Environmental enrichment in combination with Bif idobacterium breve HNXY26M4 intervention amplif ies neuroprotective benef its in a mouse model of Alzheimer’s disease by modulating glutamine metabolism of the gut microbiome
Guangsu Zhu ,Min Guo ,Jianxin Zhao,c,d ,Hao Zhang,c,d ,Gang Wang,c,d, ,Wei Chen,d
a State Key Laboratory of Food Science and Resources, Jiangnan University, Wuxi 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c (Yangzhou) Institute of Food Biotechnology, Jiangnan University, Yangzhou 225004, China
d National Engineering Center of Functional Food, Jiangnan University, Wuxi 214122, China
Keywords: Alzheimer’s disease Bif idobacterium breve Environmental enrichment Glutamine metabolism Microbiota-gut-brain axis
ABSTRACT The gut microbiota-brain axis has emerged as a novel target for Alzheimer’s disease (AD),a neurodegenerative disease characterised by behavioural and cognitive impairment.However,most previous microbiome-based intervention studies have focused on single factors and yielded only modest cognitive improvements.Here,we proposed a multidomain intervention strategy that combined Bif idobacterium breve treatment with environmental enrichment (EE) training.In this study,we found that compared with EE or B.breve treatment alone,B.breve intervention combined with EE amplif ied its neuroprotective effects on AD mice,as ref lected by improved cognition,inhibited neuroinf lammation and enhanced synaptic function.Moreover,using microbiome and metabolome profiling,we found that the combination of B.breve and EE treatment restored AD-related gut microbiota dysbiosis and reversed microbial metabolite changes.Finally,by integrating behavioural and neurological data with metabolomic profiles,we revealed that the underlying mechanism may involve the modulation of microbiota-derived glutamine metabolism via gut-brain interactions.Collectively,combined B.breve intervention with EE treatment can alleviate AD-related cognitive impairment and improve brain function by regulating glutamine metabolism of the gut microbiome.Our f indings provide a promising multidomain intervention strategy,with a combination of dietary microbiome-based and lifestyle-targeted interventions,to promote brain function and delay the progression of AD.
1.Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterised by the deposition of amyloid plaques and the formation of neurofibrillary tangles[1].AD predominantly occurs in people older than 60 years.AD symptoms involve changes in memory and behaviour,neuroinflammation and synaptic plasticity,all of which can affect brain function[1].As one of the costliest chronic diseases,the current annual cost of AD treatment and care is estimated to be $1 trillion,with forecasts predicting a cost of $2 trillion by 2030,ref lecting the growing global public health burden[2].Given that no effective therapeutic approach has been shown to halt the development of AD,there is an urgent need to develop disease-modifying interventions that can slow cognitive loss and improve quality of life[1].
The gut microbiota-brain axis has emerged as a promising novel target for neurological disorders;treatments that target this axis have recently been demonstrated to improve cognition and inhibit neuroinflammation[3].Numerous clinical and animal studies have reported that the gut microbiota of AD-affected individuals are significantly different from those of healthy controls,with differences observed in bacterial diversity and microbiome composition[4].One clinical study of patients with AD demonstrated that the AD pathologies were negatively correlated with the abundance of the generaAkkermansiaandBifidobacterium[5].Several studies on depression and AD have identifiedAkkermansiaandBifidobacteriumas key microbial modulators[6-8].The communication and biological systems involved in gut-brain connections are complex;one of the various routes by which the microbiome affects and mediates key brain processes in AD is via gut microbial metabolites[9].Recent technological advancements in metabolomic analyses have made it possible to detect gut-derived metabolites.Indeed,specific metabolites known to be strongly correlated with cognition,such as tryptophan[8,10],glutamine[11],proline[12],polyphenols[13]and poly-unsaturated fatty acids[14]have been observed in faecal samples and may serve as neuroactive agents.Despite increased awareness of the potential functions of the gut microbiota and its metabolites in AD,the mechanistic links underlying gut-brain interactions remain to be elucidated.
Placed at the intersection of neuroscience and microbiology,the gut microbiome is a dynamic entity that can change in composition and structure throughout the host’s lifespan and in response to changing environmental factors and dietary factors;this provides the opportunity to develop a novel lifestyle intervention strategy that can delay the progression of AD by modulating the intestinal microbiota.Most previous intervention studies aiming to prevent AD have targeted single factors and yielded only modest cognition improvements.Therefore,multidomain interventions that simultaneously target several risk factors and mechanisms are more likely to delay the development of AD[15].Dietary intervention with probiotic has been used as a microbiota-targeted strategy to combat the vascular and lifestyle-related risk factors of AD,including metabolic syndrome and hypertension[16-17].Psychobiotics,which produce and deliver neuroactive substances,are a class of probiotics that modulate gut microbiota composition and have been shown to benefit patients with neurodegenerative diseases[18-19].Exposing animals to environmental enrichment (EE) has yielded dramatic benefits on the brain,including improvements in memory and learning and enhancement in synaptic plasticity[20].It was recently reported that the intestinal microbiota composition and microbial metabolites of mice subjected to EE differed significantly from those of mice subjected to standard environment (SE);further,the transfer of faecal microbiota from EE-mice enhanced brain plasticity in recipient SE-mice[21].The administration ofAkkermansia muciniphila,a potentially novel probiotic,when combined with EE,was reported to reverse cognitive impairment and change the microbiota composition in a non-alcoholic steatohepatitis rat model[22].However,few studies have assessed whether a combination of probiotic and EE intervention can ameliorate AD-associated pathology and behavioural symptoms in mice;the underlying mechanisms also remain poorly understood.
