Plant-based meat analogues aggravated lipid accumulation by regulating lipid metabolism homeostasis in mice
Yunting Xie,Linlin Cai,Zhiji Huang,Kai Shan,Xinglian Xu,Guanghong Zhou,Chunbao Li
Key Laboratory of Meat Processing and Quality Control, MOE; Key Laboratory of Meat Processing, MARA; Jiangsu Innovative Center of Meat Production,Processing and Quality Control; College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
Keywords: Meat analogues Metabolomics Lipid metabolism Adipose tissue dysfunction Ectopic fat deposition
ABSTRACT To determine the effects of plant-based meat analogues on the metabolic health and the possible mechanisms,mice were fed with a real pork diet (AP),a real beef diet (AB),a plant-based pork analogue diet (PP) and plant-based beef analogue diet (PB) for 68 days.Compared with real meat,the plant-based meat analogues increased food and energy intake,body weight,white fat and liver weight and caused adipocyte hypertrophy,hepatic lipid droplet accumulation,and inf lammatory responses in mice.Metabolomics revealed that plantbased meat analogues altered the composition of serum metabolites,which regulated lipid metabolism homeostasis.The PB diet upregulated gene expression related to lipid synthesis,lipolysis and adipocyte differentiation while the PP diet upregulated expression of lipolysis-related genes but downregulated expression of adipocyte differentiation-related genes in white adipose tissue.Meanwhile,both PP and PB diets upregulated lipid inf lux-and synthesis-related genes but downregulated lipid oxidation-related genes in liver.The specif ic metabolite biomarkers may affect fat accumulation mainly by direct lipid metabolism pathways or indirect amino acid metabolism,protein digestion and absorption,bile secretion,aminoacyl-tRNA biosynthesis,neuroactive ligand-receptor interaction and ABC transporters pathways.These f indings provide a new insight into understanding the differences in nutritional functions of meat and plant-based meat analogues.
1.Introduction
Proteins are a vital component of human diet and affect a series of physiological processes such as metabolism,immune and endocrine function[1-3].The typical sources of dietary protein include meats,beans,and dairy products,of which,meat is the major source of animal protein.Meat not only provides high-quality proteins with high digestibility but also all essential amino acids in adequate proportions.Nonetheless,a growing number of studies indicate the associations between poor health outcomes and the excessive consumption of red or processed meat[4-6].Moreover,it is hard to ignore that the world population is expected to reach 9 billion by 2050 with a concomitant increasing demand for food production by 70%[7].Besides growing population,urbanization and growing income in the developing countries also drive the increasing consumption of animal protein over past decades.In terms of sustainability,continuous growth in consumption of animal-based protein will be a big burden in the future.
Based on the above reasons,plant proteins,especially plantbased meat analogues,perceived as healthy and sustainable protein foods have attracted extensive attention.Plant-based meat analogues are made of plant protein extracted from soybeans,peas,wheat and other crops as raw materials[8]and have the similar texture,chewiness and aroma to animal-based meat[9].To prepare meat analogues,plant proteins are reconstructed into the f ibrous structure of meat by a series of steps such as heating,extrusion,cooling and shaping[10].Moreover,the cross-linking enzymes,flavor protease and a variety of food additives were applied in the production process[11].Consequently,plant-based meat analogues may have different nutritional properties from those of plant or animal proteins.A comparison study has found a 90% difference in metabolite abundance between plant-based meat and beef itself,with plant-based meat analogue lacking some important physiological,anti-inflammatory or immunomodulatory nutrients compared with beef[12].Recent studies have also indicated that real meat and plant-based meat analogues have different protein digestion properties inin vitrodigestion models[13].The ingested macromolecular nutrients are usually digested in the gastrointestinal tract and absorbed into the circulatory system and finally into the liver and other tissues for different physiological functions.Therefore,the intestinal digestion and absorption function as well as digestion and absorption properties of food have a certain influence on liver metabolism,and liver metabolic homeostasis is critical for health.However,the effect of plant-based meat analogues on the physiological and metabolic health is not clear.In the case where it has been recommended to shift global protein consumption toward plant-based proteins[7],the nutritional quality of alternative protein sources should be paid more attention.Therefore,the real pork,real beef,plant-based pork and beef analogues were used as specific examples to systematically evaluate the impact of plant-based meat analogues on lipid metabolism in the present study.
2.Materials and methods
2.1 Diet preparation and animal feeding
The granule and purified type meat and plant-based meat analogue diets were prepared according to the AIN-93M diet formulation as previously described[14-15].In brief,the pork,beef and plantbased pork and beef analogues were freeze-dried and ground to a powder.Then the meat power was used to replace the nutrients in the formulation.Except for the higher fiber or fat content in plant-based meat analogues that cannot be fully balanced based on the protein content,all other nutrients were harmonized in real meat and plantbased meat analogue diets.More detailed diet preparation as well as the ingredient composition and nutritional content of diets can be seen in our previous study[15].The content of hydrolyzed amino acids in the diets was determined by an automatic amino acid analyzer (L-8900;Hitachi,Tokyo,Japan) and the data was shown in Table S1.
