Element profiles of benzo[a]pyrene malignantly transformed 16HBE cells and joint effects of copper with cisplatin or vinorelbine on cell proliferation
WANG Yu*,YAN Lailai*,FU Juanling,HAO Mingmei,CHEN Wen,YAO Biyun,CHANG Bing,ZHAO Peng
(1.Department of Toxicology,Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety,3.Department of Laboratorial Science and Technology,School of Public Health,Peking niversity,Beijing 100191,China;2.China CDC Key Laboratory of Environment and Population Health,National Institute of Environmental Health,Chinese Center for Disease Control and Prevention,Beijing 100021,China;4.Department of Toxicology,Faculty of Preventive Medicine,School of Public Health,Sun Yat-sen University,Guangzhou 510080,China;5.National Institute of Occupational Health and Poison Control,Chinese Center for Disease Control and Prevention,Beijing 100050,China)
Abstract: OBJECTlVE To assess the profiles of elements in benzo[a]pyrene(BaP)induced carcinogenesis,and explore the joint effects of copper with cisplatin or vinorelbine on cell proliferation.METHODS Forty-four elements were measured using an inductively coupled plasma mass spectrometer in 16HBE cells and BaP malignantly transformed 16HBE(T-16HBE-C1)cells.Partial least square was used to validate the robustness of cell classification of elements.Cell viability was measured by MTT assay for copper(0,237,340,487,1000 and 1432 μmol·L-1),cisplatin(0,4.4,6.1,8.6,12.0 and 16.8 μmol·L-1),and vinorelbine(0,3.8,9.8,25.0,40.0 and 64.0 μmol·L-1)to acquire their half maximal inhibitory concentration(IC50).Mixtures of copper and chemotherapeutics were prepared according to the ratio of each IC50.Their joint effects on cell viability were assessed by MTT assay and isobolographic analysis.Inhibition effect of copper(0,50,100,200,400 and 800 μmol·L-1)with IC50 of cisplatin or vinorelbine on proliferation of T-16HBE-C1 cells was also assessed.RESULTS A total of 29 elements were quantified in 16HBE and T-16HBE-C1 cells,among which concentrations of copper,zinc,silver,selenium and rubidium decreased(P<0.05,P<0.01),while those of molybdenum,arsenic,lithium,germanium,strontium,nickel,lanthanum,mercury,iron,and cesium increased(P<0.05,P<0.01)in T-16HBE-C1 cells.Element concentration could be used to distinguish T-16HBE-C1 cells from 16HBE cells.Copper concentration-dependently inhibited proliferation of both cells,with a statistically significant lower IC50 of(613±16)μmol·L-1 in 16HBE cells than(776±15)μmol·L-1 in T-16HBE-C1 cells(P<0.01).Mixtures of copper and cisplatin(1∶69.5)or vinorelbine(1∶33.4)could inhibit cell proliferation,and copper had additive effects with cisplatin or vinorelbine.When copper concentration was higher than 400 μmol·L-1,copper combined with IC50 of cisplatin or vinorelbine inhibited cell proliferation of T-16HBE-C1 cells compared with IC50 of cisplatin(11.2 μmol·L-1)or vinorelbine(23.2 μmol·L-1)alone.CONCLUSlON Element profiles and correlations can change significantly after 16HBE cells are malignantly transformed by BaP.Copper could inhibit the proliferation of T-16HBE-C1 cells and have additive effects with cisplatin or vinorelbine in higher concentration.
Key words: element;copper;cisplatin;vinorelbine;benzo[a]pyrene;cell proliferation;T-16HBE-C1 cells
Benzo[a]pyrene(BaP)is a ubiquitous environmental contaminant found in tobacco smoke,grilled food,and vehicle exhaust fumes.Given the mutagenic and carcinogenic properties,BaP is a well-known carcinogen for lung cancer,the leading cause of cancer mortality in China[1].The mechanism of BaP-induced carcinogenesis has been widely studied since 1933.In addition to a better understanding of metabolisms that initiate mutations,genomic and proteomic research has found many key molecules and potential biomarkers[2].However,despite extensive studies on the function of specific genes and proteins,element-based research on BaP-induced lung cancer remains inadequate.