To explore the neuroprotective effects of combiningBifidobacterium brevetreatment with EE on AD-associated cognitive impairment,we established a mouse model of AD by intrahippocampally injecting amyloid beta (Aβ)1-42and evaluating the changes in behavioural outcomes,neuroinflammation and synaptic proteins.Using microbiome and metabolome profiling,we investigated differences in the diversity and composition of the gut microbiota and identified altered microbial metabolites.By integrating behavioural and neurological data with metabolomic profiles,we outlined a potential mechanism by whichB.brevetreatment,in conjunction with EE,affects brain function.
2.Material and methods
2.1 Bacterial strain and culture condition
The probiotic strain ofB.breveHNXY26M4,was cultured under anaerobic conditions at 37 °C in modified De Man,Rogosa and Sharpe broth supplemented with 0.05% (m/V)L-cysteine.Freshly cultured bacterial cells were collected by centrifugation (8 000 ×gfor 10 min at 4 °C),and then washed and resuspended with 30% (V/V)glycerol.Aliquot resuspended bacteria cells in sterilized tubes and store at -80 °C until use.For oral administration,each aliquoted bacteria sample was washed twice and resuspended in phosphate-buffered saline (PBS) to reach a concentration of surviving bacteria at 1 × 109CFU/mL.
2.2 EE
For EE,mice were kept in large cages (46.1 cm × 27.4 cm ×22.9 cm) and provided with running wheels,a transparent red mouse house,coloured plastic tubes,wooden blocks,pressed cotton squares and objects of different shapes[23].Some of the items in the EE cages were replaced once a week,but the running wheel was retained for the entire experimental period.All enrichment items were disinfected or sterilized.The shape and arrangement of some of the items were changed weekly to increase novelty.
2.3 Animals
Male adult C57BL/6J mice (8 weeks old) were procured from the Model Animal Research Centre of Nanjing University (Nanjing,China) and maintained at the Animal Centre of Jiangnan University,as previously described[24].The animal experiments were approved by the Animal Experimentation Ethics Committee of Jiangnan University(JN.No.20200630c0960909[123]).
2.4 Experimental design
After adaptation for 1 week,40 mice were randomly divided into 5 groups (n=8 per group).To establish an AD model,we intrahippocampally injected the Aβ1-42oligomer (Cat.No.H-1368,Bachem,Bubendorf,Switzerland) in the hippocampus of mice,as previously described[24].The mice were housed under either SE or EE conditions (4 mice per cage).The mice were administered 200 μL of either bacterial suspension or sterile PBS daily for 6 weeks by oral gavage.The details of the treatment protocols for each group and schematic overview of the experimental timeline are presented in Fig.1.
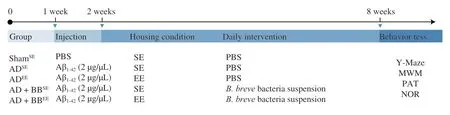
Fig.1 Schematic overview of the experimental design and study timeline.The Aβ1-42 was intrahippocampal injected to the brain of mice using a stereotaxic apparatus.Administration of PBS or B.breve (200 μL/day) was started one week after the Aβ1-42 injection and continued for 6 weeks.Vertical bars represent 1 week.
2.5 Behavioural tests
To evaluate different types of learning and memory,we performed four behavioural tests: the Y-maze,Morris water maze (MWM),passive avoidance test (PAT) and novel object recognition (NOR)task,as described previously[11,24].All of the behavioural tests were conducted in a blinded manner between 8:00 AM and 12:00 AM.All of the tests were recorded and analysed using Ethovision 11.5 (Noldus,Wageningen,Netherlands).
2.6 Faecal sample collection
After completing the behavioural tests,the mice were placed in an empty cage until they defecated.Faecal samples were immediately collected,snap-frozen and stored at -80 °C for subsequent microbiome and metabolome sequencing.
2.7 Enzyme-linked immunosorbent assays
The mice were deeply anaesthetised with isoflurane and then decapitated,and their brains were collected.Hippocampal tissue was dissected out on ice and stored at -80 °C until analysis.The tissue was then homogenised in pre-cooled PBS and centrifuged at 5 000 ×gfor 10 min at 4 °C to collect the supernatants.The supernatants were used to measure Aβ1-42,brain-derived neurotrophic factor (BDNF)and interleukin 6 (IL-6) concentrations.To quantify synaptophysin(SYP),postsynaptic density 95 (PSD95),fibronectin type III domain-containing protein 5 (FNDC5) and transforming growth factor-β1 (TGF-β1),the supernatants were further diluted in cold PBS.All of the protein concentrations were measured using enzyme-linked immunosorbent assay kits from Elabscience (Wuhan,China) in accordance with the manufacturer’s protocol.
2.8 Faecal 16S rRNA sequencing and bioinformatic analysis
Faecal samples were collected at the end of the experiment.After snap-freezing,the samples were stored at -80 °C.Microbial genomic DNA was extracted,amplified and purified as previously described[24].Specifically,differential detection ofBifidobacteriumspecies was performed using primers targeting thegroELgene[25].Purified amplicons were sequenced on a MiSeq PE300 platform in paired-end mode (Illumina,San Diego,CA,USA).
Raw data of the 16S rRNA gene sequences were processed using the QIIME 2 platform (www.qiime.org).α-Diversity andβ-diversity were calculated and visualised.A random forest model was used to identify the microbial taxa.Metagenomes of the gut microbiome were computed from the 16S rRNA sequences based on Phylogenetic Investigation of Communities by Reconstruction of Unobserved States,and functional pathways were predicted using Kyoto Encyclopaedia of Genes and Genomes (KEGG) orthology.