Male C57BL/6J mice (3 months old) were fed in pairs in a specific pathogen-free animal center (SYXK < Jiangsu > 2011-0037,Nanjing,Jiangsu,China).The environment,including temperature((22.0 ± 0.5) °C),relative humidity ((60 ± 10)%) and illumination (12 h light cycle),was kept constant during the experiment.Following 10 days acclimation,mice were randomly divided into four groups(n=16 per group) and fed a real pork diet (AP),a plant-based pork analogue diet (PP),a real beef diet (AB),and a plant-based beef analogue diet (PB),respectively.Mice were allowed to access water and dietsad libitumfor 68 days.Feed intake and body weight changes were recorded every 2 and 4 days,respectively.All experiments were performed in compliance with the relevant guidelines and regulations of the Ethical Committee of Experimental Animal Center of Nanjing Agricultural University.
2.2 Sample collection
Mice were euthanized by cervical dislocation after 20 and 68 days of dietary intervention.Blood samples were collected and centrifuged(Allegra 64R,Beckman Coulter,CA,USA) at 3 000 ×gfor 30 min to remove pellet blood cells.Serum was stored at -80 °C for subsequent analyses.The epididymal adipose and liver tissues were taken and the weights were recorded.Then,part of them were fixed in 4% (m/V) paraformaldehyde for histological analysis,and the other part were immediately frozen in liquid nitrogen and stored at -80 °C for further analyses.
2.3 Biochemical analysis
Leptin,resistin,adiponectin,interleukin-6 (IL-6),interleukin-1β(IL-1β),tumor necrosis factor-α (TNF-α),monocyte chemotactic protein 1 (MCP-1),amyloid A3 (SAA3) and F4/80 in epididymal adipose tissue were detected by commercial ELISA kits (Angle gene,Nanjing,China) according to the manufacturers’ protocols.The alanine aminotransferase (ALT),aspartate aminotransferase(AST),alkaline phosphatase (AKP),total cholesterol (TCHO),triglycerides (TG) and total bile acids (TBA) levels in liver were detected by biochemical kits (Jiancheng,Nanjing,China) following the manufacturer’s instructions.Briefly,the tissue samples were homogenized in phosphate buffer and the homogenate was centrifuged(Allegra 64R,Beckman Coulter,CA,USA) to get the supernatant.Then the supernatant was mixed with reacting reagents in 96 × plates for designated times.Finally,the absorbance of reacting solution was recorded (SpectraMax M2,Molecular Devices Ltd.,Sunnyvale,CA,USA) and the target attributes were calculated and normalized with protein content.The protein concentration of supernatant was determined using a BCA Protein Assay Kit (ThermoScientific,Rockford,IL,USA).
2.4 Histological observations
After fixing in 4% paraformaldehyde for 24 h,the liver and epididymal adipose tissues were embedded in paraffin and cut into 5 μm sections,then they were stained with haematoxylin-eosin (H&E).In addition,the frozen liver samples were sectioned to 5 μm thickness and stained with 0.2% Oil Red O.A light microscope (BX51,Olympus,Tokyo,Japan) was used for microscopic inspection and images were examined through Image-Pro Plus 7.0 software (Media Cybernetics,Inc.,Bethesda,MD) to estimate lipid droplets in the liver tissue.
2.5 RNA extraction and real-time PCR
Total RNA was isolated from the liver and epididymal adipose tissues using an RNA extraction kit (MiniBEST Universal,TaKaRa,Shiga,Japan) and TRIzol reagent (Thermo Fisher Scientific,Carlsbad,CA) according to the manufacturer’s protocols,respectively.The RNA quality was checked by 1% agarose gel electrophoresis,and the concentration was measured using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific,Rockford,IL,USA).The cDNA was synthesized using the PrimeScript RT Master Mix(TaKaRa,Shiga,Japan) according to the recommended procedures.Real-time PCR was performed on an Applied Biosystems 7500 Real-Time PCR System using the SYBR Premix Ex TaqTM (Tli RnaseH Plus) qPCR kit (TaKaRa,Shiga,Japan) according to the manufacturer’s protocols.The mRNA level ofGapdhwas used to normalize the relative amount of each measured mRNA,and the 2-ΔΔCtmethod was chosen to analyze the relative gene expression.The primers of target genes were available in Table S2.
2.6 Untargeted metabolomics analysis
The serum (100 μL) was mixed with methanol (300 μL) in a 1.5 mL centrifuge tube by vortex oscillation for 30 s.Then,the mixture was arranged in -40 °C for 1 h then 4 °C for 30 min.Finally,the mixture was centrifuged at 13 000 ×gfor 15 min to collect the supernatant filtrate.A total of 200 μL supernatant was transferred into a new vial withL-2-chlorophenylalanine (0.14 mg/mL,10 μL)as the internal standard.Quality control (QC) samples were prepared by mixing all serum samples.The mixed extraction of QC sample was subjected to assessment every ten sample injections.Metabolite profiling was acquired using LC-MS (Thermo,Ultimate 3000LC,Q Exactive) platform.Hyper gold C18analytical column(100 × 2.1 mm,1.9 μm) was used for sample separation at 40 °C.The injection volume was 2 μL,automatic injector temperature was 4 °C and flow rate was 0.30 mL/min.Mobile phase A (5 % acetonitrile and 0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile) were mixed into a gradient mobile phase under a 19.5-min gradient program.Positive mode electrospray ionization sources (ESI+) and negative mode electrospray ionization sources(ESI-) were used.Sample data was extracted by using Compound Discoverer 3.1 (CD) for analysis and comparison.The final dataset included compound name,molecular weight,peak area,and retention time.Annotated metabolites with zero values in more than 50%samples were excluded.The minimum half method was used to complete the missing value.Data was normalized with MetaboAnalyst 4.0,and the SIMCA-P V14.1 (Umetrics,Umea,Sweden) was used for orthogonal-partial least squares discriminant analysis (OPLS-DA).The potential biomarkers were identified based on precise molecular weight in the Human Metabolome Database (HMDB) and METLIN(http://metlin.scripps.edu/) database.MetaboAnalyst 4.0 (http://www.metaboanalyst.ca/) was used to visualize the enrichment pathway of potential biomarkers.