Elements are essential for human health and play an important role in tumorigenesis.Nickel,arsenic,and cadmium compounds are carcinogenic to humans[3],while platinum-based drugs have been widely used as chemotherapeutics.Silver has antimicrobial properties and silver nanoparticles have been used for cancer theranostics[4-5].Rare earth elements like lanthanum and cesium could be used as an alternative treatment for cancers due to their high affinity to tumor tissues.As for essential trace elements,epidemiological studies have shown some benefits of a higher status of selenium on the risk of lung,prostate,colorectal,and bladder cancers[6].Serum levels of selenium and zinc are often lower in cancer patients compared with normal individuals[7].There is evidence that copper levels were higher in serum and tumor tissue of lung cancer patients[8-10],but whether cellular transformation to malignancy can drive copper accumulation remained unclear[11].Although a particular element was associated with lung cancer risks,there is limited evidence regarding the integrated element alternations as cellular transformation to malignancy.
Cisplatin and vinorelbine have been used as standard chemotherapy for patients with nonsmall-cell lung cancer(NSCLC).Despite the positive effect,cisplatin-induced resistance and side effects[12]have led to exploration on anti-chemotherapy mechanisms such as element homeostasis.Cuproplasia and ferroptosis have been found as potential paths to treat cancer alone or in combination with current chemotherapeutics[13-14].In this scenario,comprehensively exploring element changes in relation to malignant transformation may provide insight into cancer therapy.To this end,BaP malignantly transformed 16HBE(T-16HBE-C1)cells were employed as a study model,element profiles were measured and copper was further investigated for its potential role in proliferation and joint effect with cisplatin or vinorelbine.
1 MATERlALS AND METHODS
1.1 Chemicals and equipments
Cupric sulfate anhydrous(CuSO4),cis-diammineplatinum dichloride(cisplatin),vinorelbine ditartrate slat hydrate(vinorelbine)and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide(MTT)were purchased from Aladdin(InnoChem.Technology Co.,Ltd.,China).Fetal bovine serum was purchased from Sijiqing(Zhejiang Tianhang Biotechnology Co.,Ltd.,China).Single element standard,mixed rare earth elements standard,rhodium,indium,and rhenium internal standard were purchased from GuoBiao(Beijing)Testing&Certification Co.,Ltd.Other chemicals used in this study were purchased from Sigma-Aldrich(St.Louis,MO,USA)unless specified.Reagents for cell culture were purchased from Invitrogen(Shanghai,China)unless specified.
Cells were cultured in a CO2humidified incubator(SCI-165D,ASTEC,Japan).Element concentrations were measured using a microwave digestion system(Ultra WAVE,Milestone Co.,Italy)and a inductively coupled plasma-mass spectrometer(ICP-MS;ELAN DRCⅡ,PerkinElmer,USA).MTT results were read using a plate reader(Bio-Rad,CA,USA).
1.2 Cell culture
Human bronchial epithelial 16HBE cells[15-16]were generously provided by Dr.D.C.Gruenert(University of California,San Francisco,USA).T-16HBE-C1 cells were malignantly transformed by BaP from 16HBE cells by our lab[17].T-16HBE-C1 cells can form tumors in nude mice through subcutaneous injection,while 16HBE cells can not.Both cells were cultured in MEM medium supplemented with 10%(V/V)heat-inactivated fetal bovine serum,penicillin 10 kU·L-1and streptomycin 10 kU·L-1at 37 ℃in a 5% CO2humidified incubator.
1.3 Total cell protein extraction
Cells were seeded in a 75 cm2(culture area)flask(4×106cells per flask)and cultured for two days before being harvested by trypsin,washed three times by PBS,and suspended in lysis buffer(NaCl 150 mmol·L-1,0.5% Triton X-100,and PMSF 1 mmol·L-1in Tris-HCl 50 mmol·L-1,pH=7.4).After collection,cell lysates were centrifuged at 14 000×gfor 15 min at 4 ℃and stored at -80 ℃before analysis.Protein concentration was determined using the Bradford protein assay.All experiments were performed ten times.
1.4 lnductively coupled plasma mass spectromy(lCP-MS)for measurement of element concentration
450 μL cell lysates were predigested with 2%(V/V)HNO3overnight at room temperature and digested in a microwave digestion system for 30 min.Concentrations of elements were measured by ICP-MS with a 0.96 L·min-1of nebulizer gas flow,1.80 L·min-1of auxiliary gas flow,16 L·min-1of plasma gas flow,1180 W of ICP RF power,9 mm of sampling depth,and 1.1 mL·min-1of sampling uptake rate.Hopping was chosen as the mode peak,and read with 10 sweeps for 3 replicates.The final concentrations of elements were calculated by dividing the original concentrations(pg)from ICP-MS by the total concentration of protein(mg)of the sample.Forty-four elements were analyzed in the Central Laboratory of Biological Elements in Peking University Health Science Center,with qualification of China Metrology Accreditation System.