2.9 Metabolomics
For metabolomic analyses,faecal metabolites were extracted as described by Mars et al.[26].The detailed protocol for fecal metabolite sample preparation is described in the Supplementary methods.The metabolite samples were analysed using an ItiMateU-3000 ultraperformance liquid-chromatography (UPLC) system (Thermo Fisher Scientific,MA,USA) coupled to a high-resolution Q Exactive mass spectrometer (MS) (Thermo Fisher Scientific,MA,USA).The UPLC-MS analysis parameters and metabolomic data analysis have been described in a previous report[27].
2.10 Statistical analysis
Statistical analysis was performed using Prism version 8.0.2(GraphPad,San Diego,CA,USA).Correlation analysis was performed in OriginPro version 2021 (Originlab,Northampton,Massachusetts,USA).Statistical tests,the number of subjects and significance cut-offs are described in each figure legend.P< 0.05 was considered statistically significant.Network correlation between the variables was determined using Spearman’s correlation coefficients and visualised with Cytoscape version 3.8.2 (Institute for Systems Biology,Seattle,WA,USA).
3.Results
3.1 B.breve treatment in combination with EE amplifies the neuroprotective benefits of EE in AD mice
To explore the neuroprotective effects of EE alone or in combination withB.brevetreatment on AD-associated cognitive deficits,mice subjected to either SE or EE were treated with either PBS orB.breve,respectively,for 6 weeks via oral gavage following the intrahippocampal injection of Aβ1-42(Fig.1).The quantification of Aβ in hippocampal tissue revealed that ADSEmice had higher hippocampal Aβ1-42concentrations than ShamSEmice.Mice that received only EE (ADEE) or EE combined withB.brevetreatment(AD+BBEE) had significantly lower concentrations of Aβ1-42in the hippocampus than ADSEmice (Fig.2A).However,no statistically significant changes in the concentration of hippocampal Aβ1-40were observed among all the groups (Fig.S1).
To evaluate the memory and learning in mice,we performed 4 behavioral tests.ADSEmice displayed robust cognitive impairments in these testing paradigms.In the Y-maze,ADSEmice exhibited a decreased percentage of alternation compared with ShamSEmice,indicating that the former preferred to venture into a familiar arm over a new arm (Fig.2B).However,EE alone or in combination withB.brevetreatment (ADEEand AD+BBEEgroups) resulted in more mice venturing into the new arm and an increased percentage of alternating choices (Fig.2B).In addition to the short-term working memory behaviours,mice in the ADEE,AD+BBSEand AD+BBEEgroups displayed similar improvements in recognition and learning memory,with an increased discrimination index in the NOR task(Fig.2C).In the PAT,ADEEmice showed no improvements in latency time;however,AD+BBSEand AD+BBEEmice had markedly longer latency times than the ADSEmice (Fig.2D).
To verify these findings,we estimated the long-term spatial learning ability of mice using the MWM,in which mice were trained for five consecutive days before formal testing.In the probe MWM test (on Day 6),the latency times required for the mice to navigate towards the hidden platform were measured.The ADSEmice took more time to reach the platform and ventured less into the targeted quadrant than the ShamSEmice (Figs.2E and F).However,EE alone or in combination withB.brevetreatment (ADEEand AD+BBEEgroups) reduced the latency time and increased the percentage of time spent in the targeted quadrant.Interestingly,there was no significant difference in the amount of time spent in the targeted quadrant between the ADSEand AD+BBSEgroups,although the latency time was significantly lower in the AD+BBSEmice (Figs.2E and F).As these behavioural tests measure different types of learning and memory,our findings suggest that the effects of EE andB.brevetreatment on cognitive behaviours are selective.
3.2 B.breve treatment in combination with EE inhibits AD-related neuroinflammation and alleviates synaptic impairments
To investigate the alleviative effect ofB.brevetreatment in combination with EE on AD-induced neuroinflammation,we examined the concentrations of cytokines in brain samples obtained from each group.Compared with ADSEmice,ADEEand AD+BBEEmice showed significantly higher concentrations of TGF-β1 in hippocampal samples (Fig.3A).Specifically,only AD+BBEEmice showed markedly higher hippocampal IL-6 concentrations than ADSEmice (Fig.3B).As both TGF-β1 and IL-6 have been reported to regulate neurogenesis and be selectively elevated following exercise,EE-induced exercise along with the psychobiotic potential ofB.brevetreatment may improve brain function under the pathological conditions of AD.
To further confirm whether the combination ofB.brevetreatment and EE can promote synaptic plasticity,we measured the concentrations of BDNF,SYP,FNDC5 and PSD95 in hippocampal samples of AD mice.Compared with ADSEmice,ADEEand AD+BBEEmice showed highly elevated hippocampal SYPconcentrations(Fig.3C).However,onlyB.brevetreatment (AD+BBSE)dramatically increased the concentration of hippocampal BDNF,an essential neurotrophin for synaptogenesis (Fig.3D).Notably,exposure of AD mice to EE alone was sufficient to elevate the hippocampal concentration of FNDC5,but not of BNDF,though its ability was not more significant than EE combined withB.breve(Fig.3E).Similarly,the hippocampal PSD95 concentrations were elevated in both ADEEand AD+BBEEmice (Fig.3F).Collectively,these findings indicate that the combination ofB.brevetreatment with EE exhibited a more prevalent ability to promote synaptic plasticity.