2.7 Statistical analysis
All values were presented as means ± standard deviations (SD).Factorial analysis of variance (ANOVA) was used for data analysis by SPSS software (Ver.26,IBM Corporation,Armonk,NY,USA).Means were compared by Tukey’st-test (P< 0.05).Figures were generated by GraphPad Prism 8.0 (GraphPad Software,Inc.,La Jolla,USA).
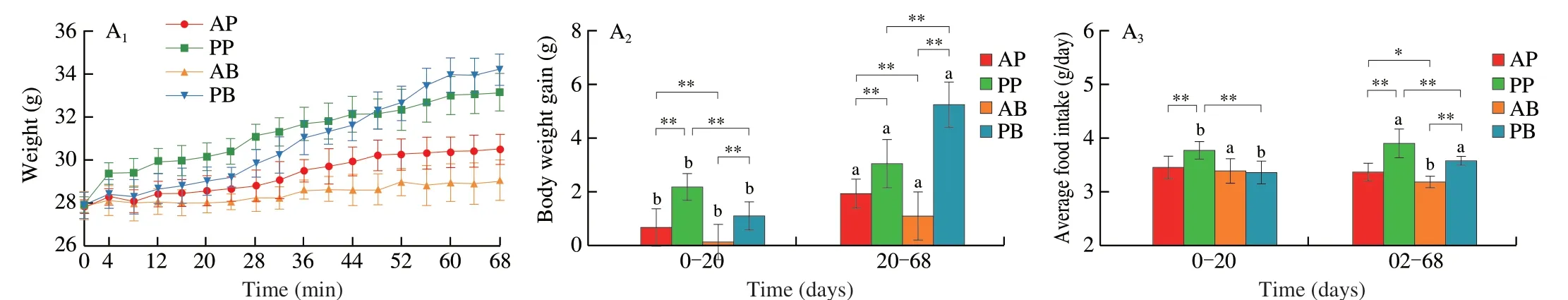
Fig.1 Effects of real meat and plant-based meat analogues on lipid accumulation.(A) Lipid accumulation;(B) Epididymal adipose tissue,H&E staining (200×);(C) Liver tissue,Oil Red staining (200×);(D) Liver tissue,H&E staining (200×).Notes: Data are presented as means ± SD.Asterisk (*) indicates significant differences between protein type or source.*P < 0.05;**P < 0.01.The “a,b” letters indicate significant differences between 20 and 68 days of dietary intervention under the same diets (P < 0.05).
3.Results
3.1 Effects of real meat and plant-based meat analogues on lipid accumulation
Mice fed the plant-based pork (PP) and beef analogue (PB) diets gained weight faster than those fed real pork (AP) and beef (AB)diets.In terms of protein source,the body weight gains,average food and energy intake as well as the food and energy efficiency of mice in the plant-based meat analogue groups (PP and PB) were significantly higher than those in the corresponding real meat groups (AP and AB)after 20 days and/or 68 days dietary intervention (Fig.1A).In terms of meat species,the body weight gain,food and energy efficiency of mice were significantly higher in the animal-and plant-derived pork groups (AP and PP) than in the corresponding beef groups (AB and PB) after 20 days dietary intervention.However,the body weight gain,food and energy efficiency of mice was significantly higher in the AP group than in the AB group while the value was significantly lower in the PP group than in the PB group after 68 days dietary intervention(Fig.1A).The weight and index of white adipose tissue in the PB were significantly higher than those in the other groups.Notably,the liver weight was significantly higher in the plant-based meat analogue groups (PP and PB) than that in the corresponding real meat groups(AP and AB),although the liver index in the PB group was lower than that in the AB group after 68 days dietary intervention.Besides,the contents of THCO and TG were higher in the PP and PB groups than in the AP and AB groups after 68 days dietary intervention(Fig.1A).The plant-based meat analogue groups,especially the PB group,significantly increased the fat cell size compared with real meat groups (Fig.1B).The Oil Red O staining also confirmed that plant-based meat analogue groups (PP and PB) showed higher lipid intensities in the liver (Fig.1C and Fig.S1A).H&E staining showed that although no serious pathological syndromes were observed in the liver tissues of the 4 dietary groups,there were significant differences among the groups (Fig.1D).Compared with real meat groups,plantbased meat analogue groups (PP and PB) showed more hepatocyte edema to balloon degeneration,cytoplasmic vacuolation (yellow arrow),with round vacuoles of different sizes (red arrow) in the PP group and more punctate lymphocyte infiltration (green arrow) in the PB group.In addition,the contents of TBA and the activities of AKP,ALT and AST were higher in the PP and PB groups than in the AP and AB groups (Fig.S1B).These results suggest that ingestion of plant-based meat analogues induces more fat accumulation and increases the potential risk of metabolic disorders in mice.
