Fabrication and characterization of multi-scale coated boron powders with improved combustion performance: A brief review
Rui Liu , Dnfeng Yng , Kunyu Xiong , Ying-Lei Wng , Qi-Long Yn ,*
a Science and Technology on Combustion, Internal Flow and Thermo-structure Laboratory, Northwestern Polytechnical University, Xi'an, 710072, China
b Xi'an Modern Chemistry Research Institute, Xi'an, 710065, China
Keywords: Boron powder coating Structure and morphology Condensed phase thermal reaction Ignition and combustion
ABSTRACT Boron has high mass and volume calorific values, but it is difficult to ignite and has low combustion efficiency.This literature review summarizes the strategies that are used to solve the above-mentioned problems, which include coatings of boron by using fluoride compounds, energetic composites, metal fuels, and metal oxides.Coating techniques include recrystallization, dual-solvent, phase transfer, electrospinning,etc.As one of the effective coating agents,the fluorine compounds can react with the oxide shell of boron powder.In comparison, the energetic composites can effectively improve the flame temperature of boron powder and enhance the evaporation efficiency of oxide film as a condensed product.Metals and metal oxides would react with boron powder to form metal borides with a lower ignition point, which could reduce its ignition temperature.
1.Introduction
At present,boron-based fuel-rich propellants are usually used in the field of solid rocket ramjets [1].The boron-based fuel-rich propellants have low oxidant content and oxygen balance in comparison to composite solid propellants (CPs) [2].But low combustion efficiency has limited its application in rocket propulsion.Boron is a great semi-metal combustible matter, and it has high mass and volume calorific values.The mass calorific value of Boron is as high as 59 kJ/g,2.3 times higher than that of Mg and 1.9 times that of Al;the volumetric calorific value is as high as 140 kJ/cm3, 3.09 times that of magnesium and 1.66 times that of Al [2,3].Because of its high calorific value,Boron has long been considered the most attractive semi-metal additive.
Despite this great energy content, boron has the following problems during its applications:a)The ignition of boron powder is quite difficult.Boron powder(melting and boiling points are 2573 K and 4139 K)is easy to react with ambient oxygen to form oxide film BmOn(m≠1).The oxide film BmOnhas a high boiling point(2133 K)and is not easy to be vaporized,so the ignition of boron powder is quite difficult [5-7].b) The combustion efficiency of boron at low pressure is usually low.Boron and its oxides have high gasification heat and need to supply a lot of heat energy,so that the combustion temperature decreases and the combustion performance becomes worse.The combustion efficiency of boron in solid rocket ramjet is only 30%-40% [4].c) Impurities such as oxide film BmOnare normally presented on the surface of boron powder, which would easily react with the hydroxyl groups of typical propellant binder such as hydroxyl-terminated polybutadiene(HTPB)to form borate esters, causing gelation, and leading to incompatibility problems[8]; d) The processability of the boron-based fuel-rich propellant slurry is poor when the content of boron is high[9].In addition,the surface impurities[10](such as oxide film and acidic impurities)of boron powder have poor reactivity with glycidyl azide polyether(GAP), but they can react with isocyanate curing agents and generate cavities or bubbles during the curing process [11].
To solve the above problems, many outstanding works have been done [12], which include coatings of boron by using fluoride compounds, energetic composites, metal fuels, and metal oxides,coating techniques include recrystallization, dual-solvent, electrospinning,continuous diffusion flow,vapor deposition methods,etc.The following sections summarize the preparation methods for modified boron by using various coating agents.Their effects on the structure, morphology, and thermal physical properties of boron have also been fully analyzed.
Abbreviations
CPs Composite solid propellants
HTPB Hydroxyl-terminated polybutadiene
GAP Glycidyl azide polyether
AP Ammonium perchlorate
KP Potassium perchlorate
FG Fluorinated graphene
NC Nitrocellulose
Viton A Fluorine rubber
THV Ttetrafluoroethylene-hexafluoropropylenevinylidene fluoride
PVDF Polyvinylidene fluoride
PTFE Polytetrafluoroethylene
PFA Perfluoroalkoxy polymer
FEP Fluorinated thylene-propylene polymer
PFNA Perfluorononanoic acid
PFUDA Perfluoroundecanoic acid
LiP Lithium Perchlorate
AN Ammonium Nitrate
NQ 2-nitroguanidine
HMX 1,3,5,7-tetranitro-1,3,5,7-tetrazocine
KH-550 A coupling agent
SEM Scanning Electron Microscope
TEM Transmission Electron Microscopy
AFM Atomic Force Microscopy
DSC Differential Scanning Calorimeter
TGA Thermal Gravimetric Analyzer
DTA Differential Thermal Analyzer
TigIgnition temperature
tigIgnition delay time
HcCombustion heat
HdDetonation heat
EfCombustion efficiency
HrHeat release
rbBurning rate
TmMaximum flame temperature
IpPeak burning intensity
tbBurning time
TacAverage combustion temperature
raAverage pressurization rate
CtThermal conductivity
pH Acidity
VViscosity
2.Coating and characterization techniques
Some literature reviews have shown that [13-17] the boron powder coating technology is one of the most effective methods to promote the ignition and combustion of boron powder.This section will introduce the boron powder coating technology from four aspects:surface treatments of boron powder,selection criteria of the coating materials, the general fabrication method for boron, and characterization techniques for coated boron powder.
2.1.Surface treatments of boron powder
Before coating boron powder, the surface treatment can partially remove the oxide film on the boron powder surface.Boron powder is insoluble in water, ethanol, and ether, but its oxide film BmOncan be dissolved in the above solvents.So we can use the above solvents to purify boron powder [18].The steps of boron powder surface treatments are:add amorphous boron powder and different types of solvent to the flask,stir,filter,and finally dry the obtained filter cake to obtain high-purity boron powder.From the content of B and O after the boron powder surface treatment[19-21],it is found that the solvents that have a greater impact on the boron powder surface treatment are sodium hydroxide,mannitol solution, and ethanol solution.Other solvents such as ethyl acetate,ammonia,and distilled water can slightly remove the oxide film on the boron powder surface.In addition, ultrasonic agitation of boron in acetonitrile followed by its processing in toluene yields a powder that has substantially less oxide than the raw material,is more stable in room air,and oxidizes more readily upon its heating[9].
2.2.Selection criteria of the coating materials
The choice of coating material is important,which should meet the following requirements: (1) It can significantly improve the ignition and combustion performance of boron powder; (2) It has good compatibility with the propellant; (3) It can improve the production process of the propellant [22].Based on the above selection conditions, it can use materials including fluoride compounds,energetic composites,metal fuels,and metal oxides to coat boron powder.Generally, when these materials are coated, the ignition delay time is reduced,and the combustion time,maximum combustion temperature and combustion efficiency are improved.The modification mechanism of these materials on boron powder will be introduced below [23].Fluorine compounds [23-25] can react with the oxide film BmOnon the surface of boron powder.Among them, metal fluorides can react [26,27] in the following chemical reaction: BmOn+ MF→MBO2+BOF (M is metal), and fluoropolymers can produce HF and react as follows:BmOn+ HF→HBO2+BOF.Different from fluorine compounds, energetic composites would release more heat,which can effectively increase the temperature around the boron powder and is beneficial to evaporating oxide film BmOn[28].Among them, azidecontaining substances such as GAP and NaN3can heat the boron powder to a high enough temperature, whereas the energetic oxidants such as ammonium perchlorate (AP) and potassium perchlorate (KP) can release to produce oxygen to promote partial boron combustion and increase combustion temperature.
