Structure,bioavailability and physicochemical properties of icariin-soymilk nanoparticle
Jinping Wang,Lingrong Wn,Yuming Jiang,Hong Zhu,Wizhng Sun,Guangyi Dai,Bao Yang,
a Guangdong Provincial Key Laboratory of Applied Botany,Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement,South China Botanical Garden,Chinese Academy of Sciences,Guangzhou 510650,China
b South China National Botanical Garden,Guangzhou 510650,China
c College of Food Science and Engineering,Jilin Agricultural University,Changchun 130118,China
d University of Chinese Academy of Sciences,Beijing 100049,China
e School of Food Sciences and Engineering,South China University of Technology,Guangzhou 510640,China
Keywords: Encapsulation eff iciency Flavonoid High pressure homogenization Loading capacity Nano-complex
ABSTRACT Soymilk is a natural nanocarrier.However,the performance of flavonoid-soymilk nano-complex remains unclear.In this work,icariin-soymilk nano-complexes (ISNCs) were successfully fabricated and characterized.The effects of high-pressure homogenization (HPH) treatment on structure and physicochemical properties of soymilk and nano-complexes were investigated.HPH treatment could signif icantly improve the surface hydrophobicity and interfacial activity of soymilk.The soymilk with HPH treatment could significantly improve the water solubility (20 folds),thermal stability and bioavailability of icariin.The highest encapsulation eff iciency (93.28%),loading capacity (39.09 µg/mg),ζ-potentia (absolute value,31.20 mV) and bioavailability (72.14%) were observed in HSI-200 (200 bar of homogenization pressure).While HSI-500(500 bar of homogenization pressure) showed the smallest particle size (183.73 nm).ISNCs showed a rougher surface and an irregular lamellar structure with large amount of f ine particles by using Cryo-SEM,suggesting that icariin was encapsulated in soymilk.These data supplied a novel strategy to improve the performance of icariin in functional foods.
1.Introduction
Icariin,a prenylated flavonoid,is present inEpimedium brevicornumMaxim (Berberidaceae)[1].Recently,icariin has attracted much attention due to its various biological activities,including anti-tumor,immunomodulatory,osteogenic,anti-depressant,anti-inflammatory and neuroprotective effects[2].However,the application of icariin in functional foods is limited due to its high hydrophobicity[3].Previous study has also pointed out that icariin is poorly absorbed after oral administration[2].Therefore,there is considerable interest in developing nanocarrier system that can overcome these problems[4].Various edible delivery systems,including emulsions,liposomes and nanoparticles have been explored to enhance the solubility,stability and bioavailability of active substances[5].Recently,an icariin nanogel loaded self-assembled thermosensitive hydrogel system has been reported to successfully prepare by a reverse microemulsion technique and present good release behavior,dissolution rate and antidepressant effect[1].However,the drug loading capacity (LC) of icariin nanogels was only 2.03%.Therefore,an eff icient nanocarrier system with high LC is required.
Proteins derived from milk,soybean and corn have been proven to be natural vehicles for bioactive substances.Previous study has indicated that soybean proteins-soybean polysaccharides complex can act as good nanocarrier for bioactive substances[6].Soybean is one of the most important legumes all over the world.Many products are processed,of which soymilk is the major one.Soymilk a stable colloidal solution that contains about 4%-6% protein,2%fat,2% carbohydrates,vitamins,soy isoflavone and other beneficial ingredients[7].Previous report indicated that emulsions showed advantages in bioaccessibility and controlling release of bioactive substances[8].As an emulsifier,soymilk have been proven to be effective for encapsulating and delivering bioactive substances[9-10].All these results suggest that soymilk may have the potential to develop as nanocarrier of bioactive substances.In addition,soymilk has a variety of biological functions,including lowering blood cholesterol level,reducing the risk of cardiovascular disease and bone loss,and restoring menopausal symptoms in women[11].
High-pressure homogenization (HPH) is a continuous,inexpensive and environment-friendly process.In addition to traditional applications in inactivating enzymes,stabilizing emulsions,and extracting functional compounds,HPH has recently been used as an effective tool to induce structural change in proteins to enhance their functional properties[12].Previous work has suggested that HPH treatment causes changes in particle shape,size and particle size distribution of the products[13],implying that the HPH treatment may be a helper for the preparation of nanocarriers.
In this work,the complexation of icariin and soymilk was prepared,and the potential of soymilk as a nanocarrier for icariin was evaluated.The effects of HPH treatment on chemical composition,microstructure,surface hydrophobicity (Ho) and interfacial tension of soymilk were investigated.The effects of HPH treatment on the properties of icariin-soymilk nano-complexes (ISNCs),including particle size distribution,ζ-potential,microstructure and stability,were characterized as well.The present results might provide new insights of icariin in functional foods.
2.Materials and methods
2.1 Materials
Mature soybeans (Wuchang Caiqiao Rice Industry Co.,Ltd.,Harbin,China) were used in this study.Icariin was purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.(Shanghai,China).Its chemical structure was further confirmed by nuclear magnetic resonance spectroscopy.Soybean oil was obtained from a supermarket (Guangzhou,China).A probe,8-anilino-1-naphthalenesulfonicate (ANS-Na) was purchased from Shanghai Macklin Biochemical Co.,Ltd.(Shanghai,China).Pepsin from porcine gastric mucosa (672 units/mg protein),pancreatin from porcine pancreas (4 × the standard USP unit),bile salts and Folin-Ciocalteau reagents were purchased from Sigma-Aldrich Chemical Co.(St.Louis,MO,USA).All the other chemical reagents were of analytical grade.
2.2 Preparation of soymilk
Soybeans (100 g) were rinsed with deionized water for 2-3 times to remove impurities,and were mixed with deionized water in the ratio of 1:13 (dry soybeans:deionized water,m/V).The mixture was ground with wall breaker (L18-Y933,Joyoung Co.,Ltd.,Jinan,China) for 39 min.After cooling to room temperature,the slurry was centrifuged (5 000 ×g,30 min,25 °C) to remove any insoluble substances,and the supernatants were named as NS (non-treated soymilk),and were collected for subsequent analysis.
2.3 HPH treatment
For HPH treatment,200 mL of NS were subjected to HPH treatment by using the APV-1000 homogenizer (Kolding,Denmark)at 200 and 500 bar for 3 times at 25 °C,and the samples were named as HS-200 and HS-500,respectively.