3.2 Effects of real meat and plant-based meat analogues on inflammatory reactions
As shown in Fig.2A,the levels of IL-1β,IL-6 and TNF-α in serum were significantly higher in the plant-based meat analogue groups (PP and PB) than in the corresponding real meat groups (AP and AB) after 20 and 68 days dietary intervention.Besides,the plantbased meat analogues increased the levels of adipose tissue proinflammatory related biomarkers,including leptin,resistin,F4/80,SAA3,MCP-1,IL-6,IL-1β and TNF-α,but decreased the level of anti-inflammatory adiponectin.In addition,the AB group had lower levels of leptin,SAA3,IL-6,IL-1β and TNF-α but higher level of adiponectin than the AP group,and the PB group had lower levels of leptin and IL-6 than the PP group (Fig.2B).These results indicate that plant-based meat analogues may induce inflammatory response than the real meat in mice.
3.3 Effects of real meat and plant-based meat analogues on the serum metabolite composition
In order to better understand the effect of real meat and plantbased meat analogues on lipid accumulation,the serum metabolites were determined by LC-MS.The sample sequencing data were confirmed to be reliable by overlaying the total ion chromatogram of quality control (QC) samples (Figs.S2A and S2B).Orthogonal projection to latent structure discriminant analysis (OPLS-DA)models for each of the acquired modes (electrospray ionization positive/electrospray ionization negative[ESI+/ESI-] demonstrated that significant separation between each of the two experimental groups,i.e.,AP versus PP,AP versus AB,AB versus PB and PP versus PB(Figs.S3 and S4).The different metabolites between the two groups are highlighted in red (upregulated) and blue (downregulated) in Fig.S5.Further identification revealed that 80,110,110,and 114 differential metabolites after 20 days dietary intervention and 134,71,108 and 115 differential metabolites after 68 days dietary intervention were found in AP versus AB,AP versus PP,AB versus PB and PP versus PB,respectively (Figs.3A and B).Among the differential metabolites from inter-group comparisons,the AP group upregulated 12 and 118 metabolites and downregulated 68 and 16 metabolites than the AB group after 20 and 68 days dietary intervention.Compared with the PP group,the AP group upregulated 18 and 14 metabolites and downregulated 92 and 57 metabolites after 20 and 68 days dietary intervention,respectively.Besides,compared with the PB group,the AB group upregulated 88 and 31 metabolites and downregulated 22 and 71 metabolites after 20 and 68 days dietary intervention,and the PP group upregulated 98 and 94 metabolites and downregulated 16 and 21 metabolites after 20 and 68 days dietary intervention,respectively (Figs.3A and B).The unique and common differential metabolites were further defined and shown in the Figs.3C-H.There were 16,7,16 and 9 unique differential metabolites after 20 days dietary intervention,corresponding to 9,28,10 and 23 unique differential metabolites after 68 days dietary intervention from the AP versus PP,AP versus AB,AB versus PB and PP versus PB,respectively (Figs.3C and D).Specifically,after 20 days dietary intervention,the AP group had higher xanthurenic acid,taurochenodesoxycholic acid,stearic acid and spermidine,but 12 differential metabolites with lower abundance,including hippuric acid than the PP group,while compared with the AB group,the AP group increased the level of MG (18:0/0:0/0:0) but decreased the levels of phosphonoacetic acid,pyrogallin,isoliquiritin,N,N-dimethylaniline,methyl indole-3-acetate and cytidine.Besides,compared with the PB group,the PP diet increased the levels of ursodeoxycholic acid,rimantadine,dihydroartemisinin,Ala-Leu,L-asparagine,curcumol,fexofenadine and deoxycholic acid but decreased dhelwangin level,and the AB diet increased levels of 13 differential metabolites including docosahexaenoic acid but decreased the levels ofL-phenylalanine,tranexamic acid and xylose (Fig.3E).After 68 days dietary intervention,the AP group had higher pantothenic acid and aliskiren but lower hippuric acid,celastrol,lysoPC (18:0/0:0),cholest-4,6-dien-3-one,leucylproline,L-glutamic acid and indoline than the PP group,while compared with the AB group,the AP group increased the levels of 27 differential metabolites including monoethylhexyl phthalic acid but decreased the stearoylethanolamide level.Besides,compared with the PB group,the PP group increased 19 differential metabolites including aldosterone but decreased the levels of guanosine monophosphate,xylulose 5-phosphate,adenosine and phosphonoacetic acid.The AB diet caused an increase in the abundances of stearic acid,cytidine,PC (16:0/0:0),stearoylcarnitine,palmitoylethanolamide and atenolol acid but a reduction in pseudouridine,thymidine,propylhexedrine and thymine (Fig.3F).In addition,19 and 15 common differential metabolites were identified in 4 groups after 20 and 68 days dietary intervention (Figs.6E and F),and the content changes of these metabolites are shown in the Figs.3G and H.Except nadolol high in the PP group and acetylcholine high in the AB group,the contents of other 17 metabolites were higher in the PP and AB groups than in the AP and PB groups after 20 days dietary intervention.Extension of dietary intervention to 68 days resulted in higher levels of eicosapentaenoic acid,nadolol and other 12 metabolites in the PP group than in the other groups (Figs.3G and 3H).The above results indicate that plant-based meat analogues induced significantly different composition of serum metabolites in mice from real meat.