1.5 MTT assay for cell proliferation
Briefly,6000 cells suspended in 200 μL MEM were incubated in 96-well plates for 24 h,and then treated with copper(0,237,340,487,1000 and 1432 μmol·L-1),cisplatin(0,4.4,6.1,8.6,12.0 and 16.8 μmol·L-1),vinorelbine(0,3.8,9.8,25.0,40.0 and 64.0 μmol·L-1),mixtures of copper and cisplatin(0,210,273,355,462 and 600 μmol·L-1),mixtures of copper and vinorelbine(0,138,207,311,467 and 700 μmol·L-1),copper(0,50,100,200,400 and 800 μmol·L-1)with half maximal inhibitory concentration(IC50)of cisplatin or vinorelbine for another 24 h.Four hours after the culture medium was replaced by 200 μL MTT(0.4 mmol·L-1),formazan was dissolved in 200 μL DMSO and plates were read at 570 nm using a plate reader to obtain the absorbance(A570nm).Five replicate wells were used for each concentration.Experiments were conducted in triplicate with five replicates each time.Cell viability(%)=A570nmof experimental group/A570nmof control group×100%.
1.6 lsobolographic analysis for additive effect
Isobolographic analysis was used to assess the joint effects of two chemicals.The line of upper 95% confidential interval(CI)of IC50,IC50,and lower 95% CI of IC50were created by connecting corresponding points,respectively.Mixtures of copper and chemotherapeutics were created according to the ratio of each IC50.Mixtures of copper and chemotherapeutics were prepared according to the ratio of each IC50.Cell viability of mixtures of copper and cisplatin(0,210,273,355,462 and 600 μmol · L-1),or mixtures of copper and vinorelbine(0,138,207,311,467 and 700 μmol·L-1)was tested by MTT assay.IC50was calculated using the least square method.Synergistic effect was assessed when IC50of the mixture lay over the upper 95% CI line.The antagonistic effect was assessed when IC50of the mixture lay below the lower 95% CI line.If IC50of mixture lay between two lines,those two chemicals had an additive effect.To further evaluate the range of joint effects,cell viability of copper(0,50,100,200,400 and 800 μmol·L-1)with IC50of cisplatin or vinorelbine was tested by MTT assay.
1.7 Statistical analysis
Data were statistically analyzed using R-3.6.0(R Development Core Team)and Graphpad Prism 7.0(GraphPad Software Inc.,CA,USA).All results were expressed as±sandP<0.05 was considered statistically significant.The Studentttest was used to analyze differences of element concentrations between 16HBE and T-16HBE-C1 cells.Correlation coefficients among elements were calculated by the Pearson method.IC50was calculated using the least square fitting in Graphpad.Two-way ANOVA was used to compare the inhibition effects of copper,cisplatin,and vinorelbine on proliferation,where Dunnett multiple comparisons test was used among concentration groups and Bonferroni multiple comparisons test was applied between cells or chemotherapeutics.Unsupervised hierarchical clustering was calculated inRwith euclidean as clustering distance and average as the clustering method.Partial least square was conducted using the "caret" package inR.
2 RESULTS
2.1 Comparison of element concentrations between 16HBE and T-16HBE-C1 cells
A total of 44 elements were measured using ICP-MS.Twenty-nine elements were successfully quantified after removing 13 elements that were below detection limits(platinum,vanadium,samarium,europium,gadolinium,terbium,dysprosium,holmium,erbium,thulium,ytterbium,lutetium,and yttrium)and 2 elements that had potential contaminants(beryllium and titanium).Tab.1 shows that 5 elements(copper,zinc,silver,selenium,and rubidium)were significantly lower(P<0.01,P<0.05),9 elements(molybdenum,arsenic,lithium,germanium,strontium,nickel,lanthanum,mercury,iron,and cesium)were significantly higher(P<0.05,P<0.01),and the other 14 elements remained unchanged in T-16HBE-C1 cells compared with 16HBE cells.Correlations among elements were different between 16HBE and T-16HBE-C1 cells(Fig.1).