When it comes to metal fuels and metal oxides, they can form metal borides with boron to release heat[27].Among them, some metal fuels such as Mg and Al can reduce the ignition temperature of boron [29].Other metal fuels such as Ti and Zr occur in highly exothermic metal-metalloid reactions, producing the respective borides(TiB2or ZrB2)[30-32].metal oxides such as Bi2O3and CuO can promote the contact between oxygen and boron and reduce the ignition delay time of boron[33].Based on the above introduction,these materials provide convenience for coating boron powder[34-37].
2.3.The general fabrication method for boron
Different types of materials use different coating methods.Table 1 has shown the experimental steps and application scopes of boron powder coating methods.Coating methods include recrystallization,dual-solvent,phase transfer,electrospinning,gas-phase condensation, etc.Recrystallization, dual-solvent, andelectrospinning methods can be used for the coating of various materials, while phase transfer and gas-phase condensation methods have a narrow application range.At the same time, LiF,PVDF,and AP materials can be coated by different methods.For the same materials,different coating methods will get different effects,which will be analyzed when introducing the microscope images of these materials.In contrast, methods for mixing powders such as mechanical grinding and high-energy ball milling are also listed in Table 1.
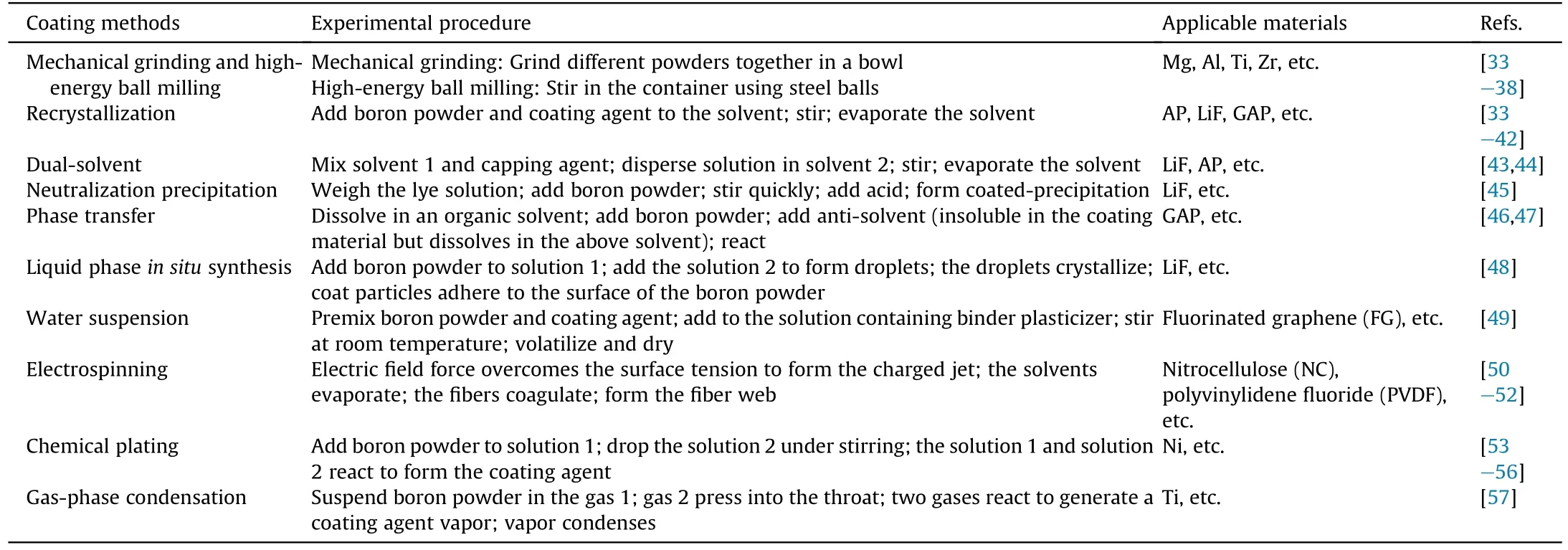
Table 1Experimental steps and application scope of boron powder coating methods.
2.4.Characterization techniques for coated boron powder
Different materials and different coating methods have different effects on boron powder coating, so it is extremely important to characterize the coated material.With the gradual development of characterization technology, more and more instruments have been used to characterize coating materials, and the evaluation methods have been more and more diversified.Table 2 has shown the different instruments used for boron powder coating characterization.It can be seen that SEM,TEM,and AFM can characterize the morphology,particle size,and distribution of the coated boron powder.Among them,SEM has a large magnification and a simple sample preparation,whereas TEM can observe the morphology and dispersion of nanoparticles,and AFM provides a three-dimensional surface map,which will not cause damage to the sample and has a wider range of practicability.In comparison,DSC,TGA,and DTA are used to characterize the thermal properties of the coated boron powder.Among them, DSC can quantitatively measure the heat change during phase transition, whereas TGA can measure the weight change of the material, and DTA can qualitatively measure thermal stability and heat resistance.Different from other instruments, Laser ignition equipment and high-speed camera are used to characterize the ignition and combustion of coated boron powder.Among them, the laser ignition equipment is used to measure the ignition delay time and spectral intensity, and the high-speed camera is used to measure the combustion wave structure and flame propagation velocity.These instruments provide convenience for characterizing coated boron powder.
3.Preparation and performance of coated boron powder
3.1.Fluorine compounds coated boron
3.1.1.Preparationprocedures
The use of fluorine compounds can greatly increase the burn rate of boron with changed oxidation mechanisms [58-60].The fluorine compounds that are widely used for coating boron powder mainly include metal fluorides, fluorinated graphene [61,62], and fluoropolymers.The promising metal fluorides used for coating boron powder are LiF, BiF3, and CoF2.As a typical example, the fabrication of B@BiF3/CoF2could be achieved by using high-energy ball milling[63]and neutralization precipitation methods[64].The preparation procedures for B@LiF [65-70] include neutralization precipitation [71], recrystallization [43,45], dual-solvent [43], and liquid-phasein-situsynthesis methods [47].In these cases, the intimate contacts between the oxide shell of boron and the metal fluorides could be realized,so that the F atom can easily react with oxide film BmOnwith faster mass and heat transfer to form BF3gas.This is so-call a pre-ignition process, which can remove oxide film and decrease the ignition delay and promote the combustion rate of boron powder.
In comparison, the coating process of B@FG is mainlyimplemented byin-siturecrystallization [72].It is quite similar to the preparation of fluoropolymer-coated boron powder.There are several fluoropolymers have been used, including fluorine rubber(Viton A), the co-polymer of tetrafluoroethylenehexafluoropropylene-vinylidene fluoride (THV), polyvinylidene fluoride (PVDF), and polytetrafluoroethylene (PTFE).For instance,the B@Viton A/THV/PVDF has been prepared mainly adopts recrystallization[73-75],whereas the B@PTFE is prepared by highenergy ball milling combined with recrystallization [76-80].

Table 2Instruments used for boron powder coating characterization.