3.3 B.breve treatment in combination with EE modulates the diversity and composition of the gut microbiota
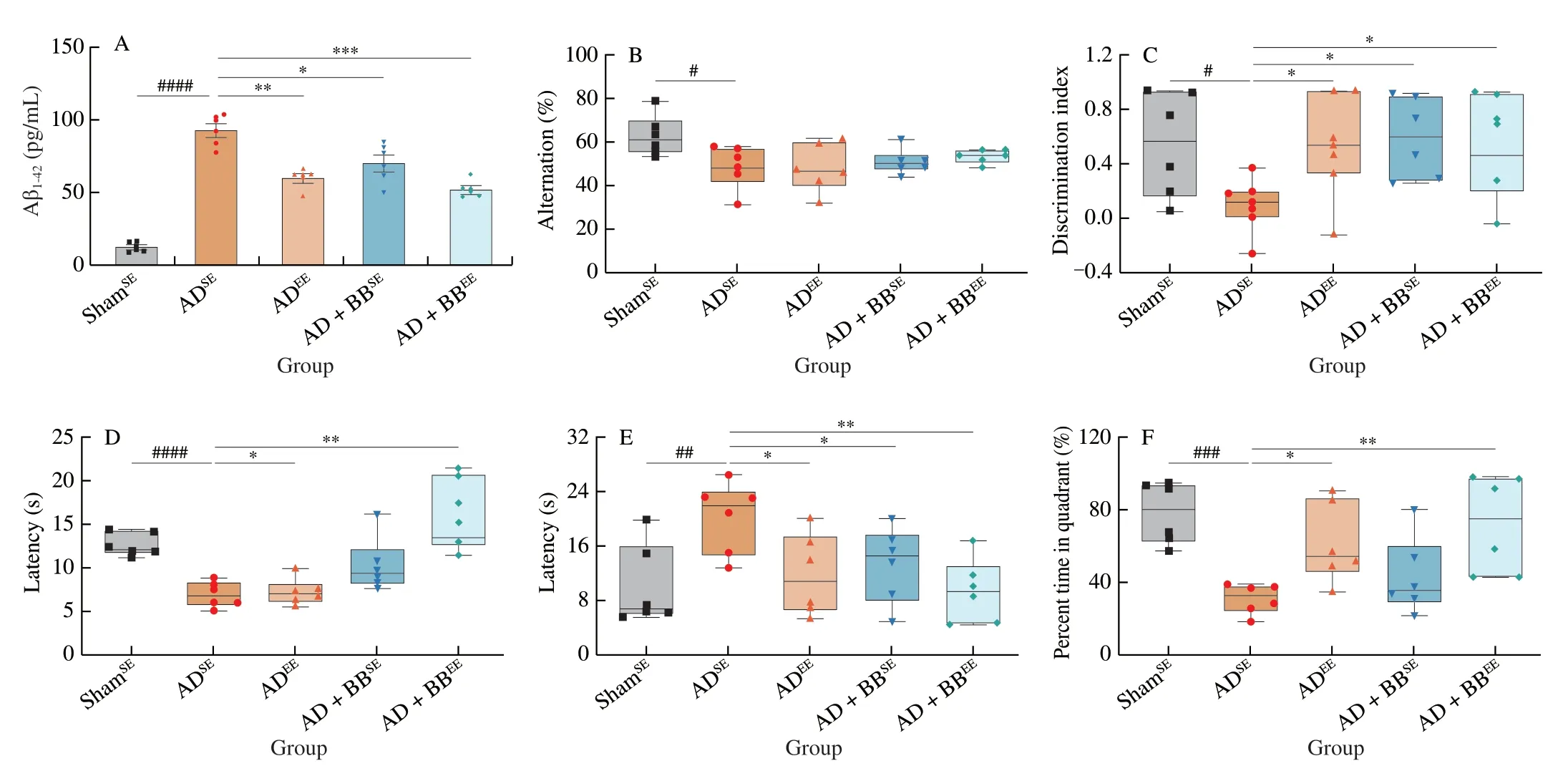
Fig.2 B.breve treatment in combination with EE amplifies neuroprotective benefits in AD mice.(A) The concentration of Aβ1-42 in the brain.(B) Spontaneous alternation behavior in Y-maze test.(C) Discrimination index in the novel object recognition task.(D) Escape latency of passive avoidance test.The escape latency (E) and percent time in target quadrant (F) during the probe phase (day 6) of the Morris water maze.For (A): Data are presented as mean ± standard error of the mean (SEM).For (B-F): In the box plot,the bottom and top are,respectively,the 25th and 75th percentile,a line within the box marks the median.Each dot represents individual mice,n=6-8 in each group.Control vs.Model: #P < 0.05,##P < 0.01,###P < 0.001,####P < 0.000 1 by unpaired student’s t-test;*P < 0.05,**P < 0.01,***P < 0.001 as determined by one-way ANOVA.

Fig.3 B.breve treatment in combination with EE inhibits AD-related neuroinflammation and synaptic impairments.(A) Hippocampal TGF-β1 levels.(B) Hippocampal IL-6 levels.(C) Hippocampal SYP levels.(D) Hippocampal BDNF levels.(E) Hippocampal FNDC5 levels.(F) Hippocampal PSD95 levels.Data are presented as mean ± SEM,n=6-8 in each group.*P < 0.05,**P < 0.01,***P < 0.001 as determined by one-way ANOVA.
To determine whetherB.brevetreatment and/or EE affected AD-associated gut microbiota dysbiosis,we profiled faecal samples using 16S rRNA sequencing.α-Diversity analysis,quantified by the Chao1 and Shannon indices,revealed that ADSEmice had reduced bacterial richness and diversity compared with ShamSEmice (Figs.4A and B).B.brevetreatment in combination with EE improved the biodiversity of the gut microbiome,but not the richness,as reflected by the significantly higher Shannon index (Fig.4B).The principal coordinate analysis plot based on the Bray-Curtis distance showed a clear separation between the ShamSE,ADSEand ADEEmice and revealed significant differences in their gut microbiome structures and signatures (Fig.4C).Notably,AD+BBSEand AD+BBEEgroup clustered together and diverged to be closer to the ShamSEgroup,suggesting thatB.breveintervention led to a similar pattern of distribution towards to normal level (Fig.4C).
