Tb3+-nucleic acid probe-based label-free and rapid detection of mercury pollution in food
Xuhn Xi,Chenxi Zhou,Yulin Zhu,Yi Dong,Qing He,Mohmmd Rizwn Khn,Yunlong Chi,Ros Busquets,Ruijie Deng,Yo Ren,
a College of Biomass Science and Engineering, Healthy Food Evaluation Research Center, Sichuan University, Chengdu 610065, China
b Department of Chemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
c School of Life Sciences, Pharmacy and Chemistry, Kingston University London, Kingston Upon Thames KT1 2EE, UK
Keywords: Mercury pollution Food safety Nucleic acid probe Label-free Terbium
ABSTRACT Mercury is a threatening pollutant in food,herein,we developed a Tb3+-nucleic acid probe-based label-free assay for mix-and-read,rapid detection of mercury pollution.The assay utilized the feature of light-up f luorescence of terbium ions (Tb3+) via binding with single-strand DNA.Mercury ion,Hg2+ induced thymine (T)-rich DNA strand to form a double-strand structure (T-Hg2+-T),thus leading to fluorescence reduction.Based on the principle,Hg2+ can be quantified based on the fluorescence of Tb3+,the limit of detection was 0.068 9 µmol/L and the linear range was 0.1-6.0 µmol/L.Due to the specif icity of T-Hg2+-T artif icial base pair,the assay could distinguish Hg2+ from other metal ions.The recovery rate was ranged in 98.71%-101.34% for detecting mercury pollution in three food samples.The assay is low-cost,separation-free and mix-to-read,thus was a competitive tool for detection of mercury pollution to ensure food safety.
1.Introduction
With the development of food technology and widespread spread of food trade,contamination monitoring has been critical issue of food safety.Mercury,as one of the most toxic heavy metals,has been found in the surroundings such as soil,water,air,and crops,and can easily transfer to food[1-2].Mercury ion (Hg2+) poses a serious threat on ecological environment and health due to persistent bioaccumulation,non-biodegradability and high biotoxicity[3-5].Pathological studies have shown that a small amount of Hg2+intake can lead to diseases of hematopoietic system,urinary system and other aspects[6-7].More seriously,excessive intake of Hg2+can cause damage to the human body,tissues and organs[8-9].Hence,cheap and rapid tools for mercury pollution detection are highly demanded[10-11].
Analytical techniques,such as atomic emission/absorption spectrometry (AAS),atomic fluorescence spectrometry (AFS),high-performance liquid chromatography (HPLC) and inductively coupled plasma-mass spectrometry (ICP-MS) provide high precision,accuracy,and sensitivity for the detection of Hg2+[12-14].However,these approaches have been suffering chronically from high cost of instruments,high requirements of detection environment,time-consuming sample pretreatments,thus hindering the on-site detection of Hg2+[15-16].The development of molecular biotechnology promotes nucleic acids for heavy metal detection[17-18].In recent years,we have witnessed the flourishing of researches of nucleic acids to be used as frameworks for functional probes[19-20].Functional nucleic acids,including DNAzyme and aptamer,have bolstered the eff icient detection of Hg2+with the advantages of being programmable,cheap and easy to preserve[21-23].In addition,with the competitive aff inity,nucleic acid-based methods can specifically recognize the target[18,24].Although the recognition principle is similar to the antigen-antibody reaction,nucleic acid-based probes are more stable and easier to be synthesized[25-27].Up to now,on-site detection tools coupling with nucleic acid probes have been applied for food safety and environment analysis,and clinical medical diagnosis[28-30].However,these tools usually used chemically labeled nucleic acid probes,thus dramatically increasing the cost for Hg2+detection,and the probe design was complexing.