The morphologies of boron powder coated with different fluorine compounds are shown in Fig.1.The higher resolution figure is shown in the supplementary material.It can be seen from Fig.1(a)and 1(b)that B@LiF obtained by the neutralization precipitation has an uneven surface and poor dispersion[45],whereas the surface of B@LiF obtained by recrystallization is relatively fully covered [45].In comparison,LiF particles in Fig.1(c)are overlain on the surface of the boron powder, forming agglomerates with the rough powder surfaces.The median diameter of B@LiF is approximately 10 μm,larger than that of the boron powder(approximately 7 μm)[48].In cases of B@BiF3and B@CoF2shown in Fig.1(d)-1(f), the ones prepared by the neutralization precipitation and the high-energy ball milling methods [64] are relatively more uniform.In Fig.1(e),it can be seen boron particles with dimensions of around 100 nm appear to be coated with a thin fluoride layer, with additional nanometer fluoride particles embedded in somewhat porous agglomerates.The coating appears to be more uniform for B@CoF2(Fig.1(f)), in which most boron particles appear lighter in color because of a thin continuous fluoride surface coating[63].When it comes to FG, as shown in Fig.1(g) and 1(h), it can be seen that a dense layer of FG is on the surface of boron powder.The coating layer on larger amorphous boron powder is thinner and slightly curled,where agglomeration is easy to occur.The composite based on 5 μm scale amorphous boron powder would be assembled in a 2D thin layered structure as graphene but with a wrinkled surface,where the FG is presented and distributed uniformly [72].
3.1.2.Solid-statereactioncharacteristics

Fig.1.The SEM images of various fluorine compounds coated boron powder:(a)B@LiF prepared by neutralization precipitation[45];(b)B@LiF prepared by recrystallization[45];(c)B@LiF prepared by liquid phase in-situ synthesis [48];(d)B@BiF3 prepared by neutralization precipitation [64];(e)B@BiF3 prepared by high-energy ball milling (400 nm)[63];(f)B@CoF2 prepared by high-energy ball milling(1 μm)[63];(g)20 μm amorphous B@FG prepared by recrystallization[72];(h)5 μm amorphous B@FG prepared by recrystallization[72].

Fig.2.(a) TG curves of boron powder coated with different fluorine compounds; (b)DTA,DTG,and DSC curves of boron powder coated with different fluorine compounds.Notes:B@CoF2 and B@BiF3 are heated in oxygen at a heating rate of 5 °C/min;B@LiF is heated in air at a heating rate of 10 °C/min;B@Viton A,B@THV,and B@PVDF are heated in air at a heating rate of 10 °C/min.
The TG,DTA,DTG,and DSC curves of boron powder coated with different fluoride compounds are shown in Fig.2.Before the analysis of Fig.2, it is particularly emphasized that the following analysis is only for materials in the same atmosphere and the same heating rate.Different materials cannot be analyzed due to different atmospheres and heating rates.As shown in Fig.2(a), it can be seen the mass gains of B@CoF2and B@BiF3obtained by mechanical grinding (the mass ratio of metal fluoride to boron is 1:1)[63]are 50.2%and 36.3%,respectively.A slow mass gain begins at about 500°C for B@BiF3,whereas it occurs at around 600°C for B@CoF2.Interestingly, the mass gain proceeds more rapidly for B@CoF2at higher temperatures in comparison to B@BiF3.When it comes to B@LiF, the total mass gains for B@LiF coated by liquid phaseinsitusynthesis (LiF:B = 1.4:10) [48] and neutralization precipitation (LiF:B = 1:10) [81] are 205.6% and 80.6%.In comparison,raw boron powder has a mass gain of 140.6%and 73.9%.It can be seen that different coating methods have a great influence on the thermal weight gain of samples.Moreover, the B@PVDF,B@THV,and B@Viton A(the mass ratio of these polymers to boron is maintained the same as 1:24 prepared by recrystallization)have different mass gains.The mass gain rate of B@PVDF (59 wt% F) is quite low(89.2%),while B@Viton A(66 wt%F)and B@THV(72 wt%F) are 91.2% and 92.9%, respectively.The mass gain is proportional to the fluorine content in these fluoropolymers[74].
In terms of DTA curves(Fig.2(b)),it can be seen the B@CoF2and B@BiF3obtained by the mechanical grinding method (CoF2/BiF3:B=1:1)[63]are slightly different.The initial exothermic slope shape of B@BiF3is shown that a variety of reactions will occur at low temperatures, and the low-temperature reaction of B@CoF2is not easy to distinguish, its main exothermic peak is relatively symmetrical.When it comes to B@LiF, the maximum mass gain rates of B@LiF prepared by liquid phasein-situsynthesis [48] and neutralization precipitation methods[81]are 52.2%/min and 25.6%/min,whereas it is 39.6%/min and 15.5%/min for original boron.This is attributed to the fact that LiF can continuously remove the oxide film BmOnproduced by the oxidation of boron, resulting in an increased oxidation rate.And different methods of coating have different impacts on oxidation rate.Moreover, the DSC curves of B@PVDF, B@THV, and B@Viton A (B:Fluoropolymer = 24:1) fabricated by recrystallization are shown the sharp exothermic peaks in the range of 700-800°C.It can also be measured that the heat release of B@PVDF, B@Viton A, and B@THV is 7.5 kJ/g,7.9 kJ/g,and 8.2 kJ/g, respectively.Compared to B@PVDF, the heats of reaction from B@Viton A and B@THV are higher,which is consistent with the increasing trend for the weight gain of the respective powders[74].
3.1.3.Ignitionandcombustioncharacteristics
The ignition processes of boron powder coated with different fluorine compounds are recorded by a high-speed camera as shown in Fig.3.It has shown in Fig.3(a)that the characteristic luminescent particles and diffused incandescence of B@BiF3and B@CoF2are shown once they are ignited in the air-acetylene flame.Under the halo of incandescent gas, B@BiF3presents relatively short green stripes, indicating that gasification occurs.The ignition temperature of B@BiF3is lower than that of B@CoF2because the formation of CoB would delay the ignition of CoF2composites[82].Moreover,the flame photos of B@BiF3and B@CoF2upon ignition by a hot filament(electric ignition wire ignition)are shown in Fig.3(d)and 3(e).If the temperature of the filament continues to increase, the burning of B@CoF2generates a bright cloud, whereas the B@BiF3produces a cloud of smoke[63].Based on the thermocouple data,it is found that the ignition temperature of B@BiF3is lower,since the formation of BF3and BOF may effectively accelerate the ignition reaction [83].
In comparison,the flame images of ignited boron powder mixed with different contents of PTFE in the air(laser ignition)are shown in Fig.3(b), where Fig.3(b1) corresponds to a sample with 7.5%PTFE prepared by mechanical grinding,Fig.3(b2)for that with 15%PTFE prepared by high-energy ball milling, Fig.3(b3) for that with 30% PTFE prepared by mechanical grinding, and Fig.3(b4) for that with 30% PTFE prepared by high-energy ball milling.It is easy to distinguish the contour of the flame on the top of the ball-milled particle, whereas the flame radiation of the samples prepared by a simple mechanical grinding method is pretty much weaker,indicating that the reactivity could be largely increased after highenergy ball milling [77].Fig.3(c) has shown the flame photos of propellants containing Fig.3(c1) B@LiF and Fig.3(c2) B@Viton A composites under an ambient atmosphere.Herein,the propellants are composed of AP, 37% of boron coating material, and 8% of Mg-Al alloy.It can be clearly observed that B@LiF containing propellant emits more incandescent particles than B@Viton A propellant due to more intensive combustion [49].
