072669) using the PTR as capping agent.In brief,aqueous PTR (0.25%) was mixed with freshly prepared ascorbic acid (100 mmol/L) under magnetic stirring prior to drop-wise addition of aqueous sodium selenite (25 mmol/L).After reconstituting with MiliQ water to 25 mL,the mixture was reacted under room temperature with stirring for 12 h,followed by extensive dialysis (Mwcutoff: 8 000 Da).Structure of the resulting PTR-SeNPs was further characterized by Zetasizer Nano ZS particle size analyzer(Malvern Instruments Ltd.),HR-TEM-EDX (JEOL 2010,Horiba EX-250),and FT-IR (Bruker Equinox 55) as previously described[26].
2.4 Antagonistic effect of PTR-SeNPs against APAP-induced cytotoxicity in HepG2 cells
Firstly,a dose-dependent study of the cytotoxicity of APAP as well as the maximal noncytotoxic concentration (MNCC) oft-BHQ(positive control) and PTR-SeNPs was conducted.In brief,HepG2 cells (1 × 105cells/well,96-well plate) were cultured inα-MEM supplemented with 10% FBS,streptomycin (100 µg/mL),and penicillin (100 U/mL) in 37 °C incubators with 95% relative humidity and 5% CO2.After treatment with different concentrations of APAP(0-50 mmol/L),PTR-SeNPs (0-40 µmol/L) ort-BHQ (0-100 µmol/L)for 12 h,their cell viability was determined by MTT assay as described previously[29].Based on the cytotoxicity of APAP as well as the MNCC oft-BHQ and PTR-SeNPs,further investigation on the protective effect of PTR-SeNPs against APAP-induced cytotoxicity was performed in HepG2 cells,which were grouped and treated as follows:
Control: Blank medium (12 h)+Blank medium (12 h) [Con.];MNCC: PTR-SeNPs (10 µmol/L,12 h)+Blank medium (12 h)[MNCC];Model: Blank medium (12 h)+APAP (30 mmol/L,12 h)[Mod.];Positive control:t-BHQ (30 µmol/L,12 h)+APAP (30 mmol/L,12 h) [Posit.];High dose: PTR-SeNPs (10 µmol/L,12 h)+APAP(30 mmol/L,12 h) [H.D.];Medium dose: PTR-SeNPs (5 µmol/L,12 h)+APAP (30 mmol/L,12 h) [M.D.];Low dose: PTR-SeNPs(2.5 µmol/L,12 h)+APAP (30 mmol/L,12 h) [L.D.].
Apart from cell viability assay,the cell morphological features of all experimental groups were visualized under an inverted phase contrast microscopy (magnification 200×,Olympus Corporation,Center Valley,PA).The experiment was performed in triplicate.Cell viability was expressed as a percentage relative to the control.
2.5 Cellular antioxidant capacity assays
Briefly,HepG2 cells (2 × 106cells/well) were seeded in 6-well plates and incubated at 37 °C for 24 h,which were grouped and treated as described above.The culture medium was collected for the spectrophotometric analysis of AST and ALT levels using commercial testing kits.For determining the intracellular MDA and GSH contents as well as the SOD,CAT,and GSH-Px activities,the cells were washed 3 times with PBS followed by homogenization in an ice bath using ultrasonic homogenizer (Ningbo Scientz,JY92-IIN,Zhejiang,China).After centrifugation (10 000 ×g,15 min,4 °C),the supernatants of cell lysates were analyzed by the commercial testing kits according to manufacturer’s instructions.
2.6 Cellular uptake efficiency and intracellular localization
A time-dependent study on cellular uptake efficiency of coumarin-6-loaded PTR-SeNPs in HepG2 cells was conducted as previously described with some modifications[26].Briefly,HepG2 cells(5 × 105cells) were seeded in 6-well plates and incubated for 24 h.After treatment with 120 µmol/L of 6-coumarin-loaded PTR-SeNPs(C6-PTR-SeNPs) at 37 °C for 0,30,60,90 and 120 min,the cellular uptake efficiency of PTR-SeNPs by HepG2 cells was observed by an inverted fluorescence microscope (200×,OlympusIX70,Tokyo,Japan).For tracing intracellular localization of PTR-SeNPs,the HepG2 cells were sequentially treated with Lysotracker Red (50 nmol/L;2 h;red),C6-PTR-SeNPs (120 µmol/L;5 min;green) and DAPI(0.3 mmol/L;30 min;blue) at 37 °C for 5 min prior to visualization using fluorescence microscopy.
2.7 Measurement of intracellular ROS generation
In brief,HepG2 cells (5 × 105cells/well) were firstly seeded in 96-well black/clear bottom plates and incubated at 37 °C for 24 h,which were grouped and treated as described above.The cells were further stained with 10 mmol/L DCFH-DA at 37 °C for 30 min in dark according to the manufacturer’s instruction (KeyGen Biotech,Nanjing,China).The intracellular ROS generation was then visualized under an inverted fluorescence microscope (200×,OlympusIX70,Tokyo,Japan) and quantified by using an Infinite M200 pro microplate reader (Tecan Trading AG,Männedorf,Switzerland) at an excitation wavelength of 485 nm and emission wavelength of 528 nm.
2.8 Observation for the ultrastructure of cellular mitochondria
Briefly,HepG2 cells (2 × 106cells/well) were seeded in 6-well plates and incubated at 37 °C for 24 h.After sequential treatment with PTR-SeNPs (5 µmol/L,12 h)/t-BHQ (30 µmol/L,12 h) and APAP(30 mmol/L,12 h),the cells were firstly digested with pancreatin and fixed in 3% glutaraldehyde at 4 °C for 2 h.Samples were then washed with PBS,post-fixed with osmic acid,dehydrated in acetone and embedded in epoxide resin.Ultrathin sections were cut using a Leica Ultracut microtome (Leica,Deerfield,IL) followed by staining with uranyl acetetate and lead citrate.The stained samples were examined in a Hitachi-7650 transmission electron microscope (TEM,Hitachi Ltd,Tokyo,Japan) using an accelerating voltage of 80 kV.
2.9 RNA extraction and real time-PCR analysis
Considering the important role of antioxidases in defending hepatocytes from oxidative damage,quantitative real-time PCR(qRT-PCR) assay was performed to examine the promoting effects of PTR-SeNPs on mRNA expression of major antioxidases such asGCLC,GCLM,HO-1andNQO-1in the HepG2 cells.Briefly,HepG2 cells (2 × 106cells) were seeded and incubated at 37 °C for 24 h,which were grouped and treated as described above.Total RNA was isolated from each group using TRIzol reagent according to manufacturer’s protocol (Life Technologies,New York,USA).RNA content and purity were determined according to the ratio of its absorbance at 260 and 280 nm.For cDNA synthesis,total RNA precipitate was air-dried and dissolved in RNAse-free water.RNA sample (2 µg) was then reversely transcribed into cDNA in a final reaction volume of 20 µL by using the PrimeScript RT reagent kit(Takara Biotechnology,Dalian,China).The cDNA templates were stored at -20 °C prior to further qRT-PCR assay.
qPCR analysis of major antioxidases such as GCLC,GCLM,HO-1 NQO-1 and GAPDH was performed by using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems,Carlsbad,CA,USA) with the following primer sequences:GCLCsense: TGTCCGAGTTCAATACAGTTGA,GCLCantisense: ACAGCCTAATCTGGGAAATGAA;GCLMsense: CATTTACAGCCTTACTGGGAGG,GCLMantisense:ATGCAGTCAAATCTGGTGGCA;HO-1sense: AAGACTGCGTTCCTGCTCAAC,HO-1antisense:AAAGCCCTACAGCAACTGTCG;NQO-1sense:GAAGAGCACTGATCGTACTGGC,NQO-1antisense: GGATACTGAAAGTTCGCAGGG;GAPDHsense: TGCACCACCAACTGCTTAGC,GAPDHantisense:GGCATGGACTGTGGTCATGAG.RT-qPCR reactions were carried out in 20 µL volumes containing 1 µL of cDNA template,10 µL of ChamQ SYBR Color qPCR Master Mix (Vazyme Biotech Co.,Ltd.,China) and 0.4 µL of each specific primer under the following amplification conditions: 95 °C for 30 s,40 cycles of 95 °C for 10 s,and 60 °C for 30 s.A melting-curve analysis was performed with the default setting of the instrument.Relative mRNA expression was calculated by the 2-ΔΔCTmethod and normalized to that of GAPDH.