In this study,we used a simple,non-labeled thymine (T)-rich DNA strand to serve as the nucleic acid probe for label-free,mix-and-read detection of Hg2+.The trivalent terbium ion (Tb3+)played a central role as a light-up fluorophore marker.This design leveraged the structural transformation that Hg2+can specifically bind to thymine-thymine (T-T) mismatch site of a DNA duplex and form stable T-Hg2+-T artificial base pairs[31].Tb3+possesses low intrinsic fluorescence in solutions but be lighted up when binding to single-strand DNA[32-34].Hence,the transformation of nucleic acid structure would trigger the fluorescence changes of Tb3+,thus leading to the quantification detection of Hg2+.The avoidance of chemically labeled nucleic acid probes reduce the test cost.The sensing procedures are rapid and simple,thus can be conducted in one-test tube at constant temperature.Attributed to the features,we applied this method for the detection of mercury contamination in food samples,including cabbage,drinking water,and milk.
2.Materials and methods
2.1 Materials
Nucleic acid probes (Table S1) were synthesized by TSINGKE Biological Technology Co.,Ltd.(Beijing,China).HgCl2·6H2O and Tb(NO3)3·5H2O were obtained from Acros Organics Co.(New Jersey,USA).PbCl2,MgCl2,Co(NO3)3,CuSO4and MnCl2were bought from Chron Chemicals Ltd.(Sichuan,China).
2.2 Probe preparation
The nucleic acid powder was centrifuged at a speed of 8 000 r/min for 5 min.Then ultrapure water was added to form a 10 µmol/L solution which and stored at 4 °C.Tb(NO3)3·5H2O was used to dissolve into Tb3+solution with 100 mmol/L and stored in a clean centrifuge tube.The reaction buffer was prepared containing 330 mmol/L Tris-acetate,100 mmol/L magnesium acetate,660 mmol/L potassium acetate,1% Tween 20 and 10 mmol/L DTT.
2.3 Hg2+ detection
The detection procedure was carried in a reaction volume of 40 µL containing 4.0 µL of reaction buffer,4.0 µL of nucleic acid solution(10 µmol/L),4.0 µL of different concentration of Hg2+solution(10 µmol/L),4 µL of the Tb3+solution (10 mmol/L),and 20 µL of H2O.The reaction assay was mixed and incubated for 10 min at room temperature.Fluorescence analysis of the mixture was performed in microplate detector Synergy H1 (BioTek,USA).For the real-time fluorescence analysis of Hg2+,the excitation wavelength was set at 290 nm,with emission spectra recorded ranging from 500 nm to 600 nm.
2.4 Establishment of standard curves
Standard solutions with Hg2+concentrations of 0.1,0.5,1,4,6,10,15 and 20 µmol/L were prepared for fluorescence detection under the above optimal conditions.The standard curve was plotted according to the relationship between fluorescence intensity and Hg2+concentration.
2.5 Selectivity experiments of Hg2+ detection
Solutions of PbCl2,MgCl2,Co(NO3)3,CuSO4and MnCl2were prepared.The final concentrations of each metal ion were 5,10 and 20 µmol/L,respectively.Fluorescence of interfering ions and Hg2+were analyzed according to the Hg2+detection procedure described before.
2.6 Detection of Hg2+ in food samples
Hg2+analysis in milk,cabbage and drinking water was conducted.For the milk and cabbage,digestion tests should be executed priorly.In a nutshell,a total of 2.5 g of samples and varying quantities of Hg2+were pre-digested by immersing in 5 mL HNO3and 0.1 mL HClO4solution.
The digestion progress was first done at 120 °C for 1 h,then at 220 °C for 3 h,and finally at 320 °C for 2 h to obtain the final mixture.When cooled,the pH of the digestion solution was adjusted to 7.5 using NaOH,in a final volume of 10 mL for the further fluorescence analysis according to the above Hg2+detection procedure.The fluorescence intensity obtained corresponds to the Hg2+concentration in the standard sample curve.The recovery was obtained through dividing the corresponding concentration by the true added concentration.For drinking water,the recovery experiment was carried out by directly adding different concentrations of Hg2+,and then further fluorescence analysis was carried out according to the same Hg2+detection procedure.The recovery was obtained through dividing the corresponding concentration of the standard sample curve by the true added concentration.