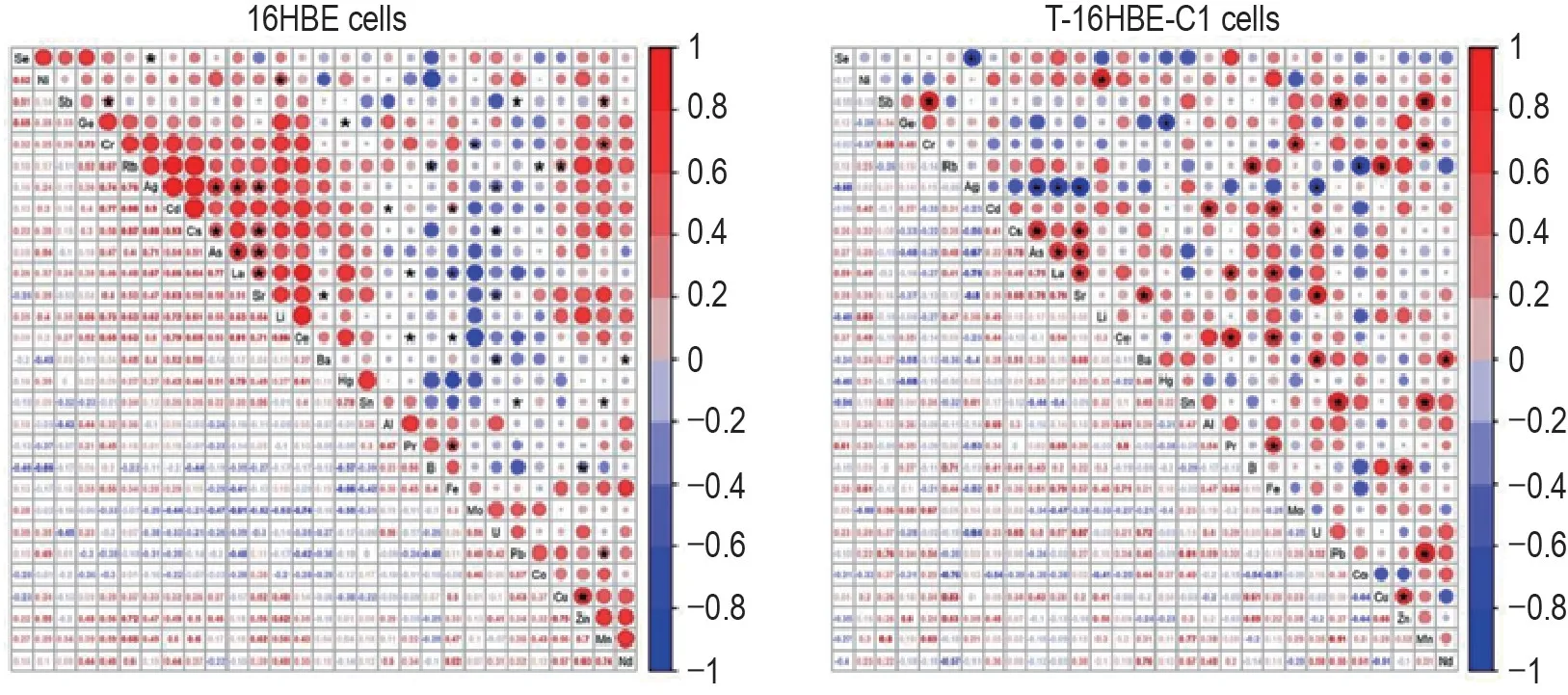
Fig.1 Comparison of element correlations between 16HBE and T-16HBE-C1 cells.Values of correlation coefficient among elements were calculated by Pearson method and displayed as the size and color of the circle in the top right corner,with statistically significant ones marked with a black start(P<0.05).