2.10 Antioxidant indexes determination in mice
In brief,WT and Nrf2 KO mice were randomly assigned to the following 7 experimental groups (n=12 per group):
Control: vehicle (0.9% NaCl)[Con.];PTR-SeNPs Control:PTR-SeNPs (10 µmol/kg) [PTR-SeNPs Con.];Model: APAP(400 mg/kg) [Mod.];Positive control: NAC (1 200 mg/kg)+APAP(400 mg/kg) [Posit.];High dose: PTR-SeNPs (10 µmol/kg)+APAP(400 mg/kg) [H.D.];Medium dose: PTR-SeNPs (5 µmol/kg)+APAP(400 mg/kg) [M.D.];Low dose: PTR-SeNPs (2.5 µmol/kg)+APAP(400 mg/kg) [L.D.].
After intraperitoneal (i.p) administration of 0.9% NaCl (vehicle),PTR-SeNPs (10,5 and 2.5 µmol/kg) or NAC (1 200 mg/kg) for 7 days,mice were i.p.injected with APAP (400 mg/kg in 0.9%NaCl) 1 h after the final dose.In addition to blood collection,livers of all experimental animals were excised for further biochemical,histopathological and immunoblotting analyses after APAP administration for 6 h.
All blood samples were centrifuged (2 000 ×g,15 min,4 °C) for serum separation.The liver tissue were homogenized in 50 mmol/L the cold phosphate buffer pH 7.4 for 5 min followed by centrifugation(2 000 ×g,15 min,4 °C) to obtain supernatants.The serum ALT,AST,ALP and LDH levels as well as hepatic SOD,CAT,MDA,GSH and GSH-Px activities were then analyzed by freedom evolyzer(Tecan,Mannedorf,Switzerland) according to manufacturer’s instruction.
2.11 Hematoxylin and eosin staining
Liver tissue were fixed,dehydrated,defatted in xylene followed by paraffin embedding.Paraffin-embedded liver sections (4 μm)were then undergone hematoxylin and eosin (H&E) staining prior to histopathological examination by optical microscopy (Leica DM4000 B LED,Wetzlar,Germany;magnification 200×).
2.12 Western immunoblot analysis
Western immunoblot analysis was further performed to find out whether Nrf2 signaling pathway and CYP 2E1 inhibition is mechanistically linked to the PTR-SeNPs mediated hepatoprotective efficacy.In brief,total and nuclear proteins were isolated from HepG2 cells or liver tissue using a commercial kit followed by determination of protein concentration with BCA protein assay.Equivalent amounts of denatured proteins were loaded and separated on 12% SDS-PAGE gels using gel electrophoresis.Separated proteins were then transferred to PVDF membranes using iBlot 2 Dry Blotting System.After blocking with 5% skimmed milk,all membranes were probed with specific primary antibodies overnight at 4 °C prior to further incubation with appropriate horseradish peroxidase conjugated secondary antibody for at least 1 h.Chemiluminescence was detected by using a ChemiDoc XRS imaging system (Bio-Rad Laboratories,Hercules,CA,USA).
2.13 Statistical analysis
All data represented as means ± standard deviation of at least three determinations.Statistical analysis was performed by oneway ANOVA with SPSS software version 22.0 (SPSS,Chicago,IL,USA).Difference with**P< 0.01 or*P< 0.05 indicated statistical significance.
3.Results
3.1 Preparation and characterization of PTR-SeNPs
By using the PTR as capping agent,standardized PTR-SeNPs(Se concentration (1.35 ± 0.12) mmol/L) were successfully prepared by using our patented nanotechnology (US patent No.: 9072669).Further structural characterization indicated that PTR-SeNPs existed as highly uniform and mono-dispensed spherical particles with an average core size of around 19.8 nm (Fig.1A).Besides,PTR-SeNPs was found to be highly stable in aqueous solution without significant change in its hydrodynamic size ((148 ± 2.20) nm) and zeta potential ((-17.6 ± 0.47) mV),over 14 weeks (Figs.1B and C).For individual PTR-SeNP,the clear lattice fringes/d-spacing (3.32 Å),SAED pattern (diffuse rings constructed with white dots) as well as EDX elemental composition collectively suggested that the resulting nanoparticle possessed a polycrystalline structure with very high level of Se (98.5%)(Figs.1A,D and E).As shown in the FT-IR spectrum (Fig.1F),absorption peaks at wavelengths 1 643 cm-1and 1 540 cm-1indicated the presence of amide groups (amide I and II) in protein moiety of PTR,while the absorption peak at wavelength 3 410 cm-1indicated the presence of the stretching vibrations of hydroxyl groups of its polysaccharides.More importantly,PTR shared a very similar FT-IR spectrum to that of PTR-SeNPs,indicating that PTR contributed as part of the PTR-SeNPs nanocomposite.
3.2 Antagonistic effect of PTR-SeNPs against APAP-induced cytotoxicity in HepG2 cells
Prior toin vitrocytoprotective studies,the cytotoxicity of APAP and MNCC of PTR-SeNPs andt-BHQ were evaluated in HepG2 cells by MTT assay.As shown in Fig.2A,APAP remarkably decreased the cell viability with dose dependent manner (P< 0.01) with an IC50value of 43.61 µmol/L for 12 h.By demonstrating a significant cytotoxicity (about 30% of growth inhibition),treatment with APAP at 30 mmol/L for 12 h was selected as an effective challenge to establish a hepatocyte model with oxidative damage for subsequentin vitrostudies.With reference to the MNCC of PTR-SeNPs (10 µmol/L)andt-BHQ (30 µmol/L) (Figs.2B and C),the dosage of PTR-SeNPs andt-BHQ (positive control) used for studying thein vitroprotective effect of PTR-SeNPs against APAP-induced cytotoxicity was 2.5-10 µmol/L and 30 µmol/L,respectively.