2.4 Determination of protein and total carbohydrates contents
Protein content was determined by using a modified Lowry method as described previously with a minor modification[14].Triplicate aliquots of soymilk samples were adjusted to 1 mL with deionized water,then mixed with 5 mL of freshly made Lowry reagent.The mixture was incubated at room temperature for 10 min prior to the addition of 0.5 mL of Folin-Ciocalteau reagent.After incubation for 30 min in the dark,the absorbance at 660 nm was measured by using a multifunctional microplate reader (Spark,Tecan Group Ltd.,Männedorf,Switzerland).Bovine serum albumin was used as standard,and the final result was expressed as milligrams of bovine serum albumin equivalents per milligram of soymilk (mg/mg).
The total carbohydrates content of the soymilk was measured by phenol-sulfuric acid method[15].An aliquot (1 mL) of tested soymilk(0.1 mg/mL) was mixed with 0.5 mL of 6% phenol.Then 3 mL of 98% H2SO4were added,and vortexed immediately.After incubation for 30 min,the absorbance at 490 nm was measured.The content of total carbohydrates was finally calculated as milligrams of glucose equivalents per milligram of soymilk (mg/mg).
2.5 Determination of Ho
As described previously[16],Hoof soymilk was measured by using ANS-Na as a fluorescence probe.Briefly,freeze-dried soymilk powder was dissolved in 10 mmol/L PBS (pH 7.0),which was diluted with 10 mmol/L PBS to various concentrations from 0.1 mg/mL to 1.0 mg/mL.An aliquot (50 µL) of ANS-Na solution (8 mmol/L) was added to 4 mL of tested sample solution.ANS-Na in PBS was used as blank control.Soy protein isolate (SPI) at the same concentration was also analyzed for comparison.After reaction in dark for 5 min,the fluorescent intensity was measured with a multifunctional microplate reader using an excitation wavelength of 370 nm and an emission wavelength of 465 nm.The relative fluorescence intensity was linearly matched to soymilk concentration and the initial slope of relative fluorescence intensity versus sample concentration plot corresponded to an index ofHo.
2.6 Interfacial tension measurement
The interfacial tension of soymilk (NS,HS-200 and HS-500)at the oil-water interface was measured as described previously[17]by using a drop shape analyzer (DataPhysica Instruments GmbH,Filderstadt,Germany) with an OCA20 video-based optical contact angle meter system at 25 °C.To avoid the adverse effect from impurities in soybean oil during the measurement,soybean oil was purified by adding 0.3% Florisil (60-100 mesh,Sigma Aldrich),and was deemed acceptable only after the interfacial tension against the deionized water kept at a constant value of (25.5 ± 0.5) mN/m for at least 30 min.The measurement was carried out as follows.Briefly,a drop of sample suspension (10 µL,0.3%) was delivered into an optical glass cuvette containing purified soybean oil,and allowed to stand for 10 800 s to achieve sample adsorption.An image of the drop was continuously recorded by a Charged Coupled Device(CCD) camera and digitalized.The tension values were calculated automatically using the SCA20 software (DataPhysica Instruments GmbH,Filderstadt,Germany).
2.7 Nano-complexes preparation
Icariin was dissolved in 70% ethanol stirred magnetically at 60 °C for 30 min with a magnetic stirrer (RT 5,IKA®,Werke GmbH &Co.,Staufen,Germany) to obtained a final concentration of 5 mg/mL.The icariin solution was added dropwise to the soymilk and the ISNCs were obtained after constant stirring at 400 r/min for 1 h using a magnetic stirrer at room temperature.The volume ratio of icariin solution in the final system was 1:5.The ISNCs were collected after centrifugation at 10 000 ×gfor 15 min to remove any insoluble matter.The nano-complexes from NS,HS-200 and HS-500 were denoted as NSI,HSI-200 and HSI-500,respectively.Icariin in water,which were added to water in the same final concentration,were used as blank control,and named as WI.
2.8 Nano-complexes characterization
2.8.1 Encapsulationefficiency(EE)andloadingcapacity(LC)The EE and LC of the ISNCs were measured and calculated as described previously[18]with some modifications.In brief,the ISNCs were centrifuged at 10 000 ×gfor 15 min to separate unbound icariin.The precipitation,which contained the unbound icariin,was collected and dissolved in anhydrous ethanol.The content of unbound icariin was measured by using an ultrahigh performance liquid chromatography (UPLC,1260,Waldbronn,Germany),which equipped with a Hypersil GOLDTMthreaded column(2.1 mm × 100 mm,1.9 µm,Thermo Fisher Scientific (China) Co.,Ltd.,Shanghai,China).The injection volume was 2.0 µL.The flow rate was 0.4 mL/min and the column temperature was 40 °C.Solvent A (H2O) and solvent B (acetonitrile) were used for gradient system.And the solvent gradient was used as follows: 3% B (0.0-3.0 min);3%-95% B (3.0-15.0 min);95% B (15.0-20.0 min);95%-3% B(20.0-20.5 min);3% B (20.5-25.0 min).The content of icariin in the test solutions was calculated according to the peak areas and the calibration curves of icariin.The EE and LC of the ISNCs were determined by the following formulas,respectively.
Wheremeandm0are the contents of icariin in the ISNCs and the initial icariin in the system,respectively.In addition,M represents the total mass of ISNCs.
2.8.2 Particlesize,polydispersityindex(PDI)andζ-potential
The particle size,PDI andζ-potential of NS,HS-200,HS-500 and ISNCs were measured at 25 °C by using a Nanosizer ZS instrument(Malvern Instruments Co.,Ltd.,Worcestershire,UK) equipped with a He-Ne (633 nm) laser.Prior to test,the prepared samples were diluted to 100-folds with deionized water in order to obtain an appropriate light intensity to make reliable measurements.The refractive index of the dispersed phase was set to 1.460 and the refractive index of the continuous phase was set to 1.330,and each sample was measured in triplicate.
2.8.3 Morphology
The treatment of the sample referred to previous literature[19].The morphology of icariin,soymilk and ISNCs were obtained by using a cryo-scanning electron microscopy (Cryo-SEM) (Regulus 8100,Hitachi,Japan).In brief,10 µL of sample dispersion were dropped on a sample stage,and was quench-frozen through liquid nitrogen mud (-210 °C).Then the quench-frozen sample was transferred to the preparation chamber.After fracturing,sublimating and gold spraying,the treated sample was transferred to the SEM chamber.Finally,the tested sample was observed and imaged after 2 500 times magnification at an acceleration voltage of 10 kV.
2.9 Nano-complexes stability
The stability of ISNCs against pH,salt,temperature and storage time were evaluated as described previously[20].