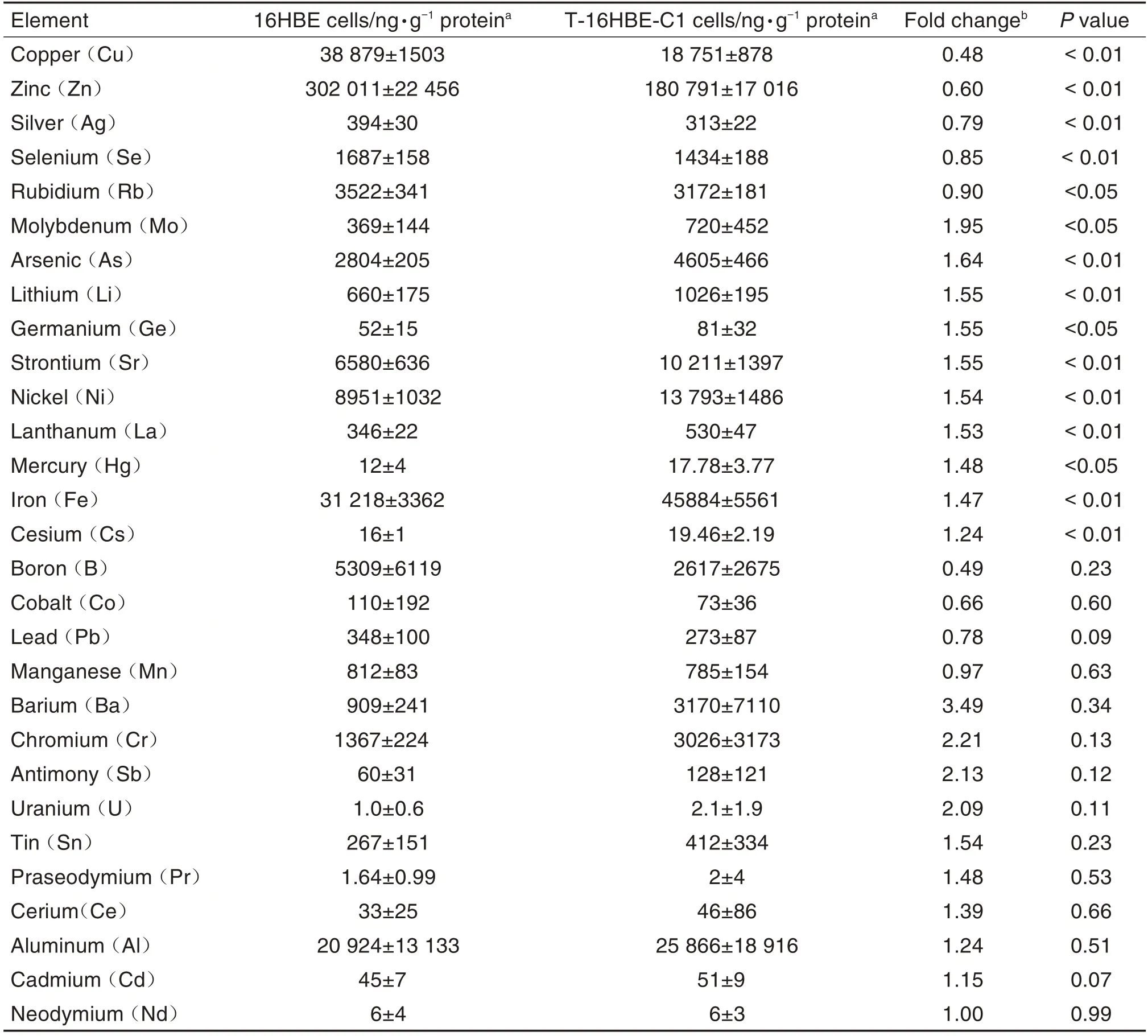
Tab.1 Comparison of element concentrations between 16HBE and Benzo[a]pyrene malignantly transformed 16HBE(T-16HBE-C1)cells
2.2 Element patterns distinguish T-16HBE-C1 cells from 16HBE cells
Fig.2A indicates that element patterns could distinguish two types of cells from each other.Due to the relatively high correlation among elements and a greater number of elements than cell samples,supervised partial least square(PLS)was then used to classify and predict cell types.Eight samples from each cell were randomly selected to build a prediction model and others were defined to validate the results.The PLS model with two components successfully separated cells into their own group(Fig.2B).PLS also predicted a probability of 71.3% and 71.9% to classify 16HBE-6 and 16HBE-8 cells into 16HBE group,68.4% and 76.5% to classify T-16HBEC1-8 and T-16HBE-C1-9 cells into T-16HBE-C1 group,respectively.The importance of elements in prediction are shown in Fig.2C.Zinc and copper ranked the highest two values of importance.
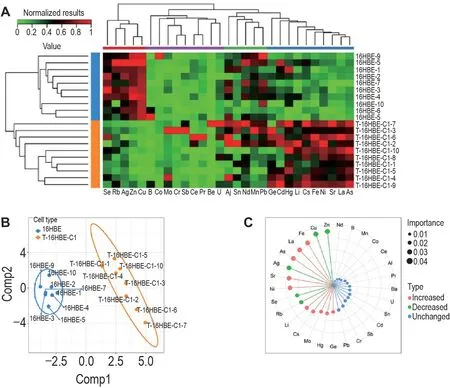
Fig.2 Performance of element concentration on cell classification.A and B:unsupervised hierarchical clustering and partial least square(PLS)analysis separated T-16HBE-C1 cells from 16HBE cells,respectively.C:importance of elements in cell-type prediction via PLS.