3.Results and discussion
3.1 Principle and verification
The assay was proceeded based on one non-labeled T-rich DNA and the feature of the light-up feature of Tb3+(Fig.1).Tb3+can emit low fluorescence in solutions but be lighted up when binding to single-stranded DNA strand.We found that the fluorescence of Tb3+was dramatically reduced when the DNA strand turned to be double stranded.Initially,Tb3+binds to single-strand T-rich probe,lead to a high fluorescence.Upon the addition of Hg2+,the probe could fold into a hairpin structure by forming stable T-Hg2+-T base pairs,leading to structural transformation of nucleic acid probe from single-strand to a double-strand structure,thus decreasing fluorescence of Tb3+.The quantification of Hg2+can be achieved by measuring the fluorescence of Tb3+.Benefited from the high affinity of Tb3+to nucleic acids and homogeneous reactions,the nucleic acid probe could quickly and sensibly response to Hg2+.The assay only involved a non-labeled DNA strand,and allow mix-and-read detection of Hg2+.
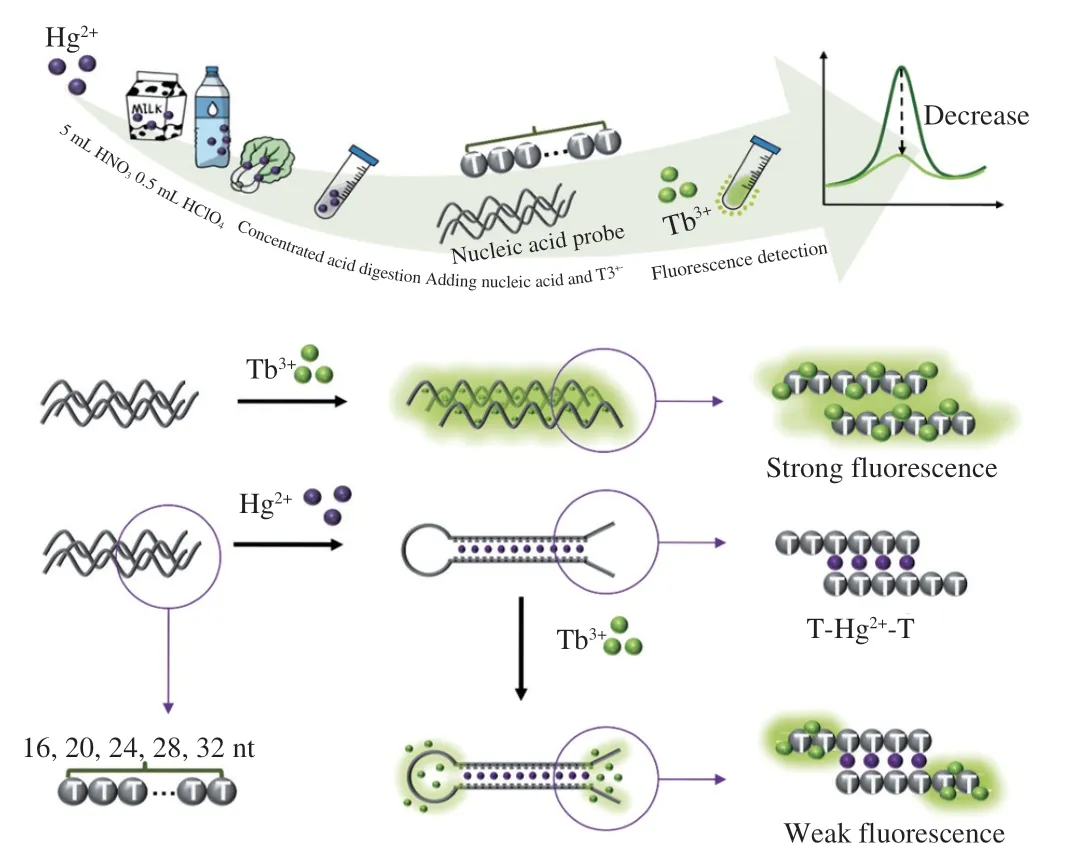
Fig.1 Schematic illustration of the design of Tb3+-nucleic acid probe and its application for homogeneous detection of Hg2+.