2.9.1 SaltandpHstability
A series of ISNCs with different pH values (pH 2.0-12.0) were prepared by using 1.0 mol/L HCl or 1.0 mol/L NaOH solution.A series of nano-complexes with different salt concentrations(0-1 000 mmol/L NaCl) were obtained by the addition of different levels of NaCl (1:1,V/V).The nano-complexes were stored overnight under refrigerated conditions at 4 °C before being analyzed.And the particle size andζ-potential were determined.
2.9.2 Storagestability
In order to investigate the storage stability of ISNCs,freshly prepared nano-complexes were stored at 4 or 25 °C for 28 days,respectively.The particle size andζ-potential of nano-complexes during long-term storage were investigated.To inhibit bacterial growth,NaN3(0.02%) was incorporated into the nano-complexes before the storage tests.Samples were regularly collected (0,7,14,21 and 28 days) from the suspensions for analysis.
2.9.3 Thermalstability
The thermal stability of ISNCs was measured by incubating at 25,60 and 90 °C for 2 h by using a water bath (HWS-26,Shanghai,Yiheng Scientific Instrument Co.,Ltd.,Shanghai,China),respectively.After cooling to room temperature using ice water,the mixture was centrifuged (10 000 ×g,15 min,25 °C).The contents of icariin in the supernatant and the whole mixture were measured by using UPLC.The remaining rate (%) of icariin was calculated using the following equation:
Wheremais the content of icariin in the supernatant after thermal treatment andm0is the content of icariin in the mixture before thermal treatment.
2.10 Simulated gastrointestinal digestion
Briefly,20 mL of the fresh made sample was adjusted to pH 2.0 using 1.0 mol/L HCl solution and stirred at 100 r/min for 10 min in a 37 °C shaker (HZQ-F160A,Shanghai,Yiheng Scientific Instrument Co.,Ltd.,Shanghai,China).Then the solution was mixed with pepsin(4%,m/m,on protein base) and incubated with stirring at 100 r/min for 60 min to mimic gastric digestion.The gastric digests were withdrawn for subsequentinvitrointestinal digestion.The mixtures were then neutralized (pH 7.0) using 2.0 mol/L NaOH solution.Subsequently,bile salts (0.5%) and trypsin (4%,m/m,on protein base) were added to the mixtures.Then the mixtures were incubated with stirring at 100 r/min for 120 min in a 37 °C shaker for intestinal digestion.
The particle size distribution and retention rate of nano-complexes and free icariin in each digestion phase were investigated.At the end of each stage,half of the tested sample was mixed with 9-fold volume of ethanol directly and then were centrifuged (10 000 ×g,10 min),while the rest was centrifuged and the supernatants were mixed with 9-fold volume of ethanol followed by centrifugation again.The content of icariin in the supernatant was determined by UPLC as described above.The retention rate and bioaccessibility were calculated by the following equation:
Where,mcandmucare the mass of icariin in the centrifuged and uncentrifuged groups,respectively;msandm0represent the mass of icariin in the centrifuged group in the small intestine and the initial mass of icariin in the whole system.
2.11 Statistical analyses
All the measurements were performed in triplicate,and the data were expressed as the mean ± standard deviation (SD).Statistical analysis was performed by means of one-way analysis of variance(ANOVA) followed by a Duncan multiple-comparison test ort-test using SPSS version 20.0 software (IBM Corporation,NY,USA).AndP< 0.05 was considered statistically significant differences.
3.Results and discussions
3.1 Effects of HPH treatment on the chemical composition of soymilk
The effects of HPH on the chemical composition of soymilk,including protein and total carbohydrates contents were investigated.The protein level of NS was (25.47 ± 0.86) mg/g,which were(25.01 ± 0.43) and (22.64 ± 0.41) mg/g in HS-200 and HS-500,respectively (Fig.1).Similarly,the total carbohydrates levels of soymilk prepared by HPH treatment were (86.65 ± 1.31) and(86.43 ± 1.64) mg/g with increasing pressure.These values were not significantly different (P> 0.05) to NS ((88.43 ± 0.96) mg/g).Present results indicated that no significant effects on the protein and total carbohydrate contents were caused by HPH treatment.However,previous report pointed out that HPH could alter the molecular conformation of proteins[12].Specifically,HPH treatment could modify the tertiary and quaternary of proteins,whereas the effects on their primary and secondary structure remained unclear.
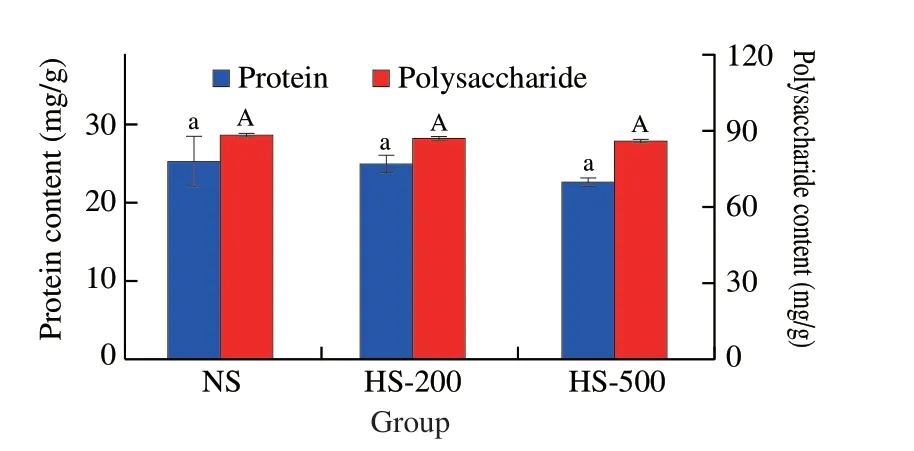
Fig.1 Effects of HPH treatment on physicochemical properties of soymilk.Values with no letters in common indicate significant (P < 0.05) difference.
3.2 Effect of HPH treatment on Ho
As a fluorescent probe,ANS-Na can bind to the hydrophobic region of protein,resulting in an increase of fluorescence intensity.Therefore,the fluorescence intensity is used as an indicator ofHo[21].As shown in Fig.2A,HS-200 (14 817.67) and HS-500 (15 523.67)showed significant (P< 0.05) higherHovalue compared to NS(13 612.67).The results suggested that HPH treatment might affect the structure of the proteins.The increasingHoin HS-200 and HS-500 may be attributed to the disruption of internal chemical bonds,and increased surface exposure of hydrophobic region[22].Hoplays an important role for the LC of hydrophobic bioactive compound-protein nano-complexes.The improvement ofHomight be favorable for the binding of phenolics to proteins[23].It’s worth noting that theHovalues of NS,HS-200 and HS-500 were significant (P< 0.05) higher than that of SPI (12 421.67),which has been proved to be a promising nanocarrier of bioactive substances[24].This information indicated that soymilk prepared by HPH might be a potential nanocarrier of bioactive substances.