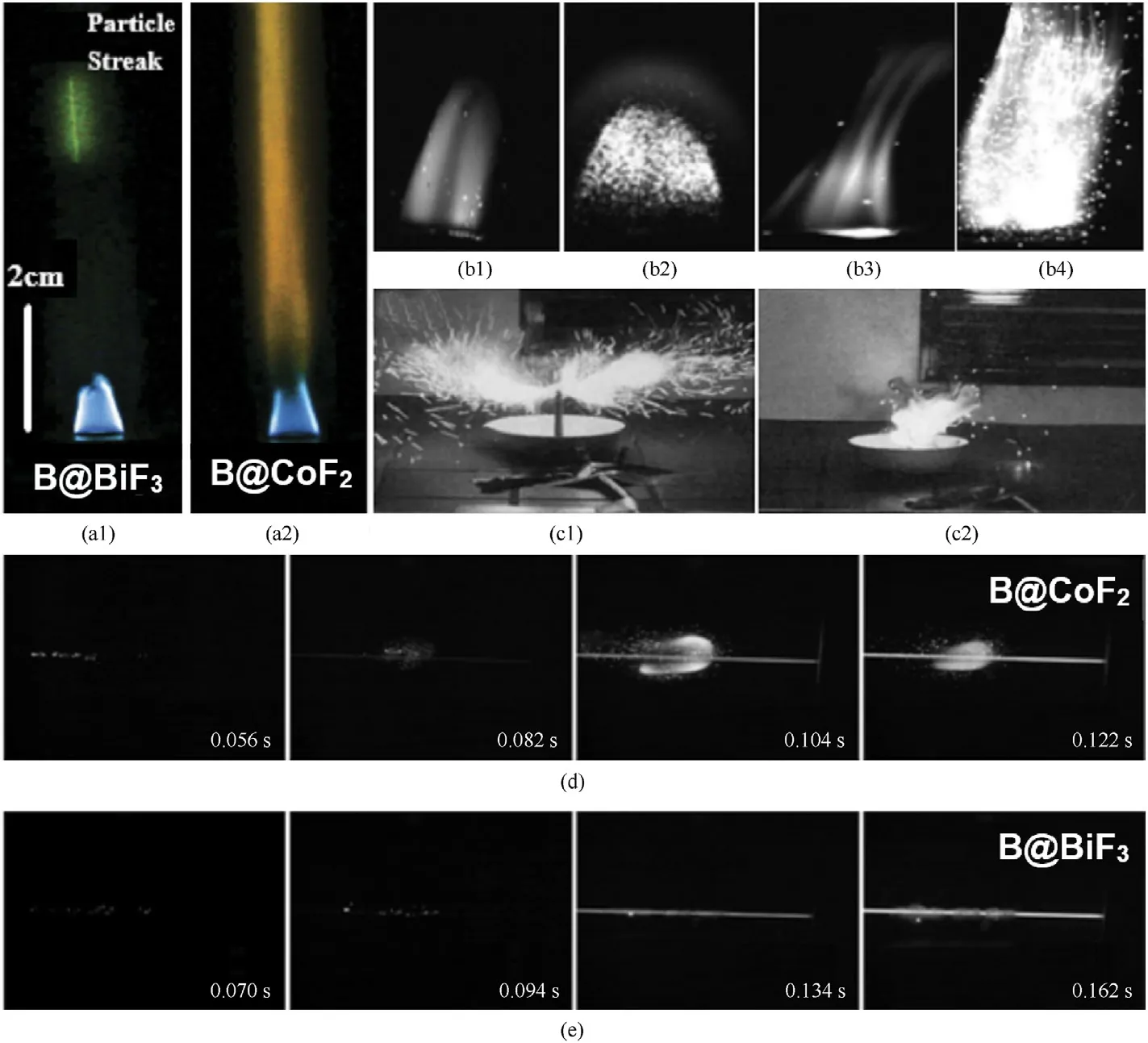
Fig.3.The flame photos of various fluorine-coated boron powders: (a) Characteristic luminescent particle streaks and diffused incandescence of B@BiF3 and B@CoF2 [82]; (b)Ignition and combustion process of boron after mixed with different contents of PTFE[77];(c)Flame photos of B@LiF and B@Viton A propellants[49];(d)Ignition process of B@BiF3 on heated filaments [63]; (e) Ignition process of B@CoF2 on hot filaments [63].
In addition to exploring the improvement of ignition and combustion of boron powder after being coated with fluoride, some works of literature also explored the effect of fluoride coated with B/Al and B/Mg.For B/Al/PVDF composites, PVDF can remove the oxide layer on the surface of aluminium and boron.The combination of PVDF and aluminium promoted the oxidation reaction of boron, which resulted in more heat release at high temperatures[84].For B/Al/FG composites, Al and FG coatings can significantly improve the reaction heat, ignition delay time, and combustion reaction of the material.Compared with Al,FG is more conducive to improving the combustion reaction and ignition performance of boron [85].Similar to B/Al/PVDF and B/Al/FG, boron in B/Mg/PTFE can increase the combustion rate, combustion temperature, and combustion calorific value [86,87].
In addition to the materials mentioned above, fluorine compounds(such as LiF,PVDF,Viton A,and THV)can also improve the ignition and combustion characteristic parameters.In a summary,it can be seen in Table 3 that coating of LiF can increase the combustion heat(experiment)and reduce the ignition delay time (the time from the beginning to the appearance of an open fire by using the electric heating wire) of boron powder.Moreover, LiF can also improve the combustion heat (experiment), detonation heat(experiment), combustion efficiency (boron powder combustion product quality/original sample quality), and burning rate (strip length/time required for the burning of the strip) of the corresponding CPs.When it comes to FG,it can improve the combustion heat (experiment)of boron powder.Fluoropolymer PVDF,Viton A,and THV can all increase the maximum flame temperature (emission spectra from tablet burning are recorded by a spectrometer and fitted using the Planck formula to obtain the temperature) of boron combustion,where the impact of THV is the most significant.
3.2.Energetic composites coated boron
3.2.1.Fabricationtechniques
Energetic composites suitable for coating boron powder mainly include energetic binders (GAP, NC, etc.), metal azide (e.g., NaN3),and oxidizers such as AP, KP, KNO3, etc.As typical examples, GAP would generate a lot of heat upon ignition heating the boron powder to a very high temperature [92-94], and also make the propellant show lower apparent activation energy, higher explosion heat value, and higher combustion rate [95].Whereas the coating with NC can help in improving the mechanical properties and combustion performance of boron-containing propellants.In comparison, the NaN3would decompose to Na2O, which can interact with oxide film BmOnto reduce the viscosity of boron powder [33].For the above reasons, boron powder can be coated with GAP,NC,and NaN3.The preparation method of B@GAP usually includes recrystallization and phase transfer [40], whereas the B@NC could be obtained by electrospinning[50,51].The fabrication of B@NaN3can be achieved by using phase transfer [47].
The principle of AP and KP is similar to that of GAP[96-99].The preparation procedures for B@AP and B@KP include recrystallization [21,22], solvent/non-solvent [100], and dual-solvent methods[20].As an improvement,lithium perchlorate(LiP)can increase the oxygen balance in comparison to AP[101].In this context,B@AP/LiP prepared by recrystallization method with AP and a minor LiP as the composite oxidizer would have a better performance [102].In addition, some other oxidizers such as KNO3[103], ammonium nitrate (AN), 2-nitroguanidine (NQ), and 1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) [104,105] can also be used as the coating agent of boron powder,and the corresponding products such as B@KNO3and B@HMX could be prepared by recrystallization method [21].
The morphologies of boron powder coated with different energetic composites are shown in Fig.4.The higher resolution figure is shown in the supplementary material.It is found that the B@GAP sample prepared by recrystallization (Fig.4(a)) is shown as large spherical particles with good fluidization behavior [106].In comparison, the thickness of the GAP coating layer prepared by phase transfer is not uniform(Fig.4(d))[46].When it comes to NC,it can be seen from Fig.4(b)that the B@NC nanofibers are not uniform in thickness and the boron particles inside the fibers are severely dispersed.It can be seen from Fig.4(c)that the dispersion of boron powder in B@NC/KH-550 nanofibers is more uniform than that in Fig.4(b), and the agglomeration is less, which indicates that KH-550 improves the dispersion of boron powder in B @NC nanofibers [50].