2.3 Copper inhibits proliferation of 16HBE and T-16HBE-C1 cells
Fig.3A shows that copper could concentrationdependently inhibit the proliferation of both cells.IC50of copper in 16HBE cells[(613±16)μmol·L-1]was significantly lower than that of T-16HBE-C1 cells[(776±15)μmol·L-1],indicating that 16HBE cells were more sensitive to the inhibition effect of copper.However,with regard to insignificant differences between the two cells when copper was over 1000 μmol·L-1,a higher sensitivity of 16HBE cells was observed only below certain copper levels.
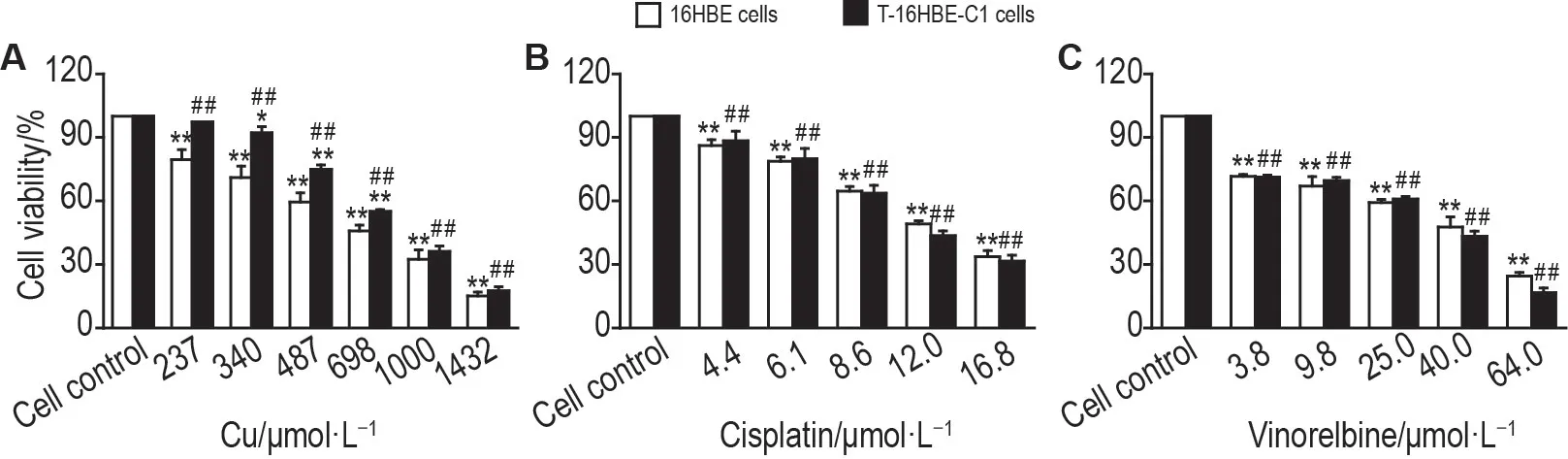
Fig.3 Copper,cisplatin and vinorelbine inhibited proliferation of 16HBE and T-16HBE-C1 cells.Cells were treated with copper,cisplatin,and vinorelbine for 24 h.Cell viability was measured by MTT assay.Cell viability(%)=A570 nm of experimental group/A570 nm of control group×100%.±s,n=3.*P<0.05,**P<0.01,compared with cell control(0 μmol·L-1)group,##P<0.01,compared with 16HBE cells.
2.4 Additive effect of copper with cisplatin or vinorelbine on inhibition of cell proliferation
IC50of cisplatin and vinorelbine was(11.2±0.8)μmol · L-1and(23.2 ± 1.7)μmol · L-1in T-16HBE-C1 cells,and(11.9±0.5)μmol·L-1and(26±4)μmol·L-1in 16HBE cells,respectively.T-16HBE-C1 cells were not sensitivitie to cisplatin or vinorelbine compared with 16HBE cells(Fig.3B and 3C).To access the joint effects of copper with cisplatin or vinorelbine,isobolographic analysis was conducted.The mixture of copper and chemotherapeutics was created according to the ratio of each IC50.Their inhibition effects on proliferation of T-16HBE-C1 cells are shown in Fig.4A and 4B.IC50of the mixture lay between the line of upper and lower 95% CI of IC50(Fig.4C and 4D),indicating that copper had additive effects with cisplatin or vinorelbine.