To verify the feasibility of Tb3+-nucleic acid probe for detecting Hg2+,fluorescence measurement for the assay was carried(Fig.2).In the absence of single-strand DNA,the Tb3+showed a weak fluorescence.Upon the addition of single-stand DNA,Tb3+was lighted up and had a maximum peak at 545 nm.Then,we explored the fluorescence of Tb3+under single-strand DNA and double-strand DNA,respectively.The result further indicated that Tb3+can only bind with single-strand DNA.In similar,the addition of Hg2+would trigger a significant reduction of fluorescence.The signal-to-noise ratio(F0/F) was 7.14,which further confirmed the successful formation of a double-strand structure (T-Hg2+-T).The result validated the feasibility of proposed Tb3+-nucleic acid probe-based label-free assay.
3.2 Optimization of detection procedure
The assay only involved one nucleic acid strand and specific dye.The effect of the length and concentration of nucleic acid probe and the reaction time for Tb3+binding on Hg2+detection was investigated.
Length of nucleic acid represented the number of binding sites of Hg2+and Tb3+,thus can influence the response of the probe towards Hg2+.Five nucleic acid probes with lengths 16,20,24,28 and 32 nt,were designed.As shown in Fig.3A,in the absence of Hg2+,the fluorescence intensity of Tb3+at 545 nm increased along with the nucleic acid probe length,in contrast,in the presence of Hg2+,a negligible change was observed when increasing the nucleic acid probe length.The highestF0/Fof 7.18 was achieved at 20 nt nucleic acid sequence length.
Optimization of nucleic acid concentration were performed.As shown in Fig.3B,the fluorescence of Tb3+enhanced significantly both in the absence and presence of Hg2+as the concentration of nucleic acid increased.More single-strand DNA can bind to more Tb3+.TheF0/Fratio fluctuated between 3.01 and 10.24,reaching the maximum value of 10.23 at a nucleic acid concentration of 50 µmol/L.
Lastly,the optimization of time was carried.The fluorescence of Tb3+increased drastically until the incubation time reached 12 min in the absence of Hg2+(Fig.3C).However,the fluorescence signal increased slowly to a lower level,without noticeable changes within 12 min in the presence of low concentrations of Hg2+(5,10 µmol/L).The real-time analysis of fluorescence revealed that the reaction would reach a plateau after 12 min with different concentrations of Hg2+at 0,5,and 10 µmol/L.Therefore,an incubation time of 12 min was employed in the further analysis.
3.3 Establishment of standard curve
Based on the optimal conditions obtained experimentally,the nucleic acid probe was validated by using a series of Hg2+solution with different concentrations (0-20 µmol/L),and then the standard curve was established.As Fig.4A demonstrated,the fluorescence intensity would recover with a rising concentration of Hg2+using nucleic acid probes,suggesting the nucleic acid probe could endow the ability of Hg2+quantification.Fig.4B showed that the slowest decrease in fluorescence was observed from 10 µmol/L to 20 µmol/L,indicating that the amount of Hg2+bound by the nucleic acid probe was close to saturation in this experimental condition.The fluorescence signal exposed at Hg2+concentration 0.1-6.0 µmol/L could be established as a standard curve,and linear regression equation was calculated asA=-374.58B+2 567.52 (R2=0.992),whereAdenoted fluorescence intensity andBdenoted Hg2+concentration.The limit of detection (LOD) of Hg2+for this method was 0.068 9 µmol/L.The LOD was defined as the concentration corresponding to the fluorescence signal at three times standard deviation of blank without Hg2+.Remarkably,this method didn’t require modification of any fluorescent probe or participation of any proteases and all of the progresses were carried in the homogeneous solution in one-test tube at room temperature.The whole procedure and could be finished within 10 min.The simple operation and fast reaction gave it an advantage in the field of on-site Hg2+detection.