At the phylum level,Firmicutes and Bacteroidetes were the most abundant phyla in all of the groups (Fig.4D).Due to the administration ofB.breve,the relative abundance of Actinobacteria was high in the AD+BBSEand AD+BBEEgroups (Fig.4D).In contrast,the abundances of Deferribacteres and Proteobacteria were high in the ADEEgroup (Fig.4D).Core microbiome analysis at the family level revealed that Muribaculaceae,Lachnospiraceae,Ruminococcaceae and Lactobacillaceae were the top four taxa in all of the groups (Figs.4E and S2).Furthermore,random forest classification analysis at the genus level identified the top 15 altered genera (Fig.4F).Indeed,the relative abundances of several genera such asBifidobacterium,Akkermansia,AngelakisellaandAnaerostipeswere higher in theB.breve-treated groups,whereas Lachnospiraceae_NK4A136_group andCandidatus_Saccharimonaswere more prevalent in the ADSEgroup (Fig.4F).
3.4 Specific taxa implicated in glutamine metabolism are associated with improved cognition
To evaluate the adherence and colonisation of probiotics,we assessed the relative abundances ofAkkermansia,BifidobacteriumandLactobacillusat the species level using thegroELgene as a marker.Specifically,exposure of AD mice to EE significantly enriched the abundance ofA.muciniphila(Fig.5A).Although the structure ofLactobacillusspp.appeared to be similar across all of the groups (Fig.S3A),the relative abundance ofL.reuteriwas higher in the AD+BBEEgroup than in the ADSEgroup (Fig.5B).Notably,the composition ofBifidobacteriumspp.was significantly different in each group (Fig.5C).As expected,a robust enrichment ofB.brevewas observed in the AD+BBSEand AD+BBEEgroups compared with the ADSEgroup,which could be due to the gavage ofB.brevefor 6 weeks (Fig.5D).The high abundance ofB.pseudolongumin ADSEmice markedly declined afterB.brevetreatment or/and EE(Fig.S3B).Specifically,B.longumwas more abundant in ADEEmice than in other mice (Fig.S3C).
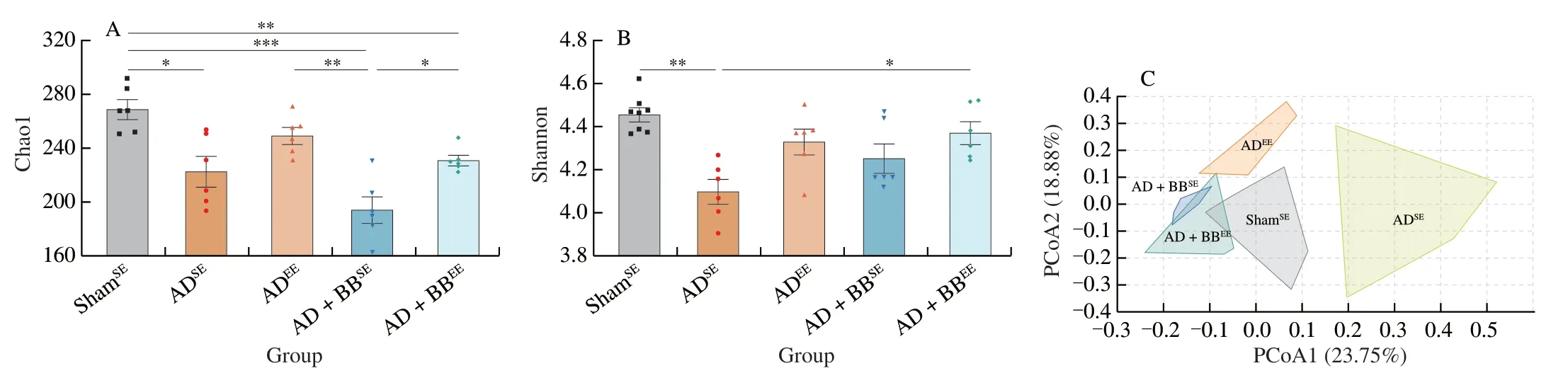
Fig.4 B.breve treatment in combination with EE modulates the diversity and composition of the gut microbiota.Chao1 (A) and Shannon (B) diversity indices representing the α-diversity of the bacterial community.Data are presented as mean ± SEM.*P < 0.05,**P < 0.01,***P < 0.001.Each dot represents individual mice, n =6 in each group.(C) PCoA plot based on Bray-Curtis distance,representing the β-diversity of the microbial structure.(PERMANOVA,P =0.000 1).(D) Stacked bar plot of the distribution of microbial community at the phylum level.(E) Heatmap of the core microbiome at the family level in AD+BBEE group.The taxa shown are presented in over 50% of the prevalence and a relative abundance of 0.01%.(F) The top 15 potential microbial biomarkers identified by random forest at the genus level.The taxa are ranked by the mean decrease in classification accuracy.Boxes are colored according to the relative abundance in each group.
To explore which bacterial taxa contribute to brain function,we performed a correlation analysis between the altered taxa and behavioural changes using Spearman’s correlation coefficient.The results indicated thatBifidobacterium,Akkermansia,and Ruminococcaceae_UCG_013 were significantly positively correlated with cognitive improvements in the AD+BBEEmice (Fig.5E).However,the abundance ofB.brevewas negatively correlated with the latency in MWM,indicating that the administration ofB.breveresulted in improved learning memory (Fig.5E).