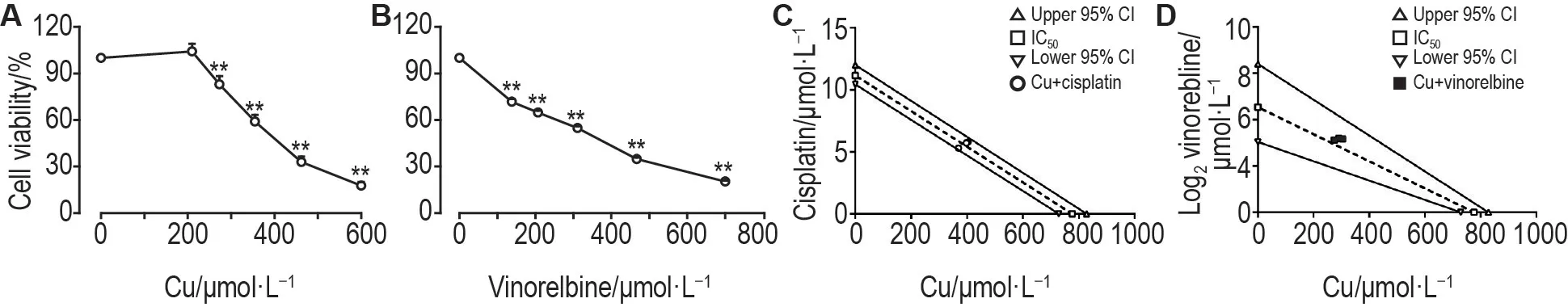
Fig.4 Copper had additive effect with cisplatin or vinorelbine on inhibition effect of cell proliferation in T-16HBE-C1 cells.Cells were treated with mixtures of copper with cisplatin or vinorelbine for 24 h.Cell viability was measured by MTT assay.A and B:cell viability of the mixture of copper with cisplatin or vinorelbine,respectively.±s,n=3.**P<0.01,compared with corresponding control(0)group.C and D:IC50 of mixture lay between the line of upper and lower 95% confidential interval(CI)of IC50 in isobologram,indicating copper had an additive effect with cisplatin or vinorelbine.The joint effects were examined using isobolographic analysis.
2.5 Range of additive effect of copper with cisplatin or vinorelbine
Fig.5 indicates that 100 and 200 μmol·L-1copper enhanced while 400 μmol·L-1copper and above inhibited proliferation of T-16HBE-C1 cells in combination with IC50of cisplatin.For IC50of vinorelbine,copper 200 μmol · L-1and below increased while copper 400 μmol·L-1and above decreased T-16HBE-C1 cell proliferation.Therefore,only some high levels of copper could additively inhibit cell proliferation with cisplatin or vinorelbine.
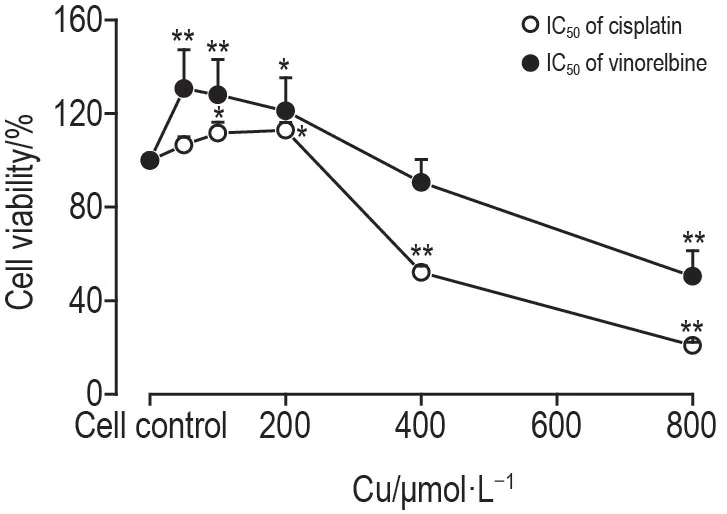
Fig.5 lnhibition effect of copper with cisplatin or vinorelbine on proliferation of T-16HBE-C1 cells.T-16HBE-C1 cells were treated with copper combined with IC50 of cisplatin or vinorelbine for 24 h.Cell viability was measured by MTT.±s,n=3.*P<0.05,**P<0.01,compared with cell control(0 μmol·L-1)group.