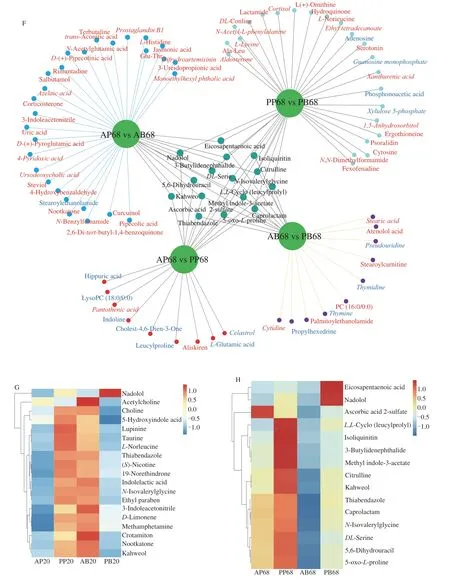
Fig.3 (Continued)
3.4 Effects of real meat and plant-based meat analogues on the metabolic pathways
To further reveal the changes in the metabolic status,the KEGG enrichment analysis for all differential metabolites was performed,and the significantly enriched pathways were shown in Fig.4.The results indicated that the differential metabolites of AB versus PB were mainly enriched in the cell growth and death,signaling molecules and interaction,immune and nervous system,lipid metabolism and membrane transport pathways after 20 and 68 days dietary intervention.Besides,they were mainly enriched in the amino acid metabolism,sensory and digestive system,signal transduction and translation pathways after 20 days dietary intervention and the metabolism of cofactors,vitamins,nucleotide,other amino acids pathways after 68 days dietary intervention.The differential metabolites of AP versus PP were mainly involved in the amino acid,cofactors,vitamins and lipid metabolism,nervous and sensory system pathways after 20 and 68 days dietary intervention,and they were also involved in the cell growth and death,digestive,excretory and immune system,membrane transport,nucleotide metabolism and signaling molecules and interaction pathways after 20 days dietary intervention.In addition,the differential metabolites of AP versus AB were mainly related to the nervous and sensory system and amino acid metabolism pathways after 20 days dietary intervention and the digestive and sensory system,amino acid,cofactors and vitamins metabolism and membrane transport pathways after 68 days dietary intervention.The differential metabolites of PP versus PB were mainly enriched in amino acid,lipid,cofactors and vitamins metabolism,nervous and sensory system pathways after 20 and 68 days dietary intervention,and they were also involved in the cell growth and death,digestive,excretory and immune system,membrane transport,nucleotide metabolism,signaling molecules and interaction pathways after 20 days dietary intervention.Combined with the published literature[16-17],the significantly differential pathways that have prominent contributions to explain the different lipid accumulation caused by different real meat and plant-based meat analogues are mainly amino acid and lipid metabolism,digestive and immune system,signaling molecules and interaction,and membrane transport pathways.
3.5 Effects of real meat and plant-based meat analogues on the expression of lipid metabolism-related genes
To further explore mechanism associated with different lipid accumulation induced by different real meat and plant-based meat analogues,the expression of lipid metabolism-related genes was measured in the adipose and liver tissue (Fig.5).In the adipose tissue,the PB group upregulated the expression of genes related to fatty acid synthesis,i.e.,stearoyl-CoA desaturase-1 (SCD1),sterol regulatory element binding protein 1 (SREBP-1),acetyl-CoA carboxylase alpha(ACCα) and fatty acid synthase (FAS),compared with the AB group after 20 and 68 days dietary intervention.No significant difference was observed between the AP and PP groups except for theSCD1expression.Moreover,the expression of the adipocyte differentiationrelated genes,including peroxisome proliferator activated receptorγ(PPARγ) and CCAAT/enhancer binding proteinα(C/EBPα) was downregulated in the PP group than in the AP group while those were upregulated in the PB group than in the AB group (Fig.5A).On the other hand,adipose triglyceride lipase (ATGL) was downregulated in the PB and PP groups after 20 days dietary intervention compared to AB and AP groups.However,ATGLwas upregulated in the PB and PP groups at time point of 68 days (Fig.5A).These results suggest that plant-based meat analogues significantly changed the lipid storage capacity of adipose tissue than real meat.In the liver,the mRNA levels of fatty acid translocase (CD36),fatty acid-binding protein(FABP4) and fatty acid transport protein (FATP2andFATP5),SCD1,SREBP-1,ACCα,FAS,acyl-CoA synthetase (ACSL1),carnitine palmitoyl transferase 1α (CPT1α) and peroxisome proliferator activated receptor α (PPARα) were significantly higher in the PB group than in the AB and PP groups after 20 days dietary intervention(Fig.5B).The PP group had higher mRNA levels ofCD36,SREBP-1andFASbut lower levels ofACSL1,CPT1α,PPARαandPGC-1αthan the AP group (Fig.5B).At 68 days of time point,the plant-based meat analogues (PP and PB) markedly upregulatedCD36,SCD-1,SREBP-1,ACCαandFAS,but downregulatedCPT1αcompared with the real meat (AP and/or AB).In addition,the PP diet induced the downregulation ofACSL1compared with the AP diet,while the PB diet upregulatedFABP4andFATP2but downregulatedPPARαandPGC-1αcompared with the AB diet (Fig.5B).These results indicate that plant-based meat analogues increase hepatic lipid synthesis but decrease fatty acid oxidation.