AP is the most widely used oxidizer in the solid propellant, it would be great if AP could combine with boron homogeneously.It has been found that AP could be tightly packed with boron powder in B@AP composites(Fig.4(e))prepared by recrystallization[15].In case of B@AP/LiP,it can be seen that the coated crystals are mainly in irregular shape,but AP and LiP are bounded tightly on the surface of boron, and the crystalline morphologies are improved (Fig.4(f)and 4(g)) [102].
3.2.2.Thermaldecompositionbehavior
The TG and DSC curves of boron powder coated with different energetic composites are shown in Fig.5.Before the analysis of Fig.5, it is particularly emphasized that the following analysis is only for materials in the same atmosphere and at the same heating rate.Different materials cannot be analyzed due to different atmospheres and heating rates.The TG curves of boron powders coated with different energetic composites are shown in Fig.5(a).It can be seen that B@GAP (B:GAP = 1:0.4) obtained by the phase transfer begin to decompose at a low temperature, whereas the mass loss of the TG curve is 27.92% [46].When it comes to B@NC,the B@NC and B@NC/KH-550 (B:NC = 1:5) prepared byelectrospinning start to lose weight from 160°C and end at 250°C,and then increase significantly due to the oxidation reaction of boron powder.In these processes, the weight gain of B@NC is 18.82%,and that of B@NC/KH-550 is 26.58%.It is indicated that the dispersion of B@NC/KH-550 is better than B@NC, and the reaction between boron powder and air is deepened [50].Moreover, the B@AP fabricated by dual-solvent(AP:B=1:10) is similar to that of the original boron,and the weight gain(90.85%)is slightly reduced relative to the original boron (109.59%), this phenomenon may be related to AP decomposition [37].

Table 3Ignition and combustion performance of boron powder coated with fluorine compounds.
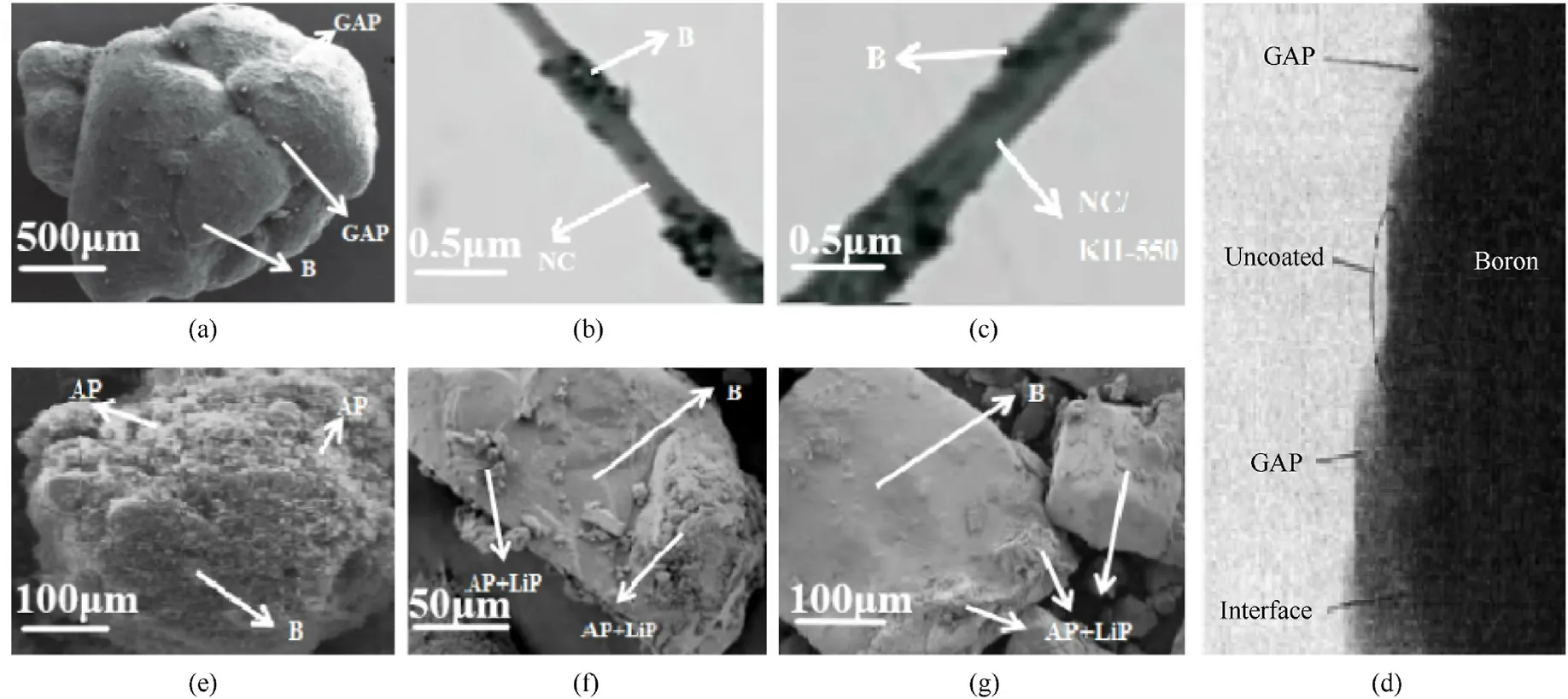
Fig.4.The SEM or TEM images of various energetic composites coated boron powder:(a)B@GAP prepared by recrystallization[106];(b)B@NC prepared by electrospinning[50];(c)B@NC modified by coupling agent KH-550 prepared by electrospinning[50];(d)B@GAP prepared by phase transfer[46];(e)B@AP prepared by recrystallization [15];(f) B@AP/LiP prepared by recrystallization at 60 °C [102]; (g) B@AP/LiP prepared by recrystallization at 80 °C [102].