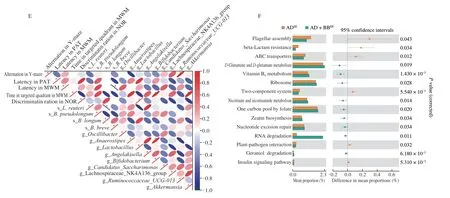
Fig.5 (Continued)
To determine the potential pathways that functionally influence host metabolic output,we predicted functions of the gut microbiome using KEGG annotation and pathway enrichment analysis.We found that that the perturbed gut microbiome in the ADSEgroup was involved in the decreased synthesis and degradation of ketone bodies (Fig.S4A).In addition,10 pathways were identified differential as being differentially regulated between the ADSEand AD+BBSEgroups,among whichD-glutamine andD-glutamate metabolism,tryptophan metabolism,and vitamin B6metabolism were significantly activated byB.breveadministration (Fig.S4B,P< 0.05).Consistently,of the fifteen pathways that were altered between the,D-glutamine andD-glutamate metabolism,and vitamin B6metabolism were also upregulated in the AD+BBEEgroup(Fig.5F,P< 0.05).Therefore,we hypothesised that metabolic activities of the gut microbiome impact host brain function by modulating gut microbiota-derived glutamine-related metabolites.
3.5 Gut microbial glutamine metabolites contribute to brain function via gut-brain interactions
To test our hypothesis and determine the role of the gut microbial metabolites in the modulation of cognition in AD,we performed a metabolomic analysis of faecal samples from ShamSE,ADSEand AD+BBEEmice.We identified 5 406 microbial metabolites (3 240 with defined names) in the faecal samples.Using sparse partial least squares discriminant analysis,we found that the ShamSEand ADSEgroups formed a distinct cluster and that the AD+BBEEgroup had a metabolite pattern similar to the ShamSEgroup,suggesting thatB.brevein combination with EE restores AD-associated microbial metabolites(Fig.6A).Next,we performed multivariate analysis to identify distinctly altered metabolites between the ADSEand AD+BBEEgroups.Compared with the ADSEgroup,17 metabolites were upregulated and another 11 were downregulated in the AD+BBEEgroup (Table S1,Fig.S5).Thus,B.brevetreatment appeared to be responsible for variations in metabolomic signature.
Functionally,pathway enrichment analysis revealed that these differentially regulated metabolites were mainly enriched in signaling pathways involved in amino acid metabolism,includingD-glutamine andD-glutamate metabolism,glutathione metabolism,arginine biosynthesis,histidine metabolism,and alanine,aspartate and glutamate metabolism (Fig.6B).This finding mirrored previous microbiome data and corresponded to changes in glutamine-related metabolites in the predicted gut microbiome functions.We found two glutamine-related metabolites that were altered in the ADSEgroup,L-pyroglutamic acid andL-glutamine,to be beneficially restored byB.brevetreatment in combination with EE (Fig.6C).Moreover,the concentrations ofL-glutamic acid and 2-oxoglutaric acid were significantly higher in AD+BBEEmice than in ADSEmice;however,these concentrations were not significantly different between the ShamSEand ADSEmice (Fig.6C).These findings prompted us to identify the metabolite underlying the possible link between cognition improvement and alterations in selective metabolites.
To elucidate the microbiota-metabolite-brain interactions and understand the mechanisms by which gut microbiota-derived metabolites impact brain function,we performed a correlation analysis between the behavioral and neurological data and glutaminerelated metabolites.We observed strong correlation between glutamine metabolites and behavioral outcomes (Fig.6D).Several behavioural changes were positively correlated withL-glutamine,but negatively correlated with 2-oxoglutaric acid,which was consistent with previous findings indicating that glutamine is critical for behavioural and cognitive responses (Fig.6D).However,no correlations were found betweenL-pyroglutamic acid and cognition outcomes.Moreover,we also found potential links between glutamine metabolites and neurological changes in the brain,as indicated by the positive association betweenL-glutamine and FNDC5 (Fig.6D).Additionally,the levels of TGF-β1 and IL-6 were negatively associated withL-pyroglutamic acid,but positively associated withL-glutamic acid (Fig.6D).Overall,these observations indicate that the microbial glutamine metabolites modulated byB.brevetreatment in combination with EE may affect brain function and inflammation via the gut-brain axis.
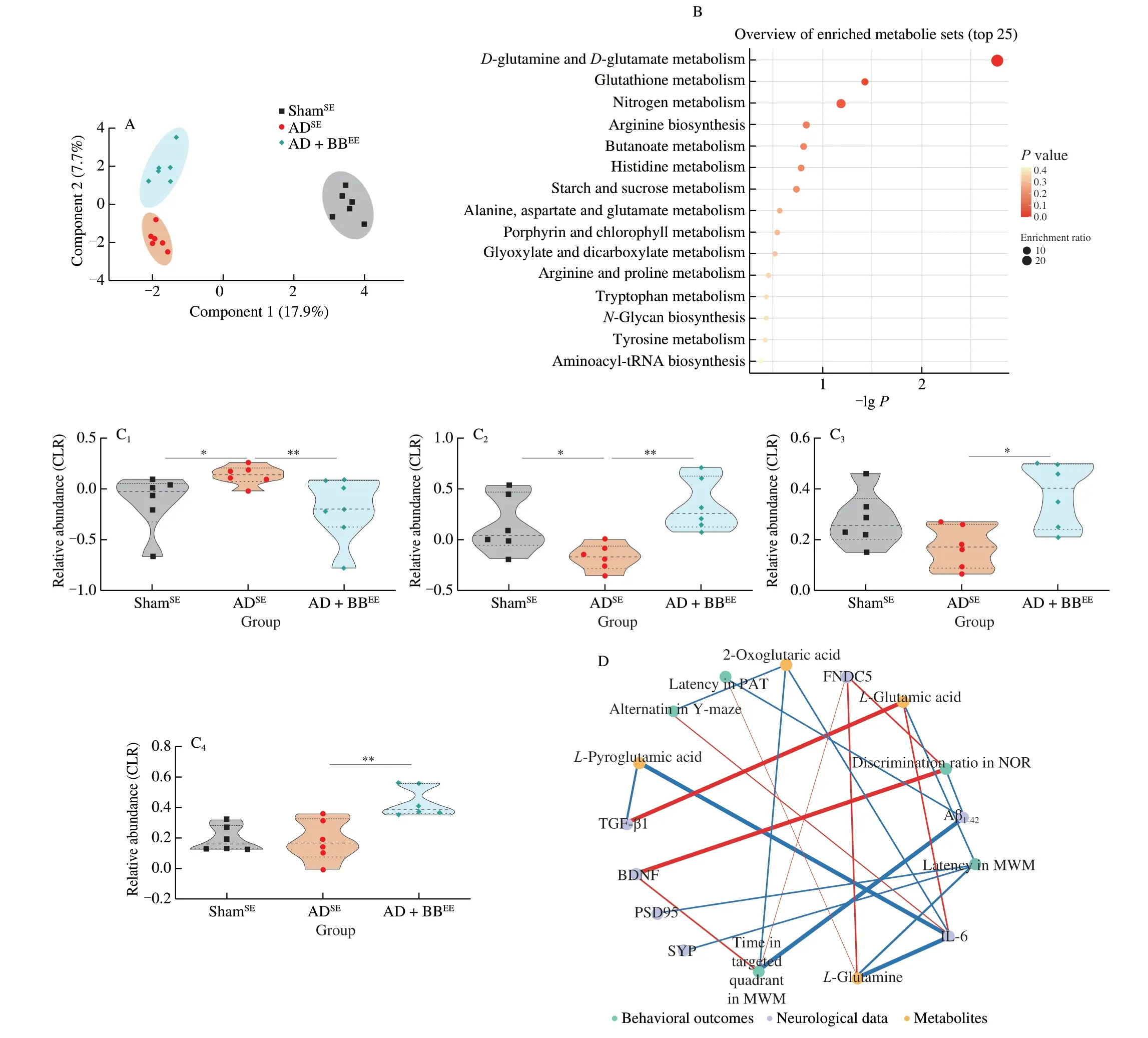