3.6 Determination of serum metabolic biomarkers associated with lipid metabolism
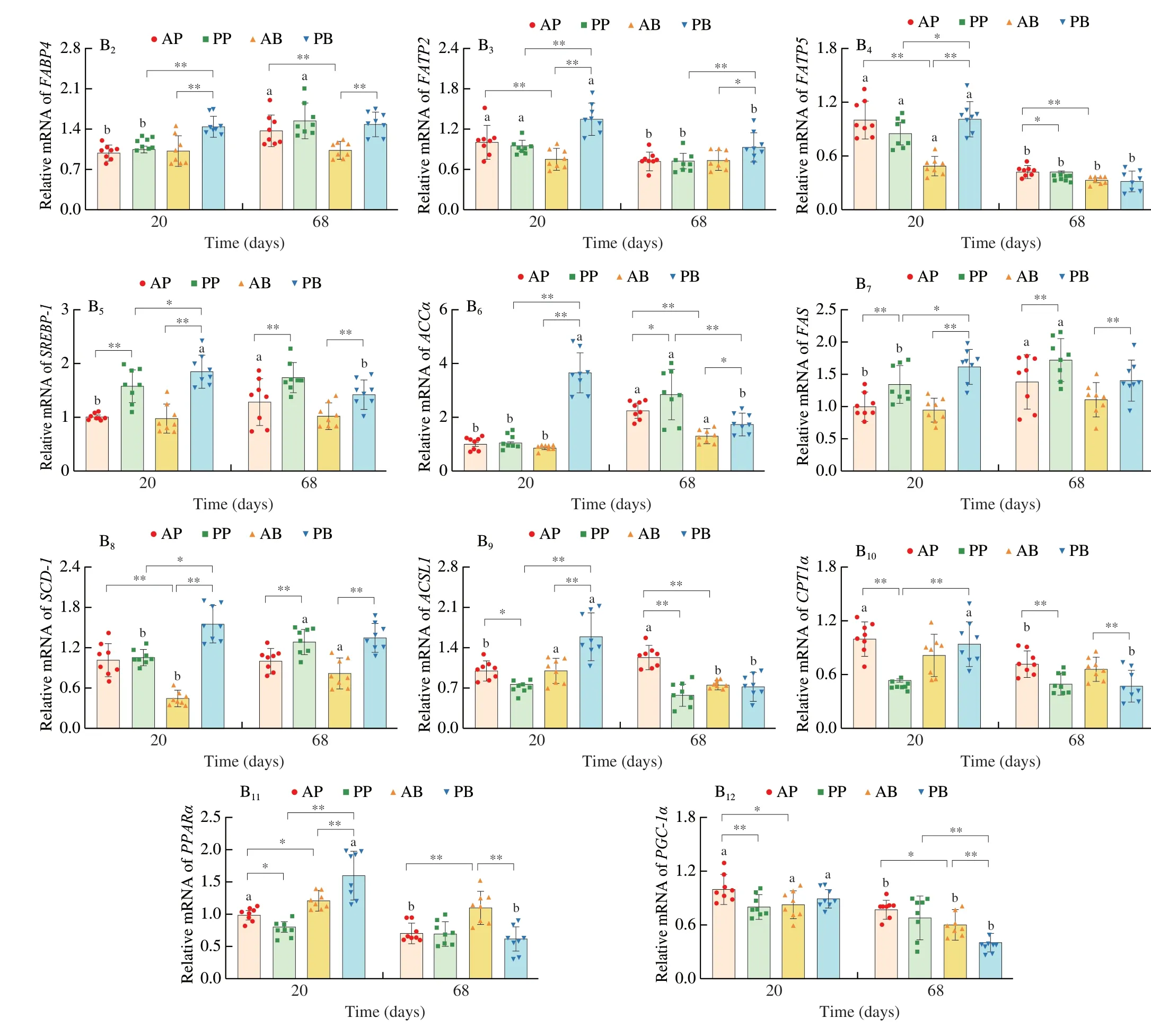
Fig.5 (Continued)
In order to further confirm the association between dietaryinduced changes in serum metabolites and lipid accumulation,the Spearman correlation analysis was performed between the differential metabolites in significantly enriched pathways and the physiological and biochemical indicators related to lipid metabolism.The results are shown in Figs.6A and B.After 20 days dietary intervention,these differential metabolites were clustered into 4 categories,and the metabolites significantly related to the lipid metabolism were mainly distributed in clusters 1,3 and 4.Specifically,the abundances of metabolites in the cluster 1 were mainly positively correlated with mRNA levels of fatty acid synthesis-related genes in liver tissues but negatively with the mRNA levels of lipolysis-related genes.However,the abundances of metabolites in the cluster 4 were negatively correlated with the mRNA levels of fatty acid synthesis-related genes in adipose tissue and liver but positively with those of lipolysisrelated genes.In addition,the levels of metabolites in the cluster 3 were mainly negatively correlated with the mRNA levels of fatty acid synthesis and oxidation-related genes in adipose tissue (Fig.6A).For samples of 68 days dietary intervention,the differential metabolites were clustered into 5 categories.In cluster 1,metabolites were mainly positively related to lipid storage capacity.In cluster 5,metabolites were negatively related to lipid storage capacity.In cluster 2,metabolites were positively linked to adipocyte differentiation-and fatty acid synthesis-related genes in adipose tissue,but negatively to fatty acid oxidation-related genes;however,metabolites in the cluster 3 showed an opposite correlation with fatty acid oxidation,fatty acid synthesis and adipocyte differentiation-related genes in adipose tissue.In cluster 4,metabolites were positively correlated with fatty acid synthesis-related genes in liver (Fig.6B).These results show that there is a complex relationship between serum metabolites and lipid metabolism.