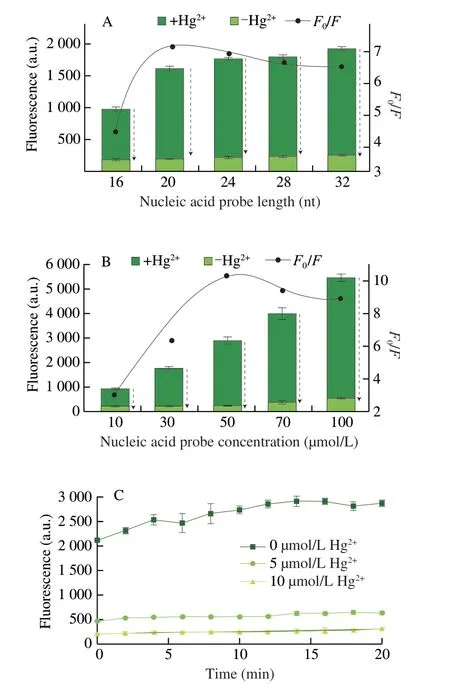
Fig.3 Fluorescence intensity and signal-to-background ratio corresponding to different optimization conditions.The fluorescence intensities of Tb3+ with or without addition of Hg2+ using different length of nucleic acid probes (16,20,24,28,32 nt) (A) and concentrations of nucleic acid probes (10,30,50,70,100 µmol/L) (B).(C) Real-time fluorescence of the nucleic acid probe for Hg2+detection.F and F0 represented the fluorescence intensity of Tb3+ in the presence and absence of Hg2+,respectively.
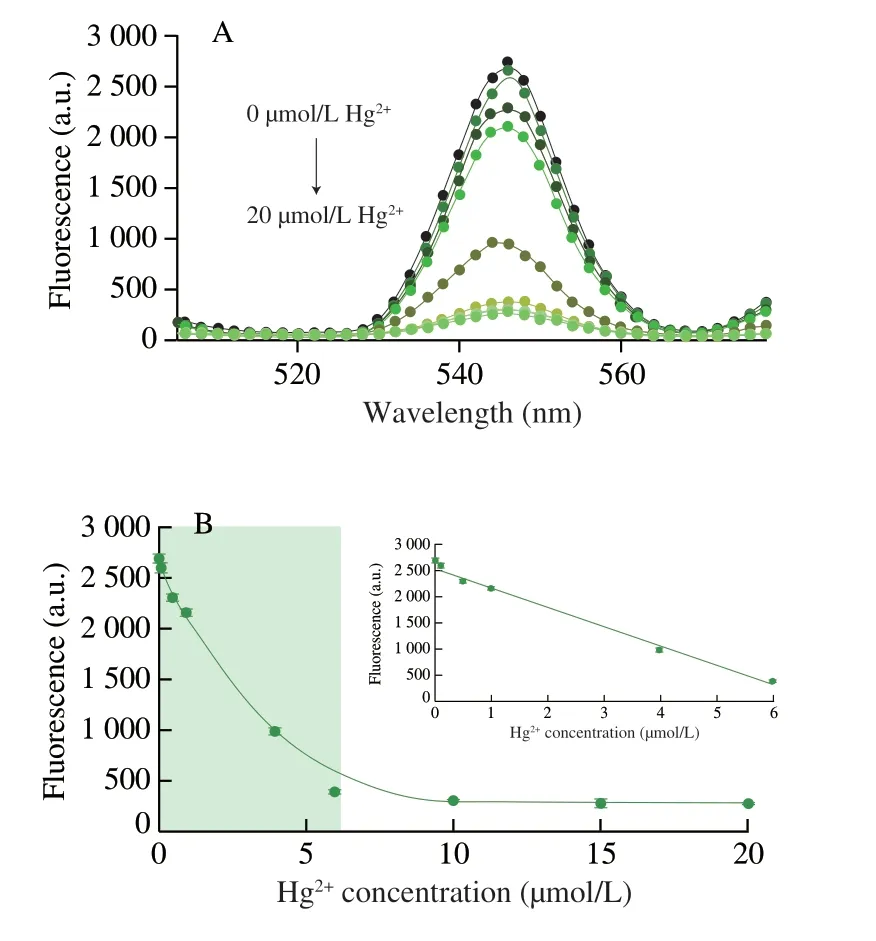
Fig.4 Quantification of Hg2+using Tb3+-nucleic acid probe.(A) Fluorescence spectra of upon the addition of different concentrations of Hg2+ (0,0.1,0.5,1,4,6,10,15,and 20 µmol/L).(B) Relationship between Hg2+ concentration and fluorescence response of Tb3+-nucleic acid probe.