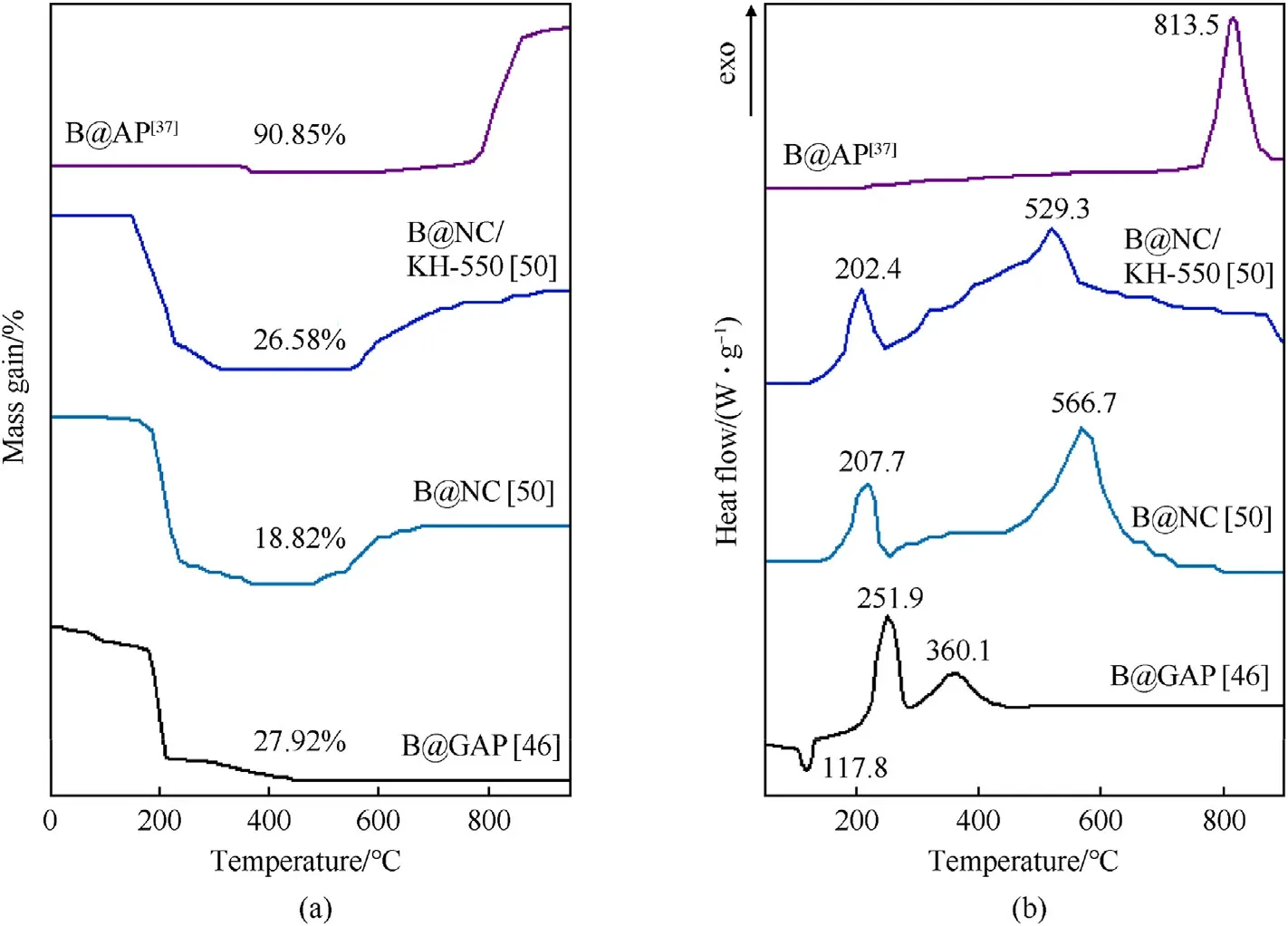
Fig.5.(a) TG curves of boron powder coated with different energetic composites; (b) DSC curves of boron powder coated with different energetic composites.
The results of the DSC curves are shown in Fig.5(b).It can be seen the B@GAP (B:GAP = 1:0.4) obtained by phase transfer has a strong exothermic peak at about 252°C,which corresponds to the process of GAP decomposition to generate water vapor.Interestingly,there is another exothermic peak near 360°C,which refers to the reaction between water vapor generated by GAP decomposition and BmOnon the surface of boron powder [46].When it comes to B@NC, the initial exothermic peaks of B@NC and B@NC/KH-550(B:NC = 1:5) prepared by electrospinning appear at about 200°C,and the top exothermic peaks of them are located at 566.7°C and 529.3°C.This is attributed to the fact that with the temperature increases, the boron powder oxidizes, whereas the contact area between air and B@NC/KH-550 is larger than that of B@NC, so the reaction of B@NC/KH-550 is faster [50].Moreover, the B@AP fabricated by the dual-solvent method(AP:B=1:10)could increase the boron exothermic peak temperature (809.9-813.5°C) and reduce the heat release of boron (3600-2199 J/g), which is proposed that the decomposition products of AP could react with boron to increase the thickness of oxide film BmOnon the boron surface, this is not conducive to boron powder coating [37].
3.2.3.Ignitionandcombustionperformances
The effects of azide-containing substances and energetic oxidants on the ignition and combustion performance of boron powder are different.Fig.6 are shown the ignition and combustion performance of the typical azide-containing substance GAP and some energetic oxidants (such as AP, AN, NQ, HMX) containing propellants.As shown in Fig.6(a)-6(c),the left image of each figure is the B@GAP ignition moment, and the right image is the B@GAP combustion process (laser ignition, high speed camera shooting).Different from the combustion processes of samples a and b,sample c with the 30% GAP content has shown a better dispersion in space.It has been observed that the boron powder has a better reaction with oxygen, so it can be burned out quickly in a short time.Moreover, it can be seen with the increase of GAP content from 10%to 30%,the average ignition delay time(the time from the beginning to the appearance of the characteristic signal peak using the laser ignition instrument)of B@GAP is shortened from 100.7 to 45.1 ms(Fig.6(d)),indicating that the higher GAP content,the more heat release from decomposition,and the faster the heating rate of boron particles in the sample.Therefore, it results in faster evaporation rate of oxide film on the boron surface during combustion,which reduces the ignition delay time of boron [106].When it comes to Fig.6(e)-6(h),it is found that there are some differences in the spectral shapes of the four samples (laser ignition,spectrometer collects spectrum).The spectra of AP and AN samples show an obvious increasing process to the maximum, and the spectral time span is large.The NQ and HMX samples reach the maximum value quickly after the spectra appear,and the maximum spectral intensity is higher than that of AP and AN samples [17].
In a summary, the ignition and combustion characteristic parameters of boron powder coated with different azide containing substances(such as GAP,NaN3)and energetic oxidants(such as AP,KP) are shown in Table 4.It can be seen that coating of GAP can increase the combustion heat (experiment) and shorten the ignition temperature (the temperature from the beginning to the appearance of an open fire by using the electric heating wire) of boron powder.In comparison,AP can increase the combustion heat(experiment) and detonation heat (experiment), and reduce the ignition delay time(the time from the beginning to the appearance of the characteristic signal peak using the laser ignition instrument)of the corresponding propellant.Similar to GAP and AP, NaN3, and KP can also reduce the ignition temperature(the same as above)of boron powder.Moreover, NQ containing propellant is the most beneficial for improving the peak burning intensity (strongest characteristic peaks by laser ignition instrument), whereas HMX can improve the burning time(the time required for the sample to start emitting light until the bright light disappears) of the corresponding propellant, and AN can decrease average combustion temperature(arithmetic mean value of temperature data within 3 s of laser ignition and combustion).
3.3.Metal fuels and metal oxides coated boron
3.3.1.Preparationmethods
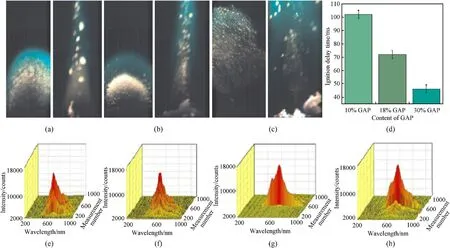
Fig.6.(a)Ignition and combustion process of 10%B@GAP[106];(b)Ignition and combustion process of 18%B@GAP[106];(c)Ignition and combustion process of 30%B@GAP[106];(d) Ignition delay time of different B@GAP samples [106]; (e) Three-dimensional spectra of the ignition and combustion process of B@AP propellant [17]; (f) Three-dimensional spectra of the ignition and combustion process of B@AN propellant [17]; (g) Three-dimensional spectra of the ignition and combustion process of B@NQ propellant [17]; (h)Three-dimensional spectra of the ignition and combustion process of B@HMX propellant [17].

Table 4Ignition and combustion performance of boron powder coated with energetic composites.