3.3 Effects of HPH treatment on the interfacial tension of soymilk
The evolution of interfacial tension values of soymilk with the adsorption time at different homogeneous pressure are shown in Fig.2B.The maximal interfacial tension of NS (17.4 mN/m),HS-200(16.1 mN/m) and HS-500 (15.8 mN/m) were lower than that of other water-soluble bioactive proteins or peptides such as lactoferricin B(19.0 mN/m),and melittin (20.5 mN/m).However,they are higher than cytochrome c (14.0 mN/m)[18].The treatment of HPH resulted in a decrease of interfacial tension in a pressure-dependent manner.The interfacial tension of soymilk decreased dramatically in the initial stage of adsorption and then decreased slowly as adsorption time prolonged.It suggested that soymilk was continuously adsorbed to oil-water interface.According to previous report,it may take 2 or 3 days for the protein to rapidly adsorb from the continuous phase to the newly formed oil-water interface,thereby reaching the adsorption equilibrium[25].
The results in this work indicated that HPH had remarkable effect on the increasing surface activity of soymilk,which might be attributed to the smaller particle size induced by HPH treatment,resulting fast migration onto the interface[26].Another possible explanation for this behavior might be the existence of a certain energy barrier to adsorption.As adsorption time prolonged,HS-500 and HS-200 with the higherHocould reduce the energy barrier for adsorption and entail a higher probability of surface adsorption[27].Moreover,it is generally recognized that the structural characteristics of protein can also affect its interfacial properties[27],implying that HPH might cause structure change of soymilk.
3.4 Effects of HPH treatment on the particle size distribution,PDI and ζ-potential of soymilk
The stability of soymilk is strongly influenced by the particle size.The smaller the particle size is,the better the stability is[28].As shown in Fig.2C,the z-average diameter (Dz) of soymilk decreased in the following order: NS (220.13 nm) > HS-200 (198.17 nm) >HS-500 (173.77 nm),indicating that theDzof soymilk were significantly (P< 0.05) affected by HPH treatment.Similarly,the PDI gradually decreased as the pressure increased (Fig.2D),suggesting an improvement of the particle size distribution by HPH treatment.Significant difference was observed between NS and HS-500(P< 0.05).Previous report indicated that HPH could disrupt protein aggregates[12],which might contribute to the smaller particle size of soymilk induced by HPH.
Zeta potential reflects the surface charge in the emulsion,and is an important index to measure the emulsification characteristics of proteins[29].The effect of HPH on theζ-potential of soymilk is displayed in Fig.2C.Theζ-potential value (absolute value) of the untreated soymilk was 27.03 mV.The value gradually increased with the pressure increased,and reach a maximum of 28.73 mV at 500 bar.The reduction of particle size might be due to the breakage and disruption of particles caused by the shear stress applied on the fluid during homogenization pressure treatment[30].The results ofζ-potential indicated the presence of increased negative charge on the surface of the soymilk droplets,suggesting an increase in the electrostatic repulsion force formed between protein molecules,which could help to prevent from coalescence,thus increasing the stability of the system[31].The present result was consisted with those of previous study,in which HPH treatment could increase theζ-potential value(absolute value) of the emulsion formed by a vegetable protein[28].
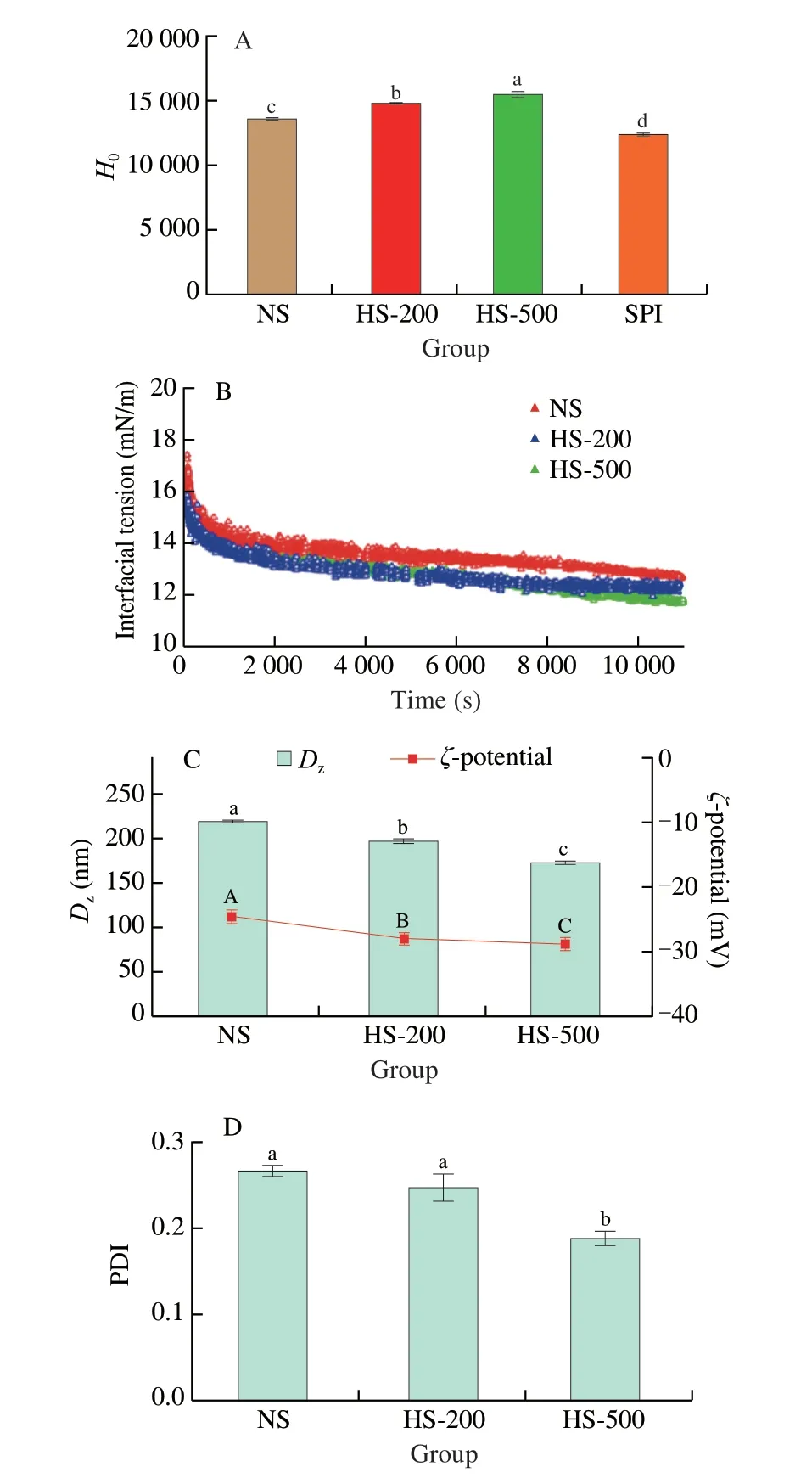
Fig.2 Effects of HPH on the structural characteristics of soymilk.(A) Ho of NS,HS-200 and HS-500;(B) Dynamic interfacial tensions of NS,HS-200,HS-500 at oil-in-water interface as a function of time;(C) Dz and ζ-potential of NS,HS-200 and HS-500;(D) PDI of NS,HS-200 and HS-500.Values with no letters in common indicate significant (P < 0.05) difference.