Further co-occurrence network analysis indicated that the metabolites significantly related to the lipid metabolism mainly included C00106 (Uracil),C00152 (L-Asparagine),C00181(Xylose),C00183 (Valine),C00245 (Taurine),C00327 (Citrulline),C00386 (Carnosine),C00429 (5,6-Dihydrouracil),C00437(N-Acetylornithine),C00719 (Betaine),C00735 (Cortisol),C00978 (N-Acetylserotonin),C01152 (1-Methylhistidine),C01996 (Acetylcholine),C06427 (α-Linolenic acid),C06428(Eicosapentaenoic acid),C06429 (Docosahexaenoic acid),C16513 (Docosapentaenoic acid) and so on.They were mainly involved in the protein digestion and absorption,bile secretion,amino acid metabolism (specifically tryptophan,histidine,betaalanine,glycine,serine,threonine,alanine,aspartate,glutamate and arginine metabolism),lipid metabolism (specifically biosynthesis of unsaturated fatty acids,linoleic acid and glycerophospholipid metabolism),aminoacyl-tRNA biosynthesis,neuroactive ligandreceptor interaction and ATP binding cassette (ABC) transporters pathways (Figs.6C and D).These results identify the serum metabolite biomarkers significantly correlated with lipid metabolism.
4.Discussion
The protein ingredients used for the manufacture of meat analogues is undoubtedly one of the most important components for product identity and product differentiation.The plant-based meat analogues in this study,mainly contained rice,pea,mung bean,soybean protein.Some studies showed that dietary proteins from different sources can change lipid metabolism in the liver.Shi et al.indicated that chicken,pork,and beef proteins at the recommended level could be more conducive to cholesterol degradation,triglyceride decomposition,and energy synthesis maintenance at a healthy level than soybean protein and casein[18].Song et al.found that soy and meat proteins induced distinct physiological and metabolic responses in rats after a short time intervention[19].The beef,pork,chicken and fish meat proteins displayed beneficial effects on growth and lipid metabolism[20].In human study,the beef-based to chicken-based diet alterations changedBacteroides-related proteins and decreased hosts’ immunoglobulins in high-and middle-BMI volunteers[21].Recent studies have also indicated that real meat and plant-based meat analogues have different protein digestion properties in anin vitrodigestion model[13].Protein digestion products may affect the release of hormones that regulate digestion rates and the anabolism of proteins,carbohydrates,and fats.Moreover,the secretion of bile acids directly affects the digestion and absorption of lipids.In the present study,the total bile acid level was significantly higher in the plantbased analogue groups than in the real meat groups.The bile acid molecule has two sides,hydrophilic and hydrophobic,which make bile acid has strong interfacial activity,promoting lipid emulsification.Meanwhile,the contact surface of fat and lipase is expanded to accelerate the digestion of lipids[22].When the body ingests a lot of fat,the liver can’t transport the fat out in time,which will lead to the accumulation of fat in the liver,damaging liver function and causing liver diseases.Ijaz et al.[23]suggested that the intake of a high-fat diet in combination with a normal level of beef protein for 12 weeks increased dyslipidemia,hypercholesterolemia and TG accumulation in the liver and led to systemic inflammation,impaired glucose metabolism and induced insulin resistance compared with casein and soy protein diet.The plant-based meat analogues were added a lot of fat and other food additives in the production process in order to simulate the texture and taste of real meat[24-25].This may have certain effects on health.