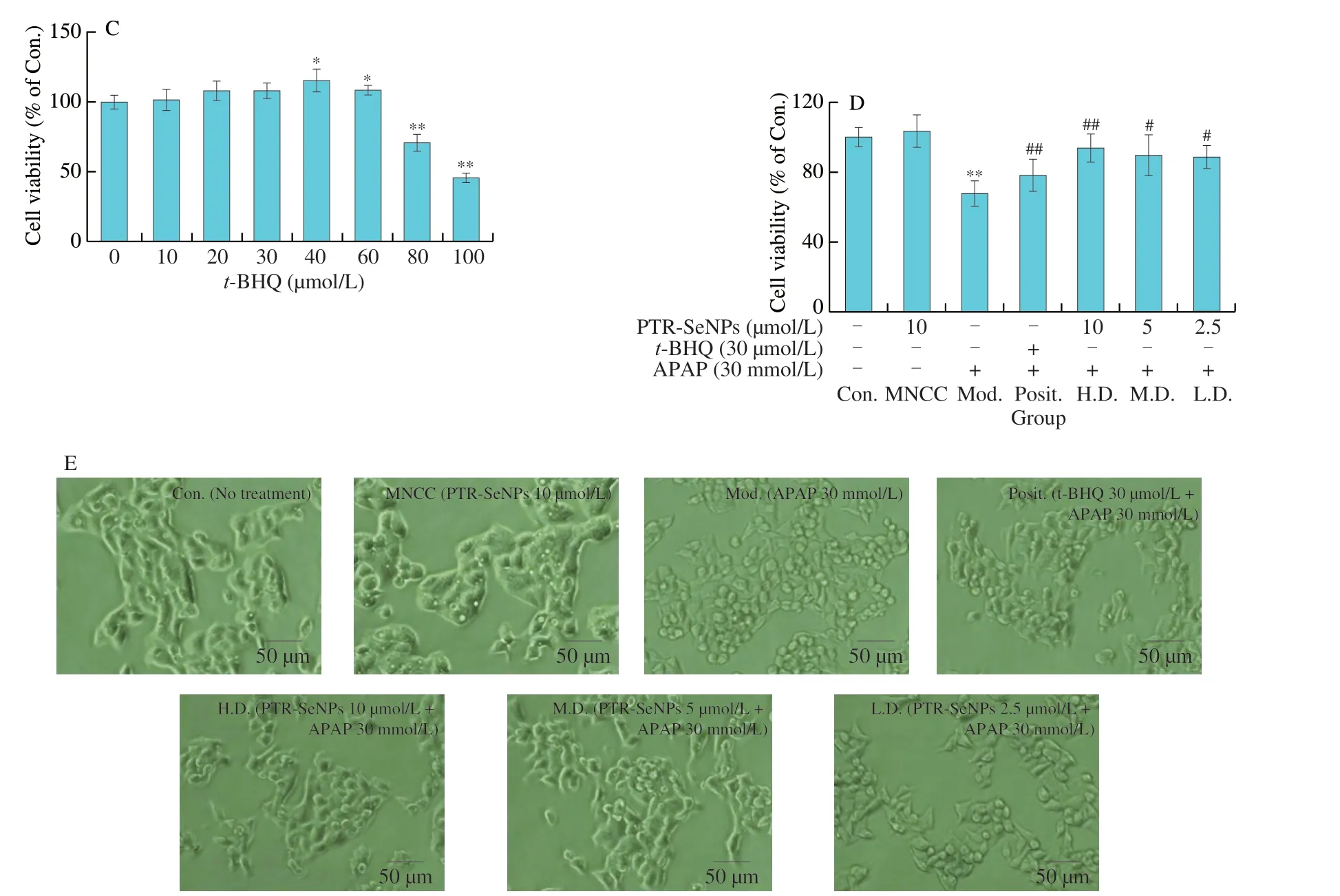
Fig.2 (Continued)
As presented in Fig.2D,compared with control cell,30 mmol/L APAP challenge obviously suppressed the cell viability of HepG2 cells (P< 0.01),which was notably antagonized by pretreatment with indicated concentrations of PTR-SeNPs ort-BHQ.The cell viability was conspicuously increased by 36.75% (H.D.),30.33% (M.D.),29.30% (L.D.) and 14.16% (Posit.),respectively,as compared with that in the Mod.group.Additionally,treatment with 30 mmol/L APAP had caused some morphological changes,appearing to be contractive and round,as compared with the Con.,which were reverted by pretreatment with PTR-SeNPs ort-BHQ,as shown in Fig.2E.The result of morphological observation was coincident with that of MTT assay,suggesting that PTR-SeNPs possessed antagonistic effect against APAP-induced cytotoxicity in HepG2 cells.
3.3 Protective effect of PTR-SeNPs against APAP-induced oxidative injury in HepG2 cells
The protective effect of PTR-SeNPs against APAP-induced cytotoxicity in HepG2 cells was evaluated in terms of the ALT and AST levels in culture medium as well as the intracellular MDA and GSH contents as well as SOD,CAT and GSH-Px activities.As shown in Fig.3A,compared with the Con.group,30 mmol/L APAP challenge (Mod.) resulted in a significant increase in the leakages of AST and ALT into culture medium by 718.1% and 143.3% (P< 0.01),respectively.In contrast,pretreatment of the cells with PTR-SeNPs (H.D.group,10 µmol/L and M.D.group,5 µmol/L) ort-BHQ (Posit.group,30 µmol/L) significantly inhibited the leakage of both ALT and AST in culture medium.These results indicated that PTR-SeNPs pretreatment protected hepatocytes from APAP-induced cytotoxicity.
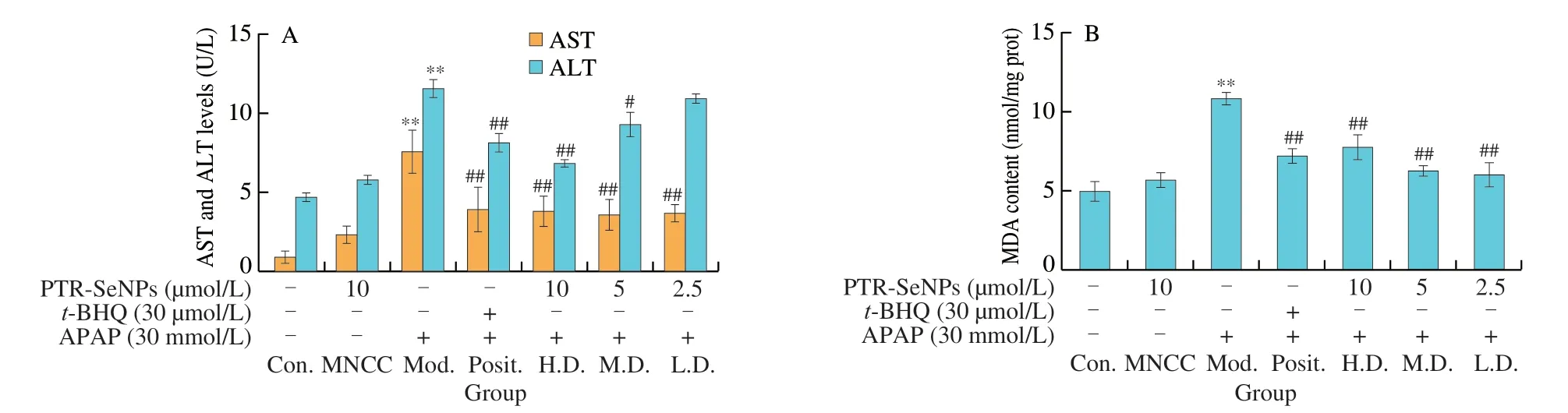
Fig.3 Biochemical analyses for the protective effect of PTR-SeNPs against APAP induced oxidative injure in HepG2 cells.(A) ALT and AST levels in the culture medium.Determination of the intracellular antioxidant status including (B) MDA and (C) GSH levels,as well as (D) SOD,(E) CAT and (F) GSP-Px activities.Cells,including control (Con.),MNCC,model (Mod.),high dose (H.D.),medium dose (M.D.) and low dose (L.D.) groups,were treated and grouped as detailed in Fig.2 caption.All data are expressed as the means ± SD (error bars) of values from three independent experiments.*P < 0.05 and **P < 0.01 were considered significant vs the control group.#P < 0.05 and ##P < 0.01 were considered significant vs the model group.