Fig.6 Gut microbial glutamine metabolites contribute to brain function via gut-brain interactions.(A) Sparse PLS-DA score plot of fecal metabolites among three groups.Number of components: 6.(B) The enriched metabolite sets in feces based on the KEGG database.(C) The relative abundance of L-pyroglutamic acid (C1),L-glutamine (C2),L-glutamic acid (C3) and 2-oxoglutaric acid (C4).The y axis shows CLR-transformed metabolite concentrations.*P < 0.05,**P < 0.01 as determined by Mann-Whitney U test.(D) Spearman correlation network representing correlations of metabolites with behavioral outcomes and neurological data.Red edges indicate a positive correlation and blue edges indicate a negative correlation.The width of the edge represents the correlation strength.
4.Discussion
The gut microbiota and its metabolites have emerged as important regulators of brain function.In addition to progressive cognitive decline,AD individuals display dramatic alterations in the composition of microbiota and microbial metabolites.In 2013,Dinan et al.[18]proposed psychobiotics as neuroactive substances that acted along the gut-brain axis to impact the brain.A recent study demonstrated that exposing mice to EE could change the composition of the gut microbiota and elevate the concentrations of short-chain fatty acids,thereby improving brain plasticity and cognition[21].However,most preventive interventions targeting microbiota modulation have focused on single lifestyle factors and yielded only modest beneficial results.Furthermore,these intervention studies were underpowered and aimed at cognition improvement and not microbiome functionality.Thus,signals from gut microbiota-derived metabolites that can delay the progress of AD have not been thoroughly explored.Here,we tackled these limitations by establishing an animal model to investigate the neuroprotective effects ofB.brevetreatment in combination with EE on AD.By integrating behavioural and neurological data with microbiome and metabolomic profiles,we outlined the mechanisms underlying the microbiota-gut-brain interplay in AD.
As the aetiology of AD is multifactorial,optimal preventive interventions that simultaneously target multiple lifestyle domains such as diet,cognitive stimulation and physical activity need to be developed[15].In this study,we compared the preventive effect of EE alone or in combination withB.brevetreatment on cognitive decline and behavioural outcomes.Compared with EE alone,we found that EE in combination withB.brevetreatment amplified its neuroprotective effects,as reflected by reduced Aβ deposition and improved learning and memory.It has been reported that probiotic interventions can effectively prevent cognitive impairment and improve cognition in both humans and other animals[24,28].Additionally,several studies have described the beneficial effects of exercise and probiotic treatment on cognition in mouse models of AD[29-31].However,these studies have mainly evaluated the effects of such interventions on specific memory domains.Our behavioural results revealed thatB.brevetreatment alone was sufficient to improve spatial learning and recognition memory,but not spontaneous short-term working memory.EE is known to have beneficial effects on brain structure and function[21].Indeed,the significantly higher improvements in cognition in mice treated withB.brevein combination with EE might be due to the effect of EE on learning and memory,as EE grants the mice more opportunities to perform species-specific behaviours[23,32].
Multiple studies have demonstrated that EE,in addition to its behavioural effects,affects neuroinflammation and synaptic plasticity[33-34].We also found that AD mice exposed to EE had lower Aβ deposition and higher concentrations of SYP,PSD95 and FNDC5,which inhibit neuroinflammation and enhance synaptic function,than those exposed to SE[35].Specifically,mice in the AD+BBEEgroup had significantly higher BDNF and IL-6 concentrations than those in the ADSEgroup.Compared with the ADEEgroup,however,this beneficial effect was modest;no significant difference was observed between the ADSEand ADEEgroups.This can be explained by a previous observation of multidomain interventions being more effective at and thus better suited to preventing the development of AD[15].As an important component of EE,physical exercise induces FNDC5 production,which stimulates the hippocampal expression of BDNF[36-37].Recently,the mechanism through which EE improves brain function was shown involve the increased expression of hippocampal BDNF[34].As EE comprises a complex combination of cognitive training,physical exercise and social interaction,further investigations into its role in alleviating neuroinflammation and improving cognition may provide innovative strategies for preventing AD progression.