3.5 Effects of HPH treatment on the characterizations of ISNCs
3.5.1 EE and LC
The soymilk had a homogenous appearance with a bright milky color.As the pressure increased,the color of soymilk gradually became light.When icariin was added,all the ISNCs had a homogenous creamy appearance with a slight yellow color,which suggested an initial stability of nano-complexes,whereas icariin was insoluble in water (Fig.3A).The EE and LC of icariin in ISNCs are presented in Fig.3B.The EE and LC of icariin in non-treated soymilk(NSI) were (88.16 ± 0.05)% and (28.44 ± 0.02) µg/mg,respectively.With HPH treatment,the EE and LC of icariin in HSI-200 were(93.28 ± 0.26)% and (30.09 ± 0.08) µg/mg,respectively.When the pressure increased to 500 bar,these values decreased to(81.68 ± 0.17)% and (26.35 ± 0.06) µg/mg,respectively.In addition,the solubility of icariin in water was 39 µg/mL,significantly (P< 0.05)lower than that in NSI,HSI-200 and HSI-500,which were 740,780 and 680 µg/mL,respectively.All these results also indicated that HPH treatment at a low pressure (like 200 bar) could improve the EE and LC of icariin,while such promotion would be reversed when a higher pressure (500 bar) was applied.
It is well-known that SPI is a promising nanocarrier of flavonoids.However,in our previous study,EE,LC and solubility of icariin were 69.96%,20.65 µg/mg and 583 µg/mL,respectively,using SPI(20 mg/mL) as a nanocarrier of icariin followed by the same protocol as the present work.All these values were significantly lower than those of HS-200,NS and HS-500 (P< 0.05).Our results indicated that soymilk was a potent nanocarrier of icariin.UPLC analysis result showed that the LC of ISNCs was 2.84%,higher than that of icariin nanogels (2.03%) using Span 80 and Tween 80 as nanocarrier[1].The proteins,polysaccharides and lipids presented in soymilk might contribute to its capacity as a nanocarrier of icariin.Proteins and polysaccharides have been proven to be potential promising materials for nano-encapsulation of bioactive compounds due to their biocompatibility and biodegradability[32].In soymilk,proteins mainly present in the form of particles.Icariin might be encapsulated by protein particles through hydrophobic and/or hydrogen bonding interactions[33].Previous report indicated that the water-soluble polysaccharides in soybean were pectin-like structure[34].Polysaccharides in soymilk might help to improve the emulsifying property,thereby playing a positive role on encapsulation and stability[35].In the case of lipids,mostly are triacylglycerols exist in oleosomes,which is a micelle-like structure.In oleosomes,the triacylglycerol matrix is surrounded by a monolayer of phospholipids embedded with proteins[36].Flavonoid glycosides,like icariin might slight interacted with the external polar side of oleosomes due to its poor lipophilicity.As mentioned above,soymilk might work as a whole to interact with icariin to generate a nano-complexes.However,further study is required in our future work to understand the mechanism.
It is worthy nothing that the LC of ISNCs significantly increased to 3.01%,with HPH treating at 200 bar.The improvement of LC of HSI-200 might partly relate to the higherHoof HS-200,as increased hydrophobic sites were available for the binding with icariin[23].Although HS-500 showed a higherHovalue than those of HS-200 and NS,the LC of HSI-500 was similar to NSI,suggesting that other factors except for hydrophobic interactions were involved in the binding with icariin.However,further investigation is needed to elucidate this issue.Treatment conditions,including pressure applied and number of passes could strongly affect the matrix characteristics and functions induced by HPH treatment.Previous study showed that over-processing conditions could induce a general reduction of functions[12],suggesting that pretreating at 500 bar might be an over-processing condition for the encapsulation capacity of soymilk,in comparison with 200 bar.
3.5.2 Particle size distribution and ζ-potential
TheDzof WI,NSI,HSI-200 and HSI-500 were (940.97 ± 8.42),(256.67 ± 2.45),(246.93 ± 3.97) and (183.73 ± 0.93) nm,respectively(Fig.3C).TheDzof NSI,HSI-200 and HSI-500 were significantly higher than that of corresponding soymilk,respectively,similar to curcumin-SPI nanoparticles[23],suggesting the formation of ISNCs.Additionally,the higher value of WI forDzmight imply the aggregation of icariin in water,which was further confirmed by the PDI value (0.97 ± 0.03).The PDI values of ISNCs varied from 0.19 to 0.27,indicated narrow size distribution of nanocomplexes.Additionally,theζ-potential of WI,NSI,HSI-200 and HSI-500 were (-15.73 ± 1.36),(-27.20 ± 0.22),(-31.20 ± 0.41) and(-29.60 ± 1.07) mV,respectively.These results indicated the more negative surface potentials of the nano-complexes in the presence of icariin.It suggested that the presence of icariin altered the surface chemistry of the nano-complexes.Moreover,HPH treatment showed benefit for the higher absolute value ofζ-potential,comparing with the untreated soymilk.