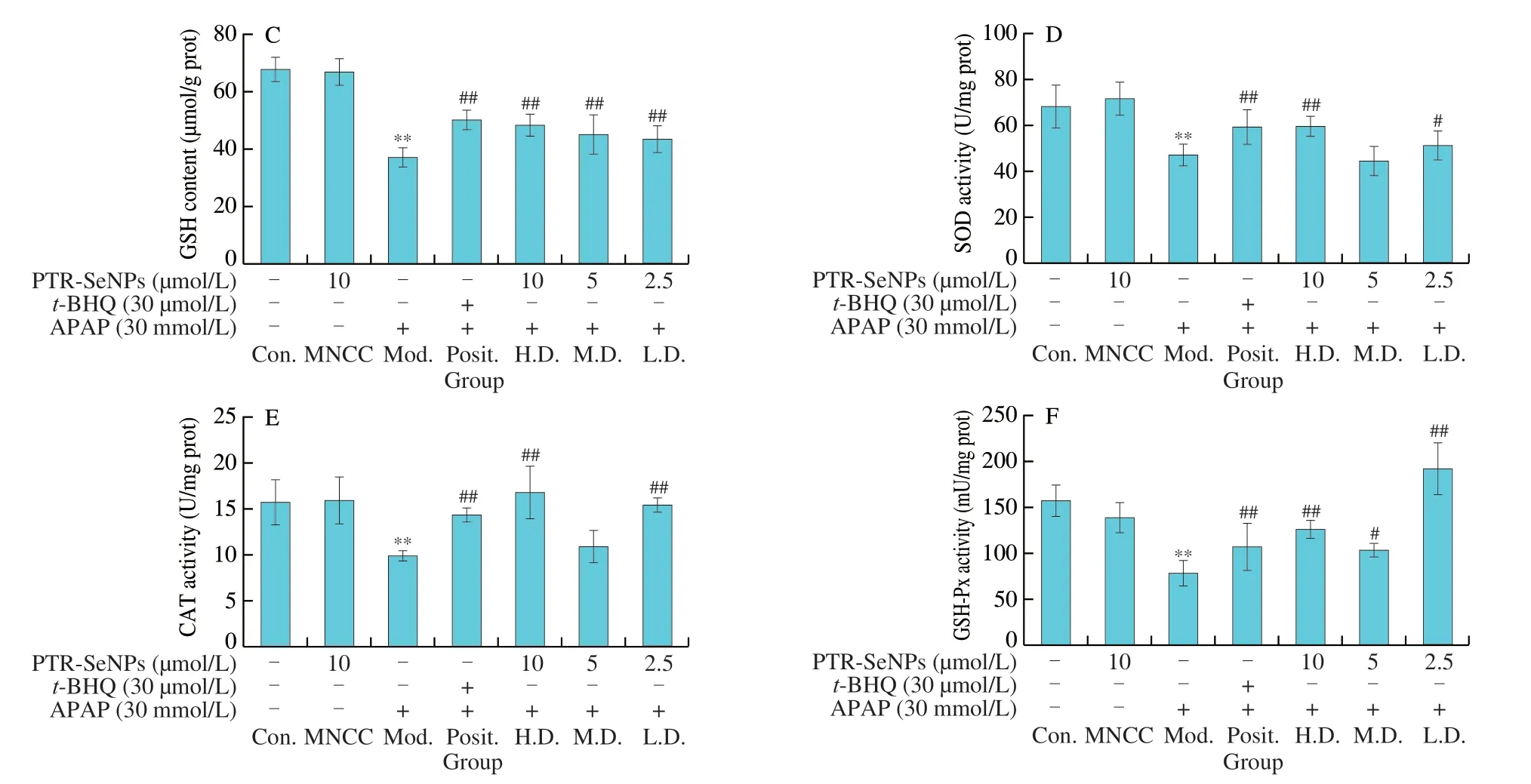
Fig.3 (Continued)
MDA and GSH are considered as reliable markers of oxidative stress in organisms.As seen in Figs.3B and C,30 mmol/L APAP challenge (Mod.) significantly increased MDA level by 1.17-fold and decreased GSH content by 45.21%,when compared with that of the Con.(P< 0.01).PTR-SeNPs ort-BHQ were found to prominently alleviate the APAP-induced oxidative stress as evidenced by significant reduction of MDA level and increase of GSH content(P< 0.01).Further investigation of SOD,CAT and GSH-Px activities also demonstrated that botht-BHQ and PTR-SeNPs (10 and 2.5 µmol/L)could withstand the APAP challenge by significant enhancing the activity of these three antioxidases (P< 0.01),as shown in Figs.3D-F.Taken together,our results collectively suggested that PTR-SeNPs enhanced cellular antioxidant status,thereby exhibiting significant protective effect against APAP-induced oxidative injury in the HepG2 cells.
3.4 Cellular uptake efficiency and intracellular localization of PTR-SeNPs
C6-PTR-SeNPs (120 µmol/L) was employed to visualize the cellular uptake behavior of PTR-SeNPs in HepG2 cells.As shown in Fig.4A,PTR-SeNPs were taken up by HepG2 cells and their intracellular concentration increased in a time-dependent manner.Endocytosis has been widely reported as the major cellular uptake mechanism for nanoparticles.Similarly,our findings demonstrated that C6-PTR-SeNPs co-localized with lysosomes in the HepG2 cells after cellular internalization (Fig.4B),suggesting the involvement of endocytosis and lysosomes is the major target organelle of PTR-SeNPs.
3.5 Inhibition of PTR-SeNPs against APAP-triggered intracellular ROS generation
As shown in Figs.5A and B,compared with the Con.group,the intracellular ROS generation of HepG2 cells was markedly elevated to 1.3-fold by APAP challenge (P< 0.01),which was not found in the MNCC (P> 0.05).Pretreatment with 10 (H.D.) and 5 (M.D.) µmol/L of PTR-SeNPs or 30 µmol/L oft-BHQ (Posit.) resulted in a significant decrease of intracellular ROS level by 28.15%,21.48% and 37.78%,respectively,compared with that in the Mod.group (P< 0.01).These results demonstrated that PTR-SeNPs could effectively attenuate the APAP-triggered intracellular ROS generation in the HepG2 cells.
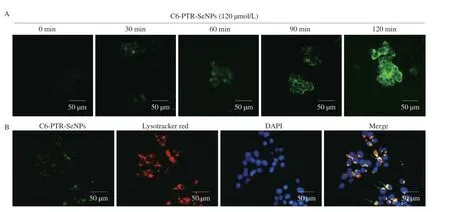
Fig.4 Cellular uptake efficiency and intracellular localization of PTR-SeNPs in HepG2 cells.(A) Cells incubated with C6-PTR-SeNPs at 37 °C for 0,30,60 90 and 120 min were visualized under fluorescence microscope.(B) Cells were pre-treated with DAPI (0.3 mmol/L;blue;nucleus),LysoTracker Deep Red (50 nmol/L;red;lysosomes) and C6-PTR-SeNPs (120 µmol/L;green) at 37 °C for 5 min followed by examination of intracellular localization under fluorescence microscope (200×).

Fig.5 PTR-SeNPs attenuated the APAP-induced ROS production in HepG2 cells.The levels of ROS in the different group cells were characterized after staining with DCFH-DA by fluorescent microscopy.(A) Microscopy characterization of ROS levels in cells (magnification 200×).(B) Quantitative analysis of intracellular ROS levels.Cells,including control (Con.),MNCC,model (Mod.),high dose (H.D.),medium dose (M.D.) and low dose (L.D.) groups,were treated and grouped as detailed in Fig.2 caption.Data were representative images or expressed as the mean ± SD of each group from three independent experiments.The levels of ROS in the control cells were designated as 100%,*P < 0.05 or **P < 0.01,compared with the control group;##P < 0.01,compared with the model group.
3.6 Protective effect of PTR-SeNPs against APAP-induced alteration of mitochondrial morphology
High resolution transmission electron microscopy showed that the mitochondrial membranes and matrix present a regular ultrastructural appearance in normal HepG2 cells (Fig.6A).After 30 mmol/L APAP stimulation,mitochondrial morphology altered with swollen and rounded appearance,vacuolization,loss of cristae and decreased electron density of the matrix (Fig.6B).Similar to that oft-BHQ(30 µmol/L),PTR-SeNPs (5 µmol/L) were found to effectively restore the APAP-induced morphological alteration of mitochondria,as shown in Figs.6C and D.