3 DlSCUSSlON
3.1 Element profile changes in T-16HBE-C1 cells
Element dysregulation is one of the features in tumor and malignantly transformed cells.We found element profiles and their correlations changed significantly after 16HBE cells were malignantly transformed by BaP.The results showed that copper,zinc,silver,selenium,and rubidium decreased while molybdenum,arsenic,lithium,germanium,strontium,nickel,lanthanum,mercury,iron and cesium increased in T-16HBE-C1 cells.Carcinogenesis can lead to substantial gene mutations related to element hemostasis.For example,changes in copper transporters[e.g.copper transport protein 1(Ctr1)and copper-transporting ATPase 1]lead to unbalanced storage and secretion of copper[18-19].Differentially expressed iron-related genes in lung cancer such as human solute carriers can disrupt iron hemostasis[20].
Copper is an essential trace element and a cofactor of enzymes that are involved in redox reaction,cell proliferation,and tumor progression[13].Although elevated levels of copper were found in serum and tumor tissue of lung cancer patients[21-22],evidence on copper accumulation during cellular transformation to malignancy was far from sufficient.In our study,copper concentration decreased by more than 50% in T-16HBE-C1 cells.Excessive intracellular copper could induce elevated oxidative stress and exert a direct cytotoxic effect[23].Nonetheless,supplementary copper in drinking water yielded inconsistent effects on tumor growth in mice[24].Epidemiological evidence has not yet concluded the association between dietary copper intake and lung cancer risks[25-26].The discrepancy could be explained partly by tumor microenvironmentinvivoand the distinct mechanism of BaP-induced carcinogenesis.Copper transporters and binding proteins induced by BaP could result in different intracellular copper homeostasis and related functions(e.g.angiogenesis),ending up with different health outcomes[11,18].Therefore,future studies are warranted to find out whether copper has pro- or anti-tumor capacity in BaP-induced lung cancer.
In terms of other essential trace elements,zinc and selenium served as antioxidants and were generally lower in serum of cancer patients[27-28].In contrast to zinc,selenium appeared to be higher in tumor tissues,which may help selenium to inhibit cell growth and disrupt energy systems[29].Dietary zinc and selenium intake were inversely associated with lung,prostate,and colorectal cancer risk[26,30-31],but supplementation of supranutritional doses of selenium might have insignificant or inverse effects[32].Changes in uptake and metabolism of iron are crucial features of cancer.High dietary iron intake and increased body iron storage were associated with increased cancer risks[26,33-34].Albeit with mixed results,disturbance of element metabolisms has been proved to be features of tumor and malignant cells.Element changes in this study provided clues for understanding the mechanism of BaP-induced carcinogenesis.
3.2 Effect of copper on cell proliferation
We found copper inhibited the proliferation of T-16HBE-C1 cells,which was consistent in HepG2 cells when copper range from 10 to 500 μmol·L-1[35].Given redox active effect of excessive intracellular copper,copper ionophores have been used to treat cancer[13].We also found that higher copper levels could additively inhibit cell proliferation in combination with cisplatin or vinorelbine.However,lower copper levels showed a contradictory effect,which was in line with A549 and H1355 cells where proliferation increased when treated with 10 μmol·L-1copper for 72 h[36].The biphasic response can be explained by the hormesis effect and insufficient oxidative pressure aroused by low levels of intracellular unbounded copper.Therefore,when combined with chemotherapeutics or ionophores to treat cancer,local treatment with high doses of copper or via effective copper transporter[22]might be favored as 16HBE cells were more sensitive to the inhibition effect of copper.
In addition,copper transporters have been reported to be associated with resistance to platinum-based therapeutics,including cisplatin[37].Malignant transformation can alter the expression of copper transporters,such as which controls the uptake of chemotherapeutics[38-39].We found that the inhibition effect of cisplatin and vinorelbine remained almost the same between T-16HBE-C1 and 16HBE cells.One explanation is that Ctr1 levels could be rapidly down-regulated by cisplatin and did not usually correlate well with cellular uptake of platinum-based drugs in NSCLC[40-41].
3.3 Conclusion
Element hemostasis c hanged significantly after 16HBE cells are malignantly transformed by BaP.Copper inhibited the proliferation of T-16HBE-C1 cells,and had an additive effect with cisplatin or vinorelbine within relatively higher concentrations.Our findings provided elementbased clues for understanding the mechanism of BaP-induced carcinogenesis.