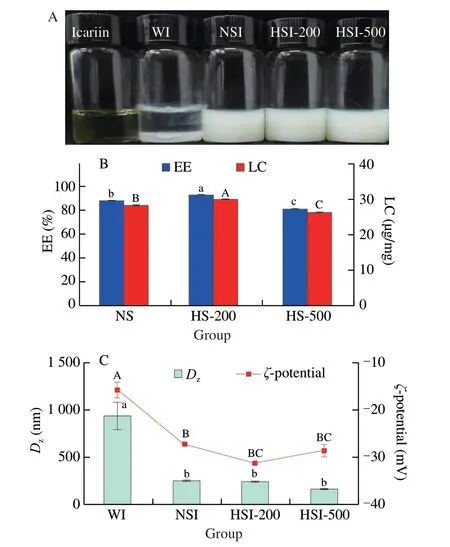
Fig.3 Effects of HPH on ISNCs characteristics.(A) The appearances of ISNCs;(B) EE and LC of icariin in ISNCs;(C) Dz and ζ-potential of WI,NSI,HSI-200 and HSI-500.Values with no letters in common indicate significant(P < 0.05) difference.
3.5.3 Morphology
Cryo-SEM was used to visualize the microstructure of the ISNCs,as well as icariin and soymilk in solution (Fig.4).The results showed that icariin exhibited a flocculent structure,consisting of a large amount of fine particles.Irregular lamellar structure,various voids and rough surface were visualized on the cross-section of NS.After HPH treatment,the rough surface turned to be smoothy.Irregular lamellar structure was still visible on the cross-sections of HS-200 and HS-500.However,fewer voids were observed for HS-200,comparing to NS and HS-500.The previous research also found that the protein was irregular and big flake before HPH,while the particle size of the protein became smaller after HPH treatment[28],which was similar to the results of present study.These results indicated that HPH treatment at different pressures had different effects on the microstructure of soymilk.And tearing might be caused by high pressure treatment.After encapsulation with icariin,the rougher surface was observed in NSI,HSI-200 and HSI-500,comparing with the corresponding NS,HS-200 and HS-500,respectively.These results were consistent with the results from particle size distribution.A very low amount of icariin was dissolved in water (WI),and a few flocculent and loose structure were visible after sublimating and gold spraying.Similarly,loose structure,which included large amount of fine particles were observed in NSI,HSI-200 and HSI-500 as well.All these results indicated that icariin was successfully encapsulated in soymilk.
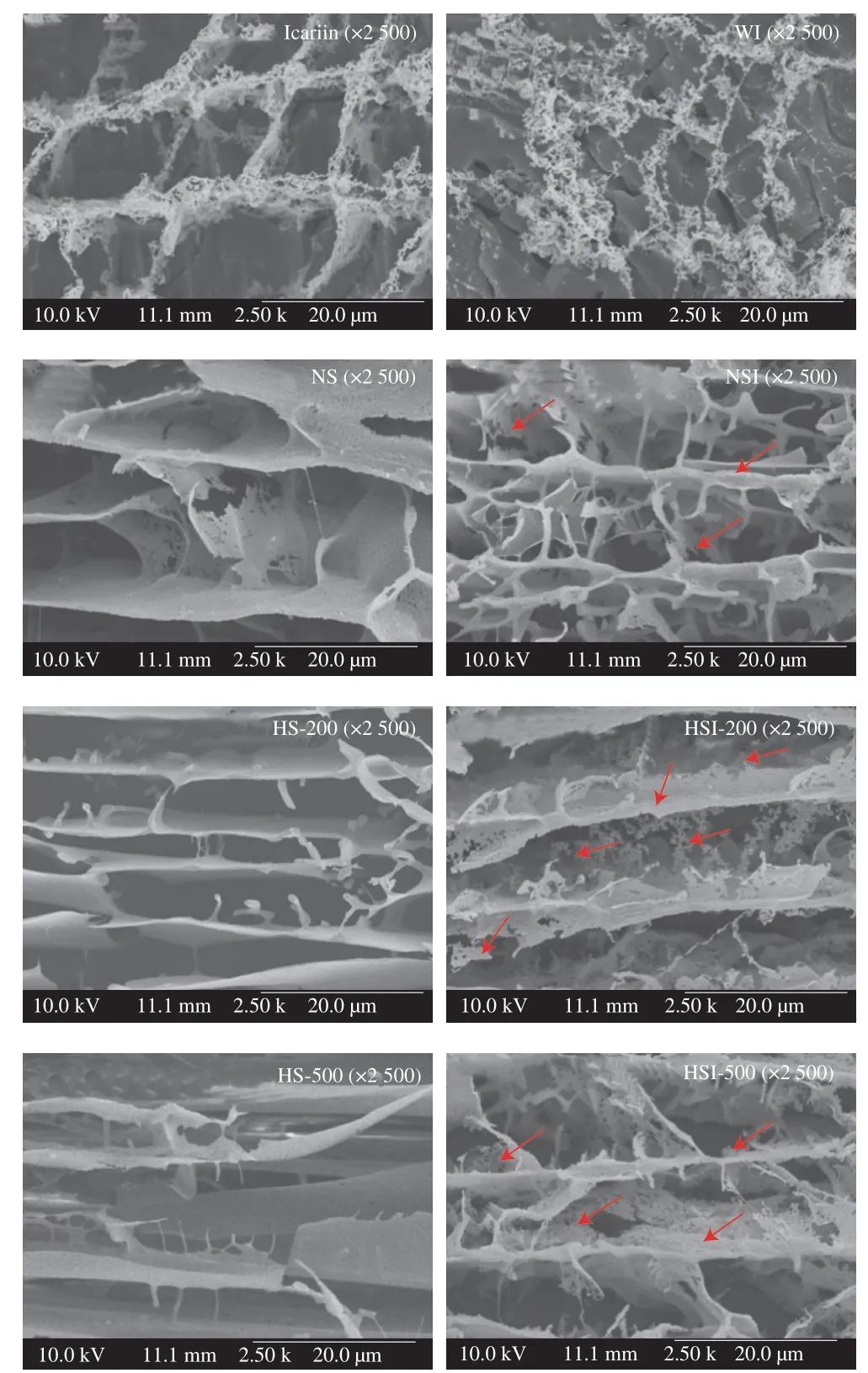
Fig.4 Cryo-SEM images of icariin,soymilk and ISNCs.The red arrow refers to the presence of icariin in ISNCs.
3.6 Physicochemical stability of nano-complexes
The stability of nano-complexes is an important factor affecting their application in food systems.The stability to pH,salt,temperature and storage time of ISNCs were evaluated in the present work.