Metal fuels (Mg, Al, Ni, Mo, Fe, Ti, etc.) and metal oxides (NiO,CuO,Bi2O3,Co3O4,Fe2O3,MoO3,CeO2,etc.)are suitable for coating boron powder.As typical examples,metal combustible agents such as Mg and Al can improve the oxidation degree, reduce the oxidation reaction temperature,and effectively shorten the ignition time of boron powder[109,110].In addition,transition metals such as Ni, Mo, and Fe have higher catalytic activity than their oxides[111-113].They can improve the burning rate and enhance the energy of boron-containing propellants.For the above reasons,these metals can be used to coat boron powder.For instance, the preparation method of B@Mg would be divided into mechanical grinding[114]and continuous diffusion flow[54,115],whereas the B@Al could be obtained by mechanical grinding [33] and binder coating [45].The fabrication of B@Ni [56,116-118] would be prepared by high-energy ball milling and chemical plating, and the B@Mo and B@Fe could be obtained by high-energy ball milling[117,119].Different from the above metal fuels, the exothermic reaction of Ti and boron is a new idea for boron combustion enhancement in rockets and ramjets.The preparation procedure for B@Ti mainly includes gas-phase condensation [120].
In comparison, metal oxides (NiO, CuO, Bi2O3, Co3O4, Fe2O3,MoO3, CeO2, etc.) can promote the contact between oxygen and boron and reduce the ignition delay time of boron[121-124].Due to their excellent properties,they can be used to coat boron powder.The fabrication strategies of B@Bi2O3/Co3O4/Fe2O3/MoO3would be obtained by mechanical grinding,whereas the B@NiO/CuO could be achieved by surface chemical reactions [125,126] and mechanical grinding, and the preparation method of B@CeO2mainly includes high-energy ball milling [127-129].
The microscopic images of boron powder coated with different metal fuels and metal oxides are shown in Fig.7.The higher resolution figure is shown in the supplementary material.It is found that B@Mg prepared by mechanical grinding includes large boron powder and irregular Mg particles.Mg particles are relatively uniformly bound on the surface of the boron powder(Fig.7(a))[33].When it comes to Al, different coating materials are obtained by using different methods (Fig.7(b) to 7(e)).After the composite of 80/200 nm Al powder and boron powder prepared by mechanical grinding,it can be seen the distribution of Al is uniform,and the Al powder is composited on the surface of boron powder(Fig.7(b)and 7(c)) [33].In addition, Al powder in B@Al obtained by mechanical alloying is uniform and firm than recrystallization (Fig.7(d) and 7(e)) [45].In comparison, the micro-graph of B@Metal oxide is different from that of B@Metal.When it comes to B@Bi2O3/Co3O4/Fe2O3/MoO3obtained by ultrasonic mixing (Fig.7(f)-7(j)), it is demonstrated that the boron powder in these samples is an irregular sphere with submicron size,and all metal oxide particles have at least one characteristic size less than 200 nm interestingly,but they do have different morphologies.The B@Metal oxides also exhibit distinct morphologies and degrees of homogeneity [130].
3.3.2.Condensedphasereactions
TG and DSC results of boron powder coated with different metal fuels and metal oxides are shown in Fig.8.Before the analysis of Fig.8, it is particularly emphasized that the following analysis is only for materials in the same atmosphere and at the same heating rate.Different materials cannot be analyzed due to different atmospheres and heating rates.It can be seen that among the prepared B@Metal(Fig.8(a)and 8(b)),the peak temperature of B@Al is the highest, which is 16.2°C higher than that of boron, and its oxidation gain is reduced by 14.3%.When it comes to B@Mg and B@Ni, their exothermic peak temperatures of them change little.Moreover, the exothermic peak temperatures of B@Fe and B@Mo are decreased by 11.0°C and 11.1°C, and the mass gains are increased by 11.1%and 4.0%,the oxidation degree of boron powder in B@Mo and B@Fe are improved [117].
The oxidation effect of boron powder is different in various B@Metal oxides coated by mechanical grinding(Fig.8(c)and 8(d)).In this experiment,5 mg of each sample(boron:metal oxide=20:1)is heated at 20°C/min.As an initial attempt, MgO has no obvious oxidation change in boron powder, and Al2O3can inhibit the oxidation reaction at 875-1000°C.Different from MgO and Al2O3,SnO2has a good effect on the oxidation of boron.The TG curve for the B@SnO2sample shows that boron oxidation begins at 720°C,and the DSC curve shows that the maximum exothermic peak appears at 750°C.Fe2O3can also catalyze the oxidation of boron powder,but its activity is lower than Bi2O3.As the most outstanding material, Bi2O3has the highest oxidation activity among all metal oxides.In its DSC curve, the maximum exothermic peak temperature of B@Bi2O3is 707°C(whereas boron is 811°C),indicating that Bi2O3would make the maximum exothermic peak temperature decrease by 104°C.On the contrary, CeO2and CuO could not promote the oxidation of boron powder,and the maximum exothermic peaks in their DSC curves change little [121].
In addition to the oxidation activity of different metal oxides on boron powder, it can also be found that the thermal reaction conversion of boron powder in each sample is about 50%, indicating that only about half of the boron powder reacts below 1000°C,and the metal oxides have no obvious effect on the extent of conversion of boron powder [122] (Fig.8(c)).
3.3.3.Ignitionandcombustioncharacteristics

Fig.7.The SEM images of various metal fuels and metal oxides coated boron powder:(a) B@Mg prepared by mechanical grinding [33]; (b) B@Al (80 nm) prepared by mechanical grinding [33]; (c) B@Al (200 nm) prepared by mechanical grinding [33]; (d) B@Al prepared by recrystallization [45]; (e) B@Al prepared by mechanical alloying [45]; (f) B@CuO prepared by ultrasonic mixing[130];(g)B@Bi2O3 prepared by ultrasonic mixing[130];(h)B@Co3O4 prepared by ultrasonic mixing[130];(i)B@Fe2O3 prepared by ultrasonic mixing[130]; (j) B@MoO3 prepared by ultrasonic mixing [130].
The ignition and combustion processes of boron powder coated with different metal fuels and metal oxides are recorded by a highspeed camera as shown in Fig.9.Under the heating of 68 W/cm2radiant flux, the ignition and burning period of B@Al, B@Ti, B@Ni,and B@Fe samples prepared by mechanical grinding in the air(laser ignition)are about 100 ms,and the maximum flame front length of these samples are in the range of 270-300 mm (Fig.9(a)-9(d)).It can be observed that the high activity of metal nanoparticles,fragmentation of Ti particles,and the catalytic interaction of Fe and Ni oxides with AP crystals in the reaction layer of the samples would increase the rate of outflow of decomposition products from the surface of the condensed phase [119].When it comes to the ignition processes and flame images of B@Co3O4@AP, B@CuO@AP,and B@Bi2O3@AP coated by solvent evaporation-induced self-assembly on the ignition wire in the air (Fig.9(e)-9(g)), their precombustion is pretty quick with a very high burning rate, and the combustion of these solid particles is complete without obvious residue.Among them, B@CuO@AP has the shortest ignition delay time(280±12 ms),whereas B@Co3O4@AP has the shortest burning time (183±15 ms), and B@Bi2O3@AP shows intensive combustion reactions, of which the ignition delay time (the time from the beginning to the appearance of an open fire by using the ignition wire)and burning time(the time required for the sample to start to emit flame until the flame disappears) are 430±26 ms and 188±10 ms, respectively [131].The above experimental phenomenon demonstrated that the MxOy(M means Co, Cu, and Bi) can largely decrease the oxidation temperature of boron due to the high reactivity of involved thermite reactions, where AP can directly interact with the active boron upon ignition with shorter mass transfer distance,and thus greatly enhancing its reaction efficiency.Moreover, B@Bi2O3@AP shows the highest combustion heat(experiment), detonation heat (experiment), and highest combustion temperature, B@Bi2O3@AP is the outstanding candidate for obtaining the boron-based energetic material with high-energy output performance.