3.4 Selectivity testing
The selectivity of label-free Tb3+-nucleic acid probe for Hg2+was investigated by detecting the fluorescence signal of other interfering ions (Pb2+,Co2+,Mn2+,Mg2+and Cu2+) at the concentration two times more than that of Hg2+.In order to further evaluate the selectivity of this detection system,three groups of different concentrations (5,10 and 20 µmol/L) of interfering ions were set up,corresponding to three groups of the same concentrations of Hg2+.The selectivity of the detection system was examined by comparingF0/F.The addition of Hg2+could significantly increase the signal-to-noise ratio of fluorescence intensity (Figs.5A and S1).Nevertheless,the addition of other interfering metal ions yielded much lowerF0/F.The signal-to-noise ratio of the interfering ions were between 1.85 and 1.02,while Hg2+would result in distinctive signals of 16.8,9.4 and 4.9,respectively.The result of this experiment showed that these interfering ions would lead to a negligible fluorescence variation,and only Hg2+would contribute to a dramatical change in the fluorescence of Tb3+.It has been demonstrated that thymine-rich nucleic acid probes have a high selectivity for Hg2+.As the recognition process of Tb3+-nucleic acid probes was based on the binding of Hg2+to its thymine on nucleic acid chains,the assay yieleded a high selectivity for Hg2+analysis.
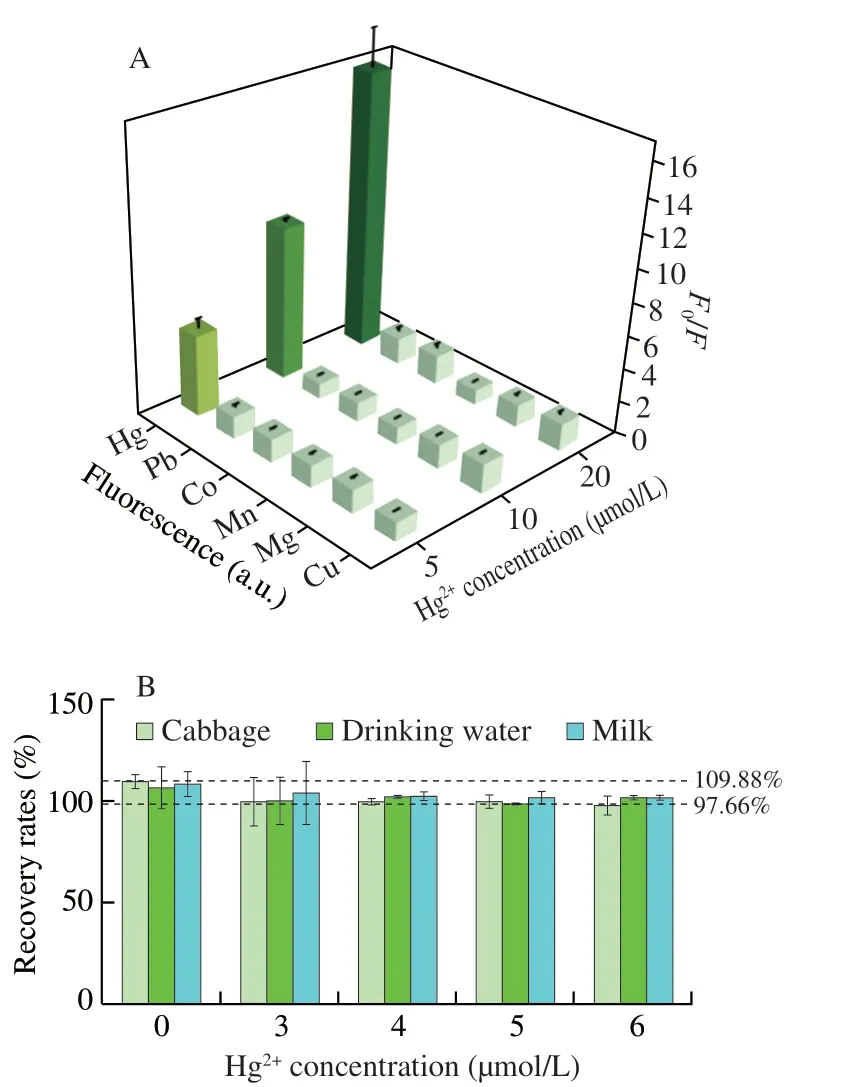
Fig.5 (A) Specificity of the Tb3+-nucleic acid probe for Hg2+ detection.The fluorescence intensity response to Hg2+ and other interfering ions (Pb2+,Co2+,Mn2+,Mg2+,and Cu2+).Different concentrations were detected including 5,10,and 20 µmol/L.(B) Recovery of Hg2+ detection in the cabbage,drinking water and milk.