3.6.1 pH stability
The particle size distribution (Fig.5A) andζ-potential (Fig.5B)of the ISNCs were all pH-dependent.Theζ-potential of the NSI,HSI-200 and HSI-500 went from negative to positive as the pH decreased from 12.0 to 2.0,with zero net charge around pH 4.2 and 4.6,respectively.When pH decreased from 3.0 to 2.0,the surface charge of all the icariin nano-complexes were decreased,which was probably due to electrostatic screening caused by the relatively high ionic strength[5].A remarkable increase ofDzwas observed at pH from 3.0 to 4.0,in all the tested samples,which might be due to the formation of protein aggregates as the reduction in electrostatic repulsion around the proteins’ isoelectric points[37].Accordingly,theDzdecreased sharply at pH from 4.0 to 6.0,and remained constantly at pH 6.0-12.0.Previous study indicated that soybean protein isolates exhibited minimal solubility at pH 4.0-5.0 (less than 10%,at a protein concentration of 2%)[38],which was partly consistent with the present results.Our results implied that ISNCs could be applied in food formulations under acidic,neutral and alkaline conditions.
3.6.2 Salt stability
As shown in Fig.5C,no significant differences in theDzof NSI and HSI-500 were observed,suggesting that NSI and HSI-500 were relatively resistant to NaCl addition.While a gradual decrease in theDzof HSI-200 was observed with increased concentration of NaCl from 0 to 1 000 mmol/L.Besides,the absolute values of electrical charge in HSI-500 decreased with the increase of NaCl concentration,which could be due to electrostatic screening effects of ions (Fig.5D),while the absolute value of the charge in HSI-200 and NSI varied.This result was consistent with previous reports[39].It is possible that nano-complexes were more resistant to salt addition because of the steric repulsion on the surface of the ISNCs[40].Additionally,the charged polysaccharides in soymilk can reduce the sensitivity of solutions to ionic strength,thereby inhibiting the aggregation[41].Meanwhile,the interaction with protein-polysaccharide can inhibit the aggregation between proteins,thereby enhancing the stability of nano-complexes[42].A decrease in average diameter of the HSI-200 may be attributed to the high ionic strength weakening the electrostatic interaction between the charged particles[43].
3.6.3 Storage stability
The impact of storage stability of the ISNCs was investigated by measuring changes in the particle size distribution andζ-potential at 4 and 25 °C within 28 days.The results indicated that the icariin nano-complexes might be more stable when stored at 4 °C(Figs.5G and H) than at 25 °C (Figs.5E and F),as slighter change in the particle size distribution andζ-potential were found when stored at 25 °C.Visual observation of all the samples at different temperatures showed that a little of light-yellow sediment formed at the bottom of the tube since Day 21 (data not shown),suggesting that little icariin in nano-complexes was precipitated during long term storage.This phenomenon may have occurred through these mechanisms:1) Dissolution of icariin molecules from inside the nano-complexes,followed by their recrystallization;2) A reduction in the repulsive interactions between the icariin nano-complexes;3) Desorption of the soymilk from the nano-complexes surfaces[5].However,icariin in water precipitated in 1 h (data not shown),at both 4 and 25 °C,indicating better storage stability of icariin after encapsulation.
3.6.4 Thermal stability
The thermal stability of the ISNCs was evaluated by the retention rate of icariin after thermal treatment.And the results are displayed in Fig.5I.After incubation for 2 h at 25 °C,the retention rate of icariin in nano-complexes was 100%.While the retention rate decreased to (93.16 ± 1.03)%,(94.27 ± 2.01)% and (95.85 ± 1.87)% in NSI,HSI-200 and HSI-500 after heating at 90 °C for 2 h,respectively.The present results suggested that icariin in nano-complexes were highly resistant to heating.This result was consistent with previous report[44].The fact could be explained in following ways.Firstly,previous study indicated that the presence of certain polysaccharides can improve the thermal stability of proteins without causing aggregation at high temperature.Therefore,the polysaccharides in soymilk might help to maintain the stability of nano-complexes,contributing to protection of icariin from release during heat treatment[45].Secondly,the protective effects of the hydrophobic cavity of nano-complexes on icariin presented.Therefore,less icariin was exposed during the heat treatment.Finally,strong surface charge repulsion among the complex could protect icariin by inhibiting aggregation during thermal treatment[46].
3.7 In vitro gastrointestinal digestion
The potential gastrointestinal fates of free and ISNCs were determined by using anin vitrosimulated gastric (60 min) and intestinal (120 min) digestion model.The change inDzof these complexes was measured and the results are shown in Fig.6A.After exposure to the simulated gastric fluids (SGF),theDzof free icariin,NSI,HSI-200 and HSI-500 were increased,and then decreased during incubation in the simulated intestinal fluids (SIF) digestion.Among them,NSI and HSI-500 showed no significant differences inDzin the whole digestion (P> 0.05).The increase ofDzmight be attributed to the low pH of the SGF,which could decrease the surface charge of the complexes,thus reducing the repulsive force among the complexes.The reduction ofDzmight be due to the presence of bile salts,which were strong emulsification agents that could inhibit protein aggregation.The hydrolysis of proteins by trypsin might also contribute to the reduction ofDz[47].
In addition,the retention rate of icariin over the intestinal digestion was measured (Fig.6B).The results showed that at the end of digestion,63.93% of icariin in the free form was degraded,indicating that free icariin was unstable during gastrointestinal digestion[2].While in nano-complexes,significantly higher retention rates were observed.The retention rate of NSI,HSI-200 and HSI-500 were 66.82%,94.06% and 69.93%,respectively.Among them,NSI and HSI-500 showed no significant differences in retention rate (P> 0.05).The highest retention rate of icariin in HSI-200 implied that the smoother structure of HS-200 might show better protective effect against digestive enzymes in the gastrointestinal tract.The possible reason for this phenomenon might be largely attributed to the strong interaction or complexation of icariin with HS-200[24],which was consistent with less change ofDzandζ-potential during digestion (196.03 nm and -33.60 mV),which might be beneficial to the protection of icariin from degradation[48].All these results indicated that ISNCs exhibited higher stability than free icariin.It suggested that the utilization of soymilk to encapsulate icariin might be an effective strategy for delivering icariin to the intestine.

Fig.5 Physicochemical stability of ISNCs.Effects of pH on the Dz (A) and ζ-potential (B) of ISNCs;Effects of NaCl on the Dz (C) and ζ-potential (D) of ISNCs;Changes in Dz (E) and ζ-potential (F) of ISNCs during 28-day storage at 25 °C;Changes in Dz (G) and ζ-potential (H) of ISNCs during 28-day storage at 4 °C.(I) Thermal stability of icariin in NSI,HSI-200 and HSI-500.“*” and “**” indicate significant difference from NSI at P < 0.05 and P < 0.01,respectively.