Fig.6 Mitochondrial morphology observation of HepG2 cells by a transmission electron microscope after treatment (30 000×).(A) Normal HepG2 cells control.(B) HepG2 cells challenged with 30 mmol/L APAP.(C) HepG2 cells were pretreated with 5 µmol/L PTR-SeNPs followed by stimulation with 30 mmol/L APAP.(D) HepG2 cells exposed with 30 µmol/L t-BHQ prior to challenge with 30 mmol/L APAP.
3.7 Promoting effect of PTR-SeNPs on the gene expression of antioxidases
Considered that the important role of antioxidases in defending cell from oxidative damage,qRT-PCR assay was performed to examine the effect of PTR-SeNPs on the related gene expression profiles,includingGCLC,GCLM,HO-1andNQO-1.Unexpectedly,APAP and PTR-SeNPs challenge did not affect the mRNA expressions ofGCLC,GCLMandHO-1significantly in HepG2 cells(P> 0.05),compared with that of the Con.group.Pretreatment with PTR-SeNPs andt-BHQ could improve the mRNA expressions ofGCLC,GCLMandHO-1with different degree,compared with those of Mod.group (Figs.7A-C).Additionally,compared with the Con.group,the mRNA expression ofNQO-1was dramatically decreased in Mod.group (P< 0.05).The mRNA expression ofNQO-1was significantly enhanced in the Posit.and H.D.groups,compared with that of the Mod.group (Fig.7D).In general,pretreatment with PTR-SeNPs could upregulate the gene expression of the four major antioxidases with different extent,suggesting that PTR-SeNPs might ameliorate the APAP-induced oxidative damage via improving the antioxidant status.
3.8 Protective effect of PTR-SeNPs against APAP-induced liver injury in mice
The hepatoprotective effects of PTR-SeNPs on APAP-induced liver injury were further evaluated in WT and Nrf2 KO mice.As shown in Figs.8A-D,compared with the Con.group,the serum ALT,AST,ALP and LDH levels in both Nrf2 KO and WT mice were significantly elevated 6 h after i.p.injection of a single dose of 400 mg/kg APAP (Mod.),while 10 µmol/kg PTR-SeNPs alone(PTR-SeNPs Con.) did not exhibit any obvious effect on their activities(P> 0.05).Pretreatment of PTR-SeNPs (10 µmol/kg,H.D.)significantly reduced their serum levels,compared with those of the Mod.group.It was worth noting that 5 µmol/kg PTR-SeNPs markedly prevented the increase in serum AST and LDH activities in WT mice but failed to do so in Nrf2 KO mice.Besides,Figs.8E-I also indicated that the APAP challenge significantly decreased the hepatic SOD,CAT,GSH and GSH-Px activities and increased MDA content in both WT and Nrf2 KO mice when compared with those of the Con.group.For WT mice,pretreatment with 10 µmol/L of PTR-SeNPs was found to significantly promote the SOD,CAT,GSH and GSH-Px activities and reduce the hepatic MDA content when compared with those of the Mod.group.However,10 µmol/L of PTR-SeNPs could only share similar hepatoprotective effect on the GSH and GSH-Px activities and hepatic MDA content in the Nrf2 KO mice,which SOD and CAT activities were limitedly affected (Figs.8E-I).Together these data indicated that PTR-SeNPs mitigated the APAP-induce hepatotoxicity more effectively in WT mice than that in Nrf2 KO mice,suggesting the involvement of Nrf2 signaling pathway in PTR-SeNPs mediated hepatoprotective efficacy.
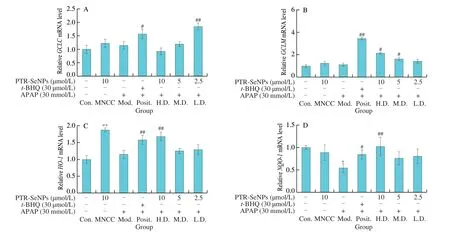
Fig.7 qRT-PCR assay for the effects of PTR-SeNPs on the gene expression profile of antioxidases,including (A) GCLC,(B) GCLM,(C) HO-1 and (D) NQO-1.Cell was grouped and treated,as described in the legend to Fig.4.Values for mean expression and standard deviation were calculated from the results of three independent biological replicates,following the method of 2-ΔΔCT.*P < 0.05 and **P < 0.01 were considered significant vs the control group (Con.).#P < 0.05 and##P < 0.01 were considered significant vs the model group (Mod.).
The hepatotoxicity of APAP and hepatoprotective effect of PTR-SeNPs were further compared in WT and Nrf2 KO mice by histopathological examination using H&E staining.As indicated in the Figs.9A and B,treatment of Nrf2 KO mice with APAP (Mod.,Fig.9B) resulted in increased haemorrhage and necrosis in the central area,which was more severe than that in WT mice (Mod.,Fig.9A),suggesting that Nrf2 played an important role in APAP-induced hepatotoxicity.Pretreating WT mice with PTR-SeNPs exhibited an obvious protective effect against the APAP-induced liver injury(Fig.9A,bottom panel).Nevertheless,the hepatoprotective effect of PTR-SeNPs was weakened in Nrf2 KO mice as visualized by the presence of obvious haemorrhage,steatosis,necrosis and inflammatory cell infiltration in the central area (Fig.9B,bottom panel).Result from histopathological examination further demonstrated the significant participation of Nrf2 in the hepatoprotective effect of PTR-SeNPs against APAP-induced liver injury.
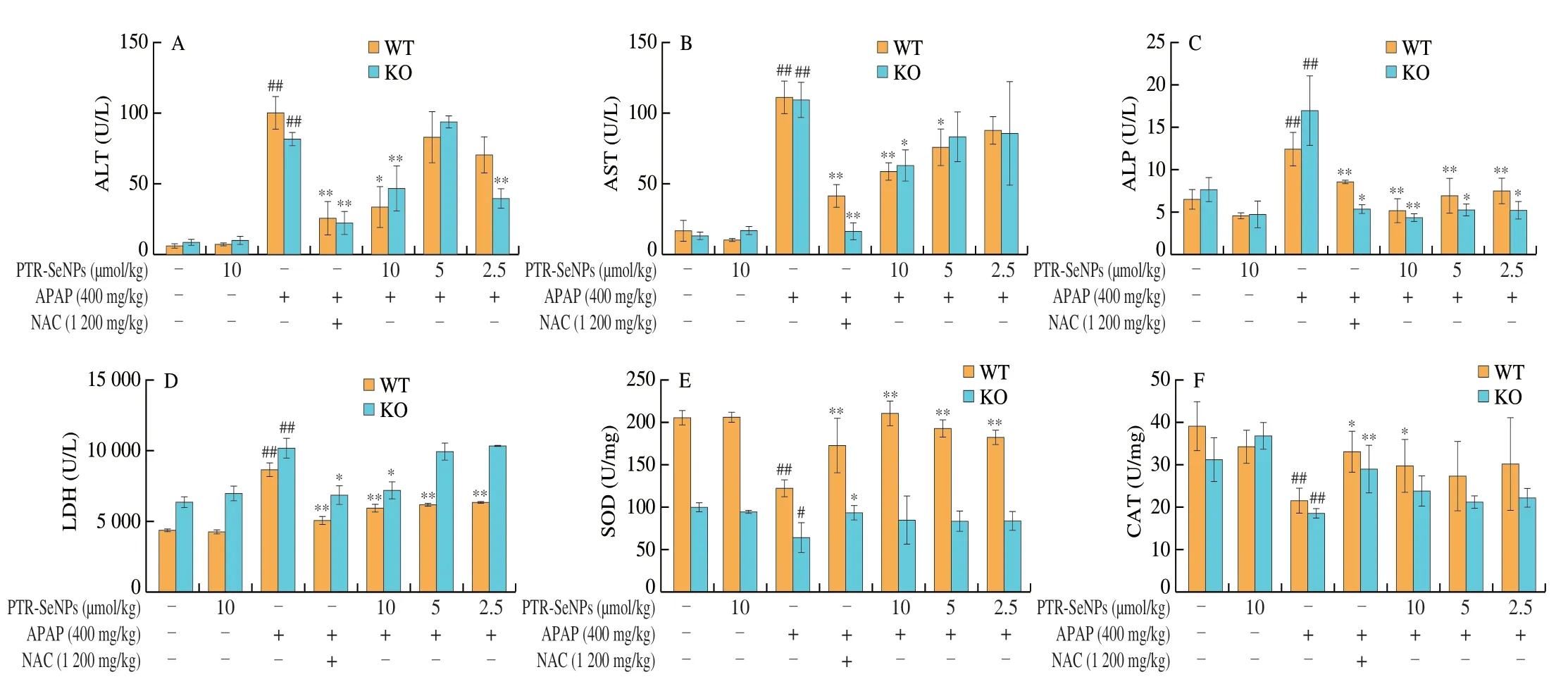
Fig.8 The effects of PTR-SeNPs pretreatment on serum levels of (A) ALT,(B) AST,(C) ALP and (D) LDH and hepatic contents of (E) SOD,(F) CAT,(G) MDA,(H) GSH,and (I) GSH-Px after APAP challenge in WT and Nrf2 KO mice.Values presented are mean ± standard deviations represented by vertical bars.#P < 0.05 and##P < 0.01 were considered significant vs normal control (Con.),*P < 0.05 and **P < 0.01 were considered significant vs APAP-challenged alone group (Mod.).