3.5 Detection of mercury pollution in food samples
Mercury pollution is featured for easy migration,enrichment and non-degradability[35],thus posing great threat in food safety regulation.With the progressively severe discharge of industrial waste water and the increased use of pesticides or veterinary drugs,the planting soil for vegetables[36],feed for dairy cows[37],and drinking water have been detected to be contaminated with Hg2+[38].Hence,to evaluate the application of this Tb3+-nucleic acid probe in food samples,milk,cabbage and drinking water were chosen as the test samples for Hg2+detection.The milk was taken from pasteurized milk and the cabbage and drinking water was picked from the supermarket.Different concentrations of Hg2+(4,5 and 6 µmol/L) were spiked to verify this detection method.Fig.5B indicated the results that the recovery rate obtained by this method was varying within a range of 97.66%-109.88%.As shown in Table S2,the recoveries obtained in milk samples were in the range of 101.36% to 108.41%,cabbage samples ranged from 97.66% to 106.46%,drinking water samples were 98.71% to 109.87%.These results indicated that the proposed T nucleic acid probe could be considered as a precise method to quantify Hg2+in the complex sample matrix.Due to its advantages of easy operation,stable results,high selectivity and high sensitivity,the assay is potentially benefiting the detection of mercury pollution in dairy products,vegetables and water resources.
As shown in Table S3,compared with the currently available methods for Hg2+detection using nucleic acid probes,the present method occupies advantages in several aspects such as probe complexity,homogeneous detection and reaction time.Therefore,this method is competitive in detection of mercury contamination in food safety.
4.Conclusions
In summary,we developed a rapid and mix-and-read assay for mercury pollution detection.The assay only involved one single-stranded,non-labeled poly T DNA as a recognition probe.The light-up feature of Tb3+upon single-strand DNA binding eliminated the chemically labeled of nucleic acid probes.Compared with conventional functional nucleic acids-based methods,our strategy possesses the advantages of low-cost,simple-design,and easy-operation.The high specificity of T-Tb3+-T artificial base pair ensured the assay with high specificity to distinguish Hg2+from other metal ions.The detection process can be carried in one-test tube at room temperature,thus was potentially applicable in on-site detection.Under the optimal condition,the assay yielded a detection limit of 0.068 9 µmol/L for detecting Hg2+.The assay allowed precise detection of mercury pollution food samples,including cabbage,drinking water,and milk.The limitation of this method is that the need of pretreatment of food samples,which is still complex and requires a digestive process.The assay was advantageous due to the features of rapid,high selectivity,and cost-effective,thus is promising for serving as tools for on-site detection of food mercury pollution.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (22074100),the Young Elite Scientist Sponsorship Program by CAST (YESS20200036),the Researchers Supporting Project Number RSP-2021/138,King Saud University,Riyadh,Saudi Arabia,and Technological Innovation R&D Project of Chengdu City (2019-YF05-317 02266-SN),Sichuan University-Panzhihua City joint Project (2020CDPZH-5).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250085.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