The ingested macromolecular nutrients are absorbed and enter the circulatory system and finally the liver to participate in different metabolic processes.Spearman correlation analysis and cooccurrence network analysis clarified that the specific metabolite biomarkers may affect lipid accumulation mainly by direct lipid metabolism pathways or indirect amino acid metabolism,protein digestion and absorption,bile secretion,aminoacyl-tRNA biosynthesis,neuroactive ligandreceptor interaction and ABC transporters pathways.ABC transporters constitute a ubiquitous superfamily of integral membrane proteins that are responsible for the ATP powered translocation of a wide range of substrates,including lipids,metabolites,and xenobiotics,across biological membranes in order to maintain normal cell metabolism[16].Circulating branched chain amino acids (BCAAs) and certain aromatic AA are associated with specific obesity-associated characteristics including hepatic insulin sensitivity and intrahepatic fat content[17].Not only the free amino acids,but also the bioactive peptides in proteins released during digestion impact lipid metabolism.Compared with real meat,the plant-based meat analogues significantly altered the expression of lipid accumulation-related genes in white adipose and liver tissue.The composition of amino acids in the diet affects the circulating amino acid profile to some extent,so the different effects of PP and PB on expression of lipid accumulation-related genes in white adipose tissue may be closely related to their amino acid composition.Adipose tissue plays a central role in maintaining energy and metabolic homeostasis.The higher body weights and larger adipocytes suggested that the mice fed plant-based analogue diets were in a state of chronically positive energy balance.To mimic the taste of real meat,some spices were added to the plant-based meat analogues during processing,which may increase food intake of mice.Moreover,the significantly more umami amino acids (UAA) in plantbased pork analogues may stimulate appetite in mice.Amino acids are the basic units of protein,and essential amino acids have important nutritional value for human health.Compared with real meat,the diet of plant-based meat analogues is lower in essential amino acids,and more dietary intake may be required to meet the body’s needs.Food intake is not only affected by the food itself,but also by the combined effects of gastrointestinal emptying and appetite regulation,and how plant-based meat analogues increase food intake in mice as well as the related mechanisms should be further explored.In addition,weight gain does not depend only on energy intake but also on activity level,so the activity of mice fed different real meat and plantbased meat analogues remains to be further explored.The surplus energy is efficiently deposited in the form of TGs in white adipose tissue,which acts to “buffer” the influx of lipid into the circulation.The excess lipids “spill over” from the dysfunctional adipose tissue,exposing other tissues to an excessive influx of lipids and then increasing the risk of ectopic lipid deposition[26-27].The liver is a prone site for fat deposition when adipose tissue is dysfunctional.The hepatic lipid homeostasis depends on influx of free fatty acids,de novolipogenesis (DNL),fatty acid oxidation,and lipoprotein secretion[28].The abnormality of any process could lead to lipid accumulation in liver.Compared with real meat,the plant-based meat analogues not only shape different adipose tissue weights by changing the lipid storage capacity but also make liver exposed to more influx of lipids due to lipolysis.Besides,the plant-based meat analogues increased hepatic lipid input and synthesis but decreased lipid oxidation.The combination of these effects resulted in lipid deposition in the liver.
Lipid accumulation is usually accompanied by the elevation of circulating pro-inflammatory cytokine levels and accumulation of adipose tissue inflammation-related cytokines and adipokines[29-30].Growing evidence indicates that adipose tissue dysfunction[31]and increased secretion of adipokines[32]are implicated in the systemic nature of some liver pathogenesis.In the present study,the plantbased pork and beef analogues increased the food intake leading to more energy intake compared with real meat,which may be closely related to the inflammatory responses.Moreover,van Vliet et al.indicated that plant-based meat analogues lacked some important physiological,anti-inflammatory or immunomodulatory nutrients compared with real meat[12].Inflammation affects the normal development of preadipocytes to be fully differentiated into adipose cells[33]and increases lipolysis[34],which decreased lipid storage capacity of adipose tissue.Salles et al.found that TNF-αknockout (TNF-α-KO) mice significantly increased adipose fat mass than control after a 2-week high fat diet (HFD),while TNF-α-KO mice showed lower hepatic TG level,with significantly declined adipose inflammatory biomarkers[35].This implies that decreased levels of inflammatory cytokines in adipose tissue might improve fat storage capacity of adipose tissue to prevent abnormal lipid deposition in non-adipose tissues.Furthermore,in vitrostudies found that IL-6 induced higher FABP level in both HepG2 cells and primary mouse hepatocytes,leading to increased intracellular lipid content[36].Therefore,the inflammatory response that accompanies fat accumulation caused by ingestion of plant-based meat analogues in turn affects lipid accumulation.Ectopic fat deposition may be an important factor determining the onset of type 2 diabetes mellitus and obesity-associated comorbidities[37-38].Excessive hepatic fat accumulation predisposes to non-alcoholic fatty liver (NAFLD),nonalcoholic steatohepatitis (NASH),which may progress to cirrhosis and hepatic cancer[39-40].Therefore,more fat accumulation and inflammatory response induced by plant-based meat analogues in mice increase the potential risk of metabolic disorders.In term of the sustainable development,the plant-based meat analogues can relieve stress to a certain extent,but the intake should also be paid attention to.Although these results from animal experiments cannot be extrapolated directly to humans,they do provide some evidence and references for the composition of a healthy human diet.More indepth related mechanisms need to be further explored.
5.Conclusion
The effects of plant-based meat analogues on metabolic health and the underlying mechanism were investigated for the first timein vivothrough rigorous intervention trials.Compared with real meats,the plant-based meat analogues increased food intake of mice and further induced more body weight gain,lipid accumulation and inflammatory responses.These were closely related to changes in the composition of serum metabolites.The specific metabolic biomarkers may affect fat accumulation directly by lipid metabolism(specifically biosynthesis of unsaturated fatty acids,linoleic acid and glycerophospholipid metabolism) pathways or indirectly by protein digestion and absorption,bile secretion,amino acid metabolism(specifically tryptophan,histidine,beta-alanine,glycine,serine,threonine,alanine,aspartate,glutamate and arginine metabolism),aminoacyl-tRNA biosynthesis,neuroactive ligand-receptor interaction and ABC transporters pathways.The findings provide a new insight into the different nutritional functions between real meats and plantbased meat analogues,and more scientific basis for rational diet.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by Jiangsu Innovative Group of Meat Nutrition,Health and Biotechnology and the Postgraduate Research&Practice Innovation Program of Jiangsu Province (grant number:KYCX21_0575).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250081.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