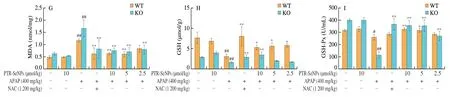
Fig.8 (Continued)
3.9 Modulation of Nrf2 signaling pathway and CYP 2E1 inhibition by PTR-SeNPs
Immunoblotting analysis was further performed to validate the mechanism potentially involved in Nrf2 activation and CYP 2E1 inhibition.As shown in Figs.10A and C,the APAP challenge(Mod.) for both WT mice and HepG2 cells significantly upregulated the protein expression of nuclear Nrf2 and decreased that of the downstream antioxidases (such as GCLC,GCLM,HO-1 and NQO-1)when compared with those of the Con.group.And pretreatment with PTR-SeNPs resulted in a significant increase in nuclear Nrf2 and those four antioxidases compared with those of the Mod.group.Nevertheless,the promoting effect of PTR-SeNPs on antioxidases was largely weakened in the Nrf2 KO mice but not fully abolished,as Nrf2 was depletedinvivo(Fig.10B).Moreover,the APAP challenge(Mod.) significantly increased expression of CYP 2E1 in WT mice or HepG2 cells,which was obviously reversed by PTR-SeNPs pretreatment (Figs.10A and C).However,in the Nrf2 KO mice,only 10 µmol/L PTR-SeNPs pretreatment (H.D.) could notably inhibited the CYP 2E1 expression (Fig.10B).In agreement with the findings of biochemical analysis and histopathological examination,the results further supported that Nrf2 signaling pathway is mechanistically linked to the PTR-SeNPs mediated hepatoprotective efficacy.It is worth noting that PTR-SeNPs not only markedly reduced the Keap1 expression,but also significantly enhanced the protein expression of PKCδ and its phosphorylation at Tyr311 in HepG2 cells,when compared with that of the Mod.(Figs.10C and D).
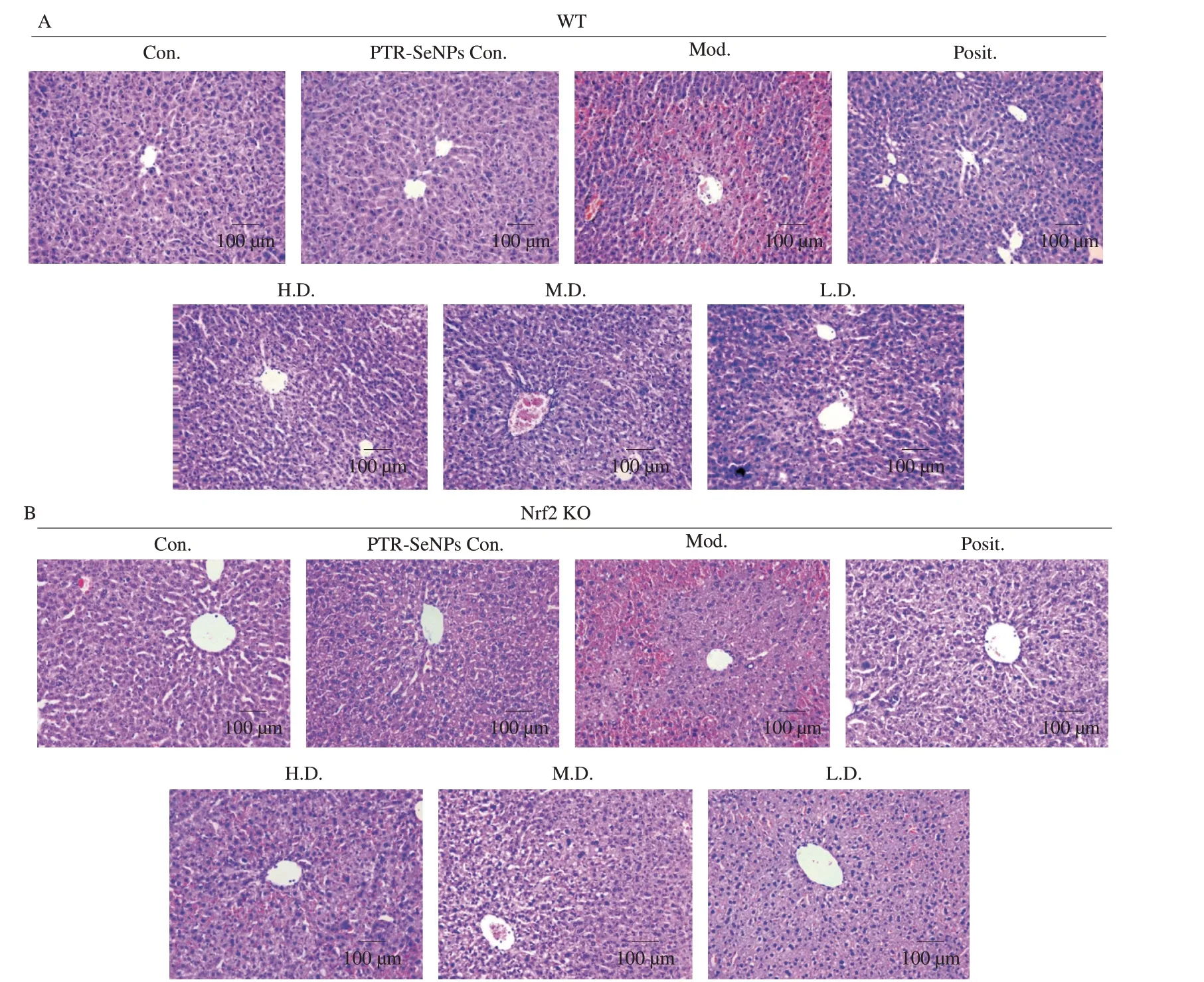
Fig.9 H&E stained liver tissue from (A) WT mice in the upper pannel and (B) Nrf2 KO mice in the lower pannel (magnification 200×).Both WT and Nrf2 KO mice were randomly divided into 7 groups (n=12 in each group),including normal control group (Con.),10 µmol/kg PTR-SeNPs only control group (PTR-SeNPs Con.),APAP-challenged alone group (Mod.),NAC treated group (Posit.,positive control),10 (H.D.,high dose),5 (M.D.,medium dose) and 2.5 µmol/kg (L.D.,low dose) of PTR-SeNPs treated groups.