Many studies have shown that the gut microbiota is a complex and critical regulator of the central nervous system through the gut-brain axis,which may be regulated by neuronal and immunemediated signalling[38].Decreased gut microbiota diversity and dramatically altered microbiota composition have been reported in patients with AD[4].Consistently,we found that Aβ injection resulted in the dysfunction of the gut microbiota,exemplified by a reduction in the complexity and richness of bacteria community and changes in microbiota structure.Notably,B.brevetreatment restored the composition of the gut microbiota.This effect was specific toB.breveand was independent of the housing condition as AD+BBSEand AD+BBEEmice both exhibited similar microbial modulations.The most striking difference was observed in AD+BBEEmice,in which species belonging to genusBifidobacteriumwere significantly enriched.The more prominent effect ofB.brevethan of EE on microbiota modulation may be becauseB.brevecan colonise and shape the gut ecosystem,thus maintaining homeostasis of the gut microbiome[39].Previously proposed mechanisms by which EE impacts cognition and behaviour have focused on the brain itself,and little is known about the link between EE stimulation and the gut microbiota[22].Recently,Lupori et al.[21]reported that the gut microbiota was a key contributor to the effects of EE on visual brain plasticity as the microbiota altered sensitivity to different environmental features.In a non-alcoholic steatohepatitis rat model,A.muciniphilatreatment,a potentially novel probiotic,in combination with EE reversed cognitive impairment and changed the microbiota composition[22].Consistently,we found that changes in specific bacterial groups,such as Bacteroidetes,Lachnospiraceae,BifidobacteriumandAkkermansia,afterB.brevetreatment in combination with EE were associated with behavioural alterations.Thus,the colonisation ofB.breveand increase in EE-induced exercise may contribute to modification of the gut microbiome.
Given the complexity of the gut microbiome ecosystem,signals from the products of intestinal microbes may shift their metabolic potential towards specific pathways[40].As communication via the gutbrain axis is bidirectional,such gut microbiota-derived metabolites can cross the blood-brain barrier (BBB) and convey signals from the gut to the brain[38].The most extensively studied microbiota-derived metabolites involved in the regulation of neurodegenerative diseases are amino acids,especially tryptophan,glutamate and arginine.As precursors for the biosynthesis of important neurochemicals and neurotransmitters,these amino acids can affect the function of the central nervous system[38].A recent clinical study reported thatB.breveCCFM1025 treatment could improve cognition and ameliorate depression by regulating tryptophan metabolism of the gut microbiome[8].We also found that tryptophan,D-glutamine andD-glutamate metabolism were significantly activated byB.breveadministration.Further,when we compared the ADSEand AD+BBEEgroups,only one bacterial pathway involving amino acids,theD-glutamine andD-glutamate metabolic pathway,was upregulated after treatment withB.breveand EE.Additionally,using metabolomic analysis,we identified significant differences in the composition of microbial metabolites in ADSEand AD+BBEEmice.Therefore,we focused on metabolites related toD-glutamine andD-glutamate metabolism,such asL-pyroglutamic acid,L-glutamine,L-glutamic acid and 2-oxoglutaric acid,to discover the underlying mechanisms.A recent study demonstrated that glutamine was a potential driver of the mechanism by which the gut microbiota from young mice offset age-associated behavioural deficits and restored the hippocampal metabolome[11].Another study demonstrated that dietary proline supplementation exacerbated depression-like behaviour in mice via microbial translocation and glutamine metabolism[12].We also found that glutamine levels were consistently linked to behavioural outcomes and cognition-related neurological changes.Numerous microbiota-derived metabolites can cross the BBB and thus enter the brain and affect its function[41-42].Taken together,we propose thatB.brevetreatment in combination with EE is an effective multidomain intervention strategy that can ameliorate AD-associated cognitive deficits by modulating microbiota-derived glutamine metabolism.
The current study has some limitations.First,despite our increased awareness of the contribution of the gut microbiome and its metabolites to brain function,the mechanistic links and signalling molecules involved remain to be determined.Second,although the functional effects of the gut microbiome were validated by a metabolomic approach,the use of 16S rRNA gene sequencing did not provide enough taxonomic resolution and yielded limited functional information.Future studies should explore the causal role of the gut microbiome in impacting AD through shotgun metagenomic sequencing,thereby providing additional validation.Third,our study explored metabolome alterations in faecal samples.As microbial metabolites can influence brain function and metabolic output through circulation as well,deeper investigations that examine hippocampal metabolome changes may strength our conclusions.Finally,we demonstrated thatB.brevetreatment in combination with EE provided a cognitive benefit to mouse models of AD.The applicability of this multidomain lifestyle intervention strategy to patients with AD needs to be validated by future studies.
5.Conclusions
In summary,compared withB.breveor EE treatment alone,a combination ofB.breveintervention with EE treatment can alleviate AD-related cognitive impairment and improve brain function by regulating glutamine metabolism of the gut microbiome.This finding will not only shift the therapeutic focus to targeted manipulation of the gut microbiota and its metabolites,but also provide a promising multidomain lifestyle intervention strategy,with a combination of physical exercise and psychobiotic interventions,to promote brain function and delay the progression of AD.
Declaration of competing interests
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31972052,32021005,31820103010),the Fundamental Research Funds for the Central Universities(JUSRP22006,JUSRP51501),the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250084.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