In a summary, the ignition and combustion characteristic parameters of boron powder coated with different metal fuels (such as Mg, Al) and metal oxides (such as MgO, Al2O3) are shown in Table 5.It can be seen that pure metal fuels such as Mg,Al,Mo,Fe,and Ni can improve the burning rate (the target line records the time required for a certain length of the propellant strip to burn)and average pressurization rate(the maximum pressure reached by combustionPm/time required from minimum pressure to maximum pressuretm) of the corresponding composite propellants.Among them,Fe has the most significant improvement in these parameters.Moreover, metal oxides such as Bi2O3, CuO,MoO3,Fe2O3,and SnO2can reduce the ignition time(the time from the beginning to the appearance of the characteristic signal peak using the laser ignition instrument)of boron powder,of which the Bi2O3is the most effective one.In addition, the pure metal Ni can increase the thermal conductivity (measurement of the circular sample with thermal constant analyzer)of the sample,whereas the metal oxides would have a strong catalytic effect on the decomposition of the organic oxidizer so that the corresponding composite propellants have higher burning rates.
4.Summary and perspectives
This paper reviews the fabrication and characterization of multiscale coated boron powders with improved combustion performance.The coating agents suitable for boron powder modification include fluorine compounds such as BiF3, CoF2, and LiF, energetic composites including GAP, AP, and AP/LiP, metal fuels such as Fe,and metal oxides such as Bi2O3.By coating boron powder, these materials can improve the thermal properties of boron powder and improve the combustion performance of the corresponding CPs.At present, there are great challenges in the modification of boron powder.The research ideas at present mainly include the following aspects.
(1) In case of pretreatment of boron powder.The key factor affecting the ignition and combustion of boron powder is the oxide film impurities on the surface of boron powder.However, most of the reports on the modification of boron powder have not treated the original boron powder.Section 2.1 of this article has shown that some solvents such as acetonitrile and sodium hydroxide are beneficial to removing oxide film impurities.In the future,the pretreatment process can be improved and new pretreatment solvents can be explored.
(2) Improve the boron powder coating techniques.Nowadays,the preparation of boron powder coating materials mainly includes recrystallization method and so on (As shown in Table 1).These methods are not accurate for interface structure control and difficult to achieve a uniform coating.Finding a coating method that can more accurately control the coating material is crucial.

Fig.8.(a) TG curves of boron powder coated with different metal powders;(b) DSC curves of boron powder coated with different metal powders;(c) TG curves of boron powder coated with different metal oxides; (d) DSC curves of boron powder coated with different metal oxides.Note: the data in Fig.8(c) refers to the reaction conversion rate of weight gain after metal oxide coated with boron powder.
(3) Looking for suitable coating agents.At present, the coating agents suitable for coating boron powder include fluorine compounds, energetic composites, metal fuels, and metal oxides.About the fluorine compounds, in addition to the materials reported in the literature such as LiF and PVDF,polymers such as PFA (Perfluoroalkoxy polymer) and FEP(Fluorinated thylene-propylene polymer) and fluorine acids such as PFNA (Perfluorononanoic acid) and PFUDA (Perfluoroundecanoic acid) may improve the combustion behavior of boron powder.And inspired by metal fluoride and metal oxide,some agents include metal hydrides such as MgH2and TiH2, and alkali metal perchlorate/periodate such as LiClO4and LiIO3could be considered.At the same time,coating boron powder with a variety of different types of materials may get more excellent results.
From a long-term perspective, the future research direction of boron powder coating should focus on: (a) On experimental research,continue to optimize the preparation conditions of boron powder coating, explore novel coating agents, and research the effects of various coating agents co-coated boron powder; (b) On numerical simulation, use software to study the influence of the coating on the generation and evolution mechanism of BmOnon the boron powder surface;(c)On weapon applications,coordinate the development of coated boron powder and fuel-rich propellants,and research the suitable oxidant and binder for coated boron powder to assist weapon equipment innovation.

Fig.9.The images of ignition and combustion processes of (a) B@Al [119]; (b) B@Ti [119]; (c) B@Ni [119]; (d) B@Fe [119]; (e) B@Co3O4@AP [131]; (f) B@CuO@AP [131]; (g)B@Bi2O3@AP [131].
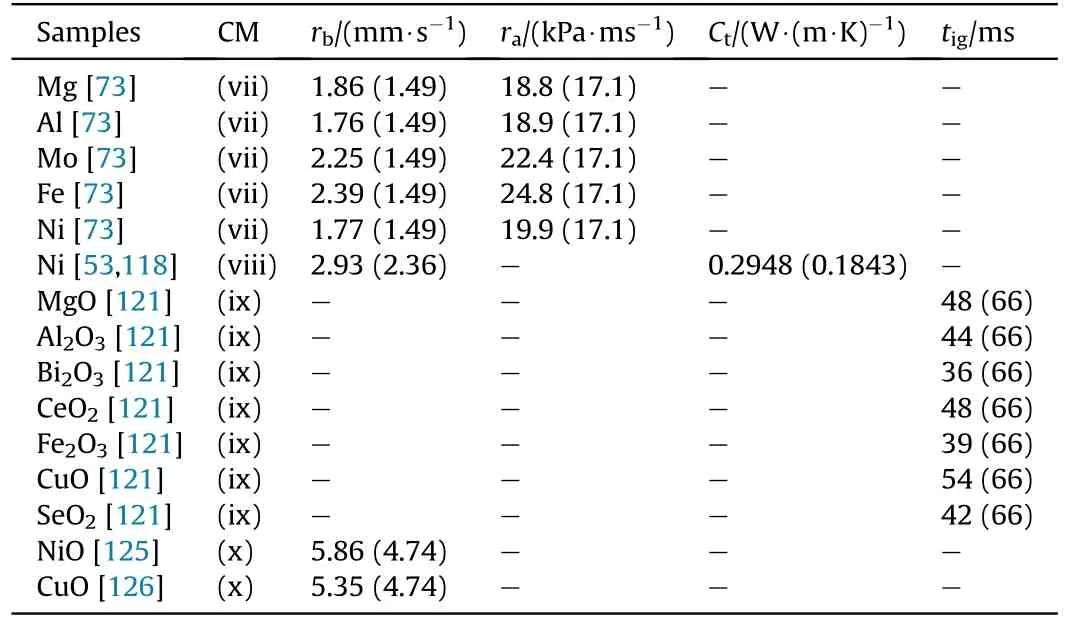
Table 5Ignition and combustion performances of boron powder coated with various metalbased materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This paper has been funded by Shaanxi Provincial Key Research and Development Program of China(Grant No.2021ZDLGY11)and partially supported by NSAF Project of China(Grant No.U2030202).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.03.015.
- Defence Technology的其它文章
- The interaction between a shaped charge jet and a single moving plate
- Machine learning for predicting the outcome of terminal ballistics events
- Experimental research on the launching system of auxiliary charge with filter cartridge structure
- Dependence of impact regime boundaries on the initial temperatures of projectiles and targets
- Experimental and numerical study of hypervelocity impact damage on composite overwrapped pressure vessels
- On the effect of pitch and yaw angles in oblique impacts of smallcaliber projectiles