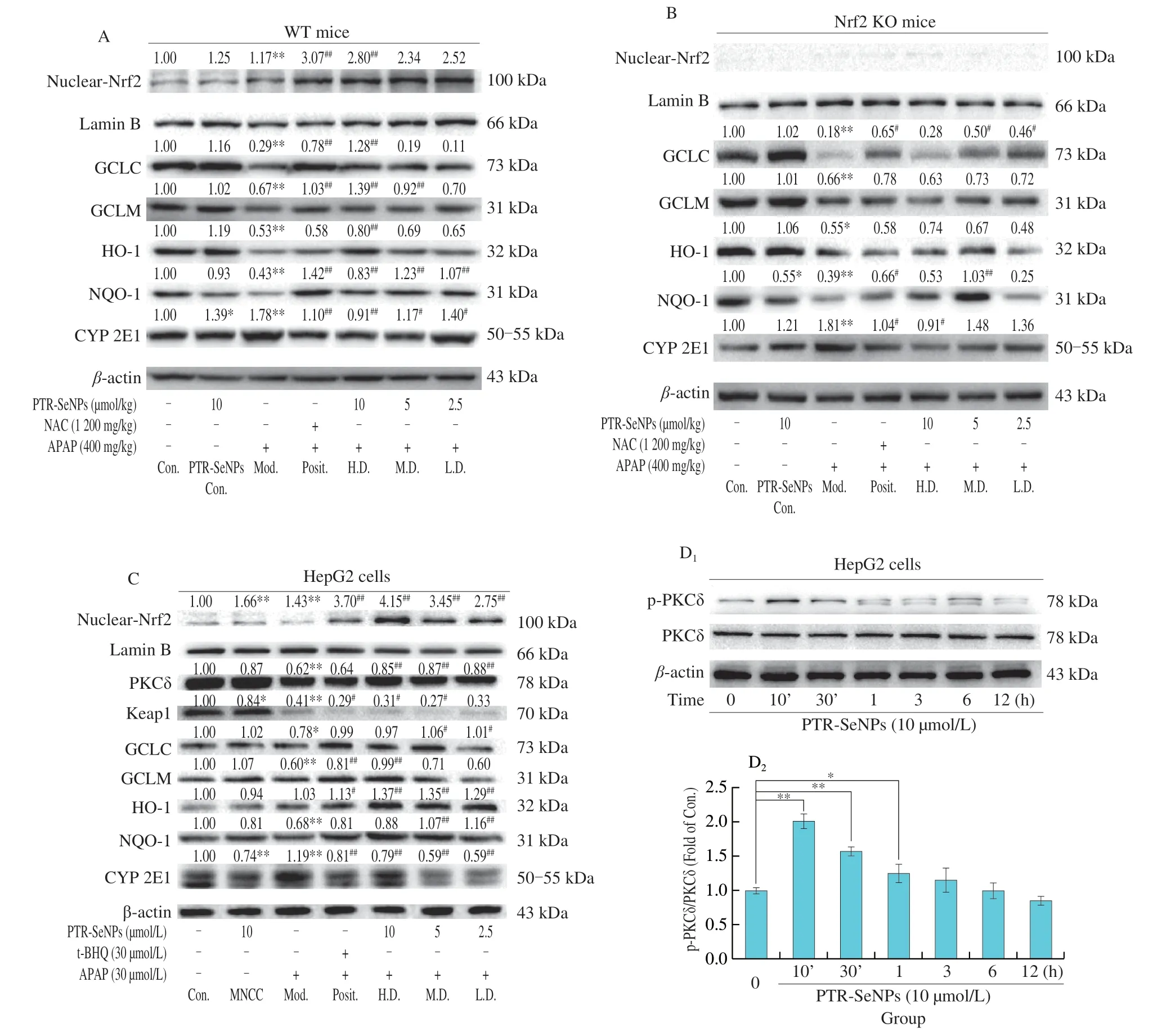
Fig.10 Immunoblotting analysis of PKCδ signaling pathway and CYP 2E1.(A) WT,(B) Nrf2 KO mice and (C) HepG2 cells were pretreated by PTR-SeNPs followed by APAP challenge,NAC and t-BHQ were used as positive control in mice and HepG2 cells,respectively.(D) Phosphorylation of PKCδ in HepG2 cells treated by PTR-SeNPs with different times.*P < 0.05 and **P < 0.01 were considered significant vs the control (0 h).
4.Discussion
APAP overdose-induced liver dysfunction is very common worldwide,which has been widely recognized as the leading cause of drug-induced liver injury in the Western world.APAP-induced hepatotoxicity is characterized by an extensive oxidative stress,consequently highlighted as a prime therapeutic target[30].SeNPs exhibit excellent antioxidant and pro-oxidant activity at different conditions,strongly implying the promising utilization in the oxidative stress related diseases[31].Size of nanoparticle is a crucial factor responsible for the way of nanoparticle internalization,and smaller nanoparticles tend to be imported into cells via endocytosis,particularly[32].According to our previous study,PTR-SeNPs showed the smallest diameter (12.5 nm) among the different mushroom polysaccharides decorated SeNPs,resulting in the higher stability,cell-penetrating ability and anticancer efficacy[25,27].In this study,PTR-SeNPs (Se concentration: (1.35 ± 0.12) μmol/L;particle size:19.8 nm) were prepared and well characterized according to our patented nanotechnology.HepG2 cells,WT and Nrf2 KO mice were employed to investigate the protective effect of PTR-SeNPs against APAP-induced oxidative injuryinvitroandinvivo.Further elucidation of the underlying molecular mechanism,in particular their modulation of Nrf2 signaling pathway and CYP 2E1 inhibition was also performed.
APAP overdose triggers oxidative stress,leading to the MDA formation,GSH depletion,and alteration of the antioxidant defense systems such as SOD,CAT and GSH-Px in bothinvitroandinvivomodels[33].MDA is a secondary product of oxidative stress formed during lipid peroxidation,which is a cornerstone of the pathogenic pathway of APAP-associated hepatotoxicity.Meanwhile,increases in serum levels of ALT,AST,ALP,and LDH as a result of APAP overdose also lead to hepatic damage and dysfunction[34].The activities of SOD,CAT and GSH-Px constitute the first line antioxidant defense system against oxidative stress[35].SOD provides efficient dismutation of superoxide radicals in mitochondria,resulting in the formation of H2O2,which is scavenged by a number of antioxidant enzymes in hepatocytes,such as CAT and GSH-Px[36].Our results showed that PTR-SeNPs treatment could prominently alleviate the APAP-induced oxidative stress as evidenced by significant reduction of ALT,AST and MDA levels as well as elevation of GSH content and SOD,CAT and GSH-Px activity.