The bioaccessibility of icariin in a sample was defined as the bioaccessible amount of icariin transferred to the aqueous phase of its digest after the whole digestion[49].From Fig.6C,it can be well observed that the bioaccessibility of icariin in ISNCs were significantly higher (P< 0.05) than free icariin (58.47%-72.14%vs10.59%).There was no significant difference (P> 0.05) between NSI and HSI-500.While the bioaccessibility of icariin in HSI-200 was significantly higher than that of NSI and HSI-500,implying that HPH had different effects on the bioaccessibility of soymilk-loaded icariin.The extremely low bioavailability of free icariin was observed in the present work.When loaded with soymilk,the amount of icariin in the aqueous phase remarkably increased in SIF,indicating an improved bioavailability.All these results implied that soymilk-based nano-encapsulation was effective to enhance the bioavailability of icariin.Proteins and/or polysaccharides-based nanoencapsulation can improve the bioavailability of phenolics,such as curcumin,resveratrol and quercetin[32].
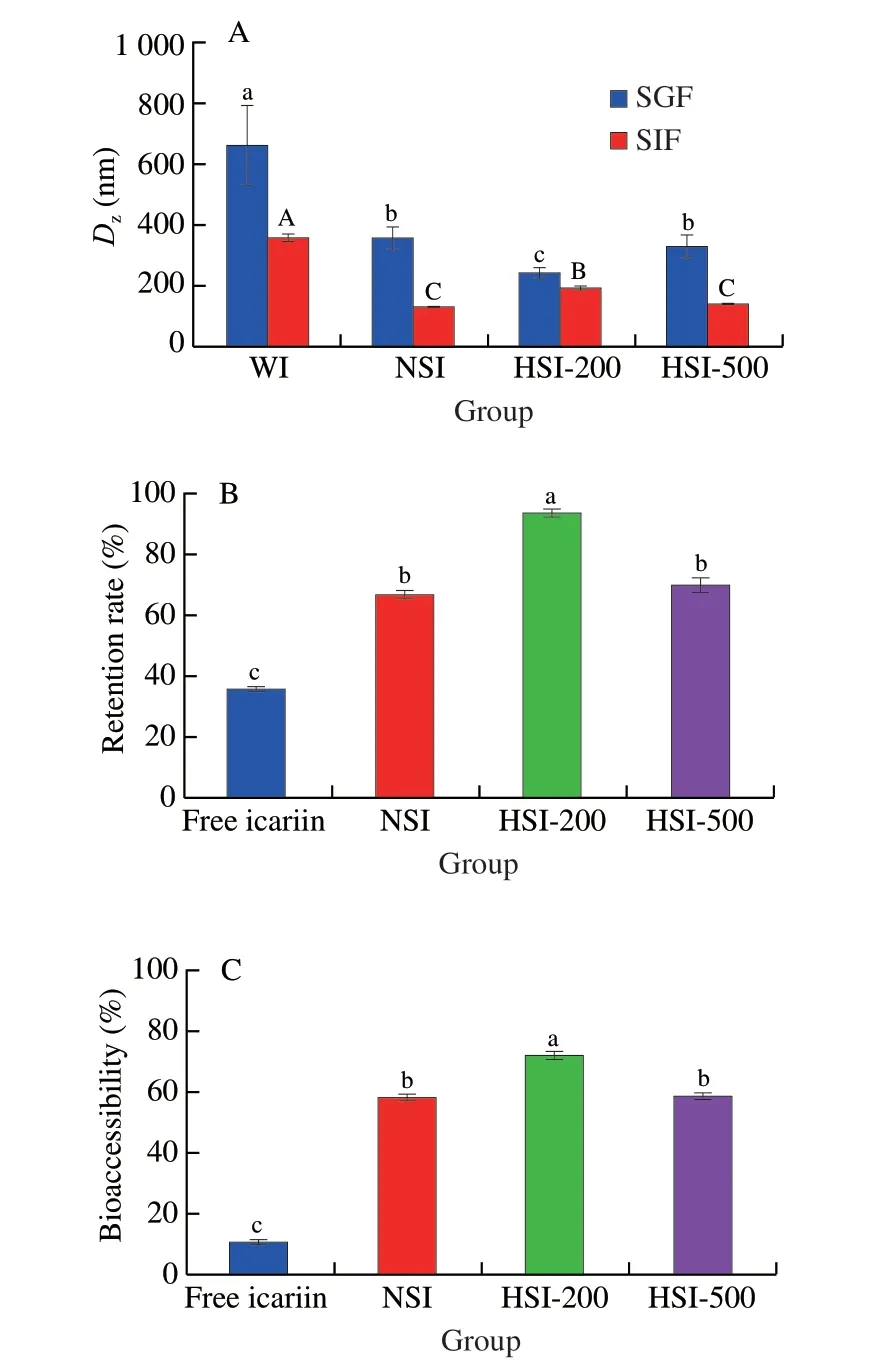
Fig.6 Stability and bioaccessibility of ISNCs during in vitro gastrointestinal digestion.(A) Dz of free icariin,NSI,HSI-200 and HSI-500 during in vitro digestion;(B) The retention rate and (C) bioaccessibility of icariin after simulated gastric-intestinal digestion.Columns with different letters indicate significant difference (P < 0.05).
4.Conclusions
The above results indicated that icariin-soymilk nano-complexes with or without HPH treatment were successfully fabricated and characterized.The nano-complexes remarkably improved the water solubility (680-740 µg/mLvs39 µg/mL in water),storage stability(21 daysvs1 h) and bioavailability (58.47%-72.14%vs10.59%) of icariin.Although ISNCs were stable in the presence of NaCl and in thermal treatment,they aggregated around their isoelectric point.After HPH treatment,soymilk showed a higher surface hydrophobicity and interfacial activity.A smoothy surface was presented.HPH treatment also showed some positive effects on the formation of ISNCs.Specifically,HSI-200 showed the highest EE (93.28%),LC(30.09 µg/mg) and bioavailability (72.14%) of icariin.HSI-500 had smallest particle size (183.73 nm).This work might promote the green and sustainable development of soymilk as multifunctional biomaterials for functional food formula.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors are grateful to the financial support from Youth Innovation Promotion Association,Chinese Academy of Sciences(2022353),Guangdong Basic and Applied Basic Research Foundation(2020A1515011025),and Science and Technology Planning Project of Guangdong Province (2022A0505050055).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