Nrf2,which is involved in the regulation of APAP-induced oxidative stress,is considered as a potential therapeutic target for APAP.Nrf2 activation results in the transcriptional activation of antioxidases,which catalyzes the initial and rate-limiting step in GSH synthesis and acts as the cellular antioxidant defense system[37].Experimental studies have indicated that Nrf2-deficient mice are more susceptible to organ injury induced by APAP[38].Consistently,acute exposure of Nrf2-deficient mice to APAP was shown to possess enhanced liver injury by histopathological examination compared to their wild type counterparts.However,there were no significant statistical differences in the biochemical indices of hepatotoxicity between Nrf2 KO and WT mice,as examplyfied by ALT,AST,LDH,ALP and MDA.The result was partially in accordance with the prior study reported by Chan et al.[39].Pretreatment with PTR-SeNPs conferred strong protection against APAP-induced hepatotoxicity in both WT and Nrf2 KO mice,with significant amelioration on indices of hepatotoxicity as well as histopathological lesions.Of note,PTRSeNPs antagonized the APAP-induce hepatotoxicity more effectively in WT mice than Nrf2 KO mice as evidenced by the decreased serum AST and LDH contents and increased hepatic SOD,CAT and GSH levels,as well as improvement in the histopathology of liver tissue.Intriguingly,hepatic GSH-Px activity,depending on Selenium concentration,was not more elevated in WT mice than Nrf2 KO mice,which may be attributable to the direct regulation of PTR-SeNPs[40].Immunoblotting result showed that PTR-SeNPs pretreatment could further promote Nrf2 nuclear translocation in response to APAP challenge,and upregulated the protein expression of GCLC,GCLM,HO-1 and NQO-1.Deletion of Nrf2 partially abolished the upregulating effect of PTR-SeNPs on these antioxidases,which was in agreement with results of biochemical analysis and histopathological examination,highlighting an important role of Nrf2 signaling pathway in the antidotal effect of PTR-SeNPs.
The excessive generation of NAPQI caused by APAP overdose causes mitochondrial oxidative stress and mitochondrial dysfunction,which are considered to be the predominant cellular processes in APAP hepatotoxicity[41].Supplementation of Se could significantly increase mitochondrial respiration and biogenesis,thus improving mitochondrial function[42].Therefore,mitochondrial morphology alteration along with intracellular ROS level in HepG2 cells was examined to evaluate the degree of mitochondrial damage caused by oxidative stress.PTR-SeNPs significantly suppressed APAP-induced intracellular ROS generation and effectively restored the morphological alteration of mitochondria,demonstrating the similar protective effect to a classic Nrf2 activator,t-BHQ[43].Further study was conducted to investigate the modulation of Nrf2 signaling pathway by PTR-SeNPs in HepG2 cells.We found that PTR-SeNPs increased PKCδ phosphorylation at Tyr311,suppressed Keap1 expression and enhanced the Nrf2 nuclear translocation,thus promoting the activities of downstream antioxidases.Therefore,PTRSeNPs could exert hepatoprotective activity partially through PKCδ/Nrf2 signaling pathway.
In hepatocytes,the cytochrome P450,prominently CYP 2E1,mediates the metabolism of APAP,resulting in a highly hepatotoxic metabolite NAPQI.Any subtle interference with the formation of reactive metabolite NAPQI can produce a profound impact on the outcome of APAP-induced oxidative injury[44].Thus,one important concern regarding this study is the assessment of the impact of PTRSeNPs on CYP 2E1 activity.In consistent with the previous findings,APAP challenge significantly up-regulated the CYP 2E1 activity in HepG2 cells,WT mice and Nrf2 KO mice livers[45-46],which were markedly antagonized by PTR-SeNPs pretreatment.Furthermore,CYP 2E1 activity was more decreased by PTR-SeNPs in WT mice than in Nrf2 KO mice,which again highlighted the pivotal role of Nrf2 in the protective effect of PTR-SeNPs against APAP induced liver injury.Taken together,our results clearly demonstrated the hepatoprotective effect of PTR-SeNPs against APAP,and revealed some possible antidotal mechanisms,including the activation of PKCδ/Nrf2 signaling pathway,the enhancement of GSH-Px and GSH synthesis,and the inhibition of CYP 2E1 activity,as shown in Fig.11.This study generates solid evidences and insights for future exploration and translational application of PTR-SeNPs in nanomedicine and nutritional sciences.
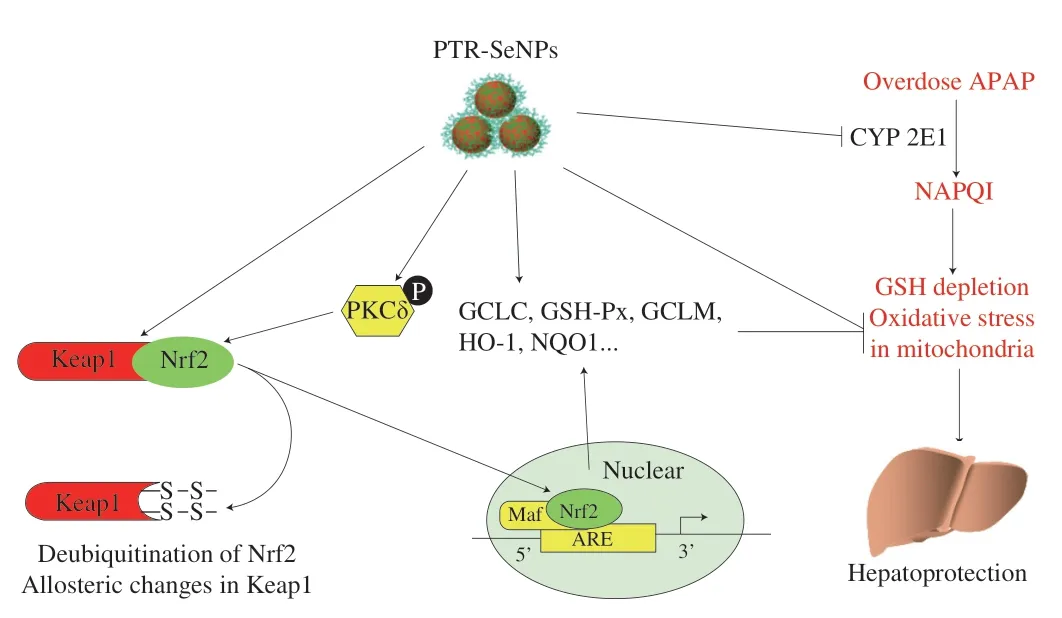
Fig.11 The suggested mechanisms of the antidotal effect of PTR-SeNPs against APAP-induced hepatotoxicity.
5.Conclusions
Excessive NAPQI induced by APAP overdose causes GSH exhaustion and oxidative stress,which is responsible for the APAP-induced liver injury.Thus,agents that can block NAPQI formation with GSH preserving ability and/or antioxidant properties may serve as potential hepatoprotectants[33].In this study,we have successfully employed PTR as a capping agent to functionalize SeNPs with high stability.PTR-SeNPs were found to possess strong hepatoprotective effect against APAP-induced oxidative injury by activation of the PKCδ/Nrf2 signaling pathway to accelerate nuclear translocation of Nrf2,thereby upregulating the expression of the detoxifying enzymes,and enhancing the synthesis of GSH-Px and GSH directly or indirectly.Moreover,PTR-SeNPs inhibited the CYP 2E1 expression to mitigate the biotransformation of APAP and the accumulation of NAPQI,thus reducing the generation of ROS and the oxidative injure in mitochondria.In conclusion,our results suggested that PTR-SeNPs mediated redox regulation to improve antioxidant status,which in turn endowed the hepatocytes with a more powerful resistance against APAP-induced toxicity.Being a potential hepatic protectant,PTRSeNPs could serve as a new source of selenium supplement for health-promting and biomedical applications.
Declaration of conflicting interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Funding information
This work was financially supported by National Natural Science Foundation of China (81700524),Natural Science Foundation of Fujian Province (2022J01866) from Fujian Provincial Department of Science and Technology,Key Project of Fujian University of Traditional Chinese Medicine (X2021019) and Collaborative Innovation and Platform Establishment Project of Department of Science and Technology of Guangdong Province (2019A050520003).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

