Mesoporous Silica with Different Morphology Synthesized by Self-assembling of Lipid Molecule
JIN Hai-ying(School of Environmental Engineering, Shanghai Second Polytechnic University, Shanghai 201209, P. R. China)
Mesoporous Silica with Different Morphology Synthesized by Self-assembling of Lipid Molecule
JIN Hai-ying
(School of Environmental Engineering, Shanghai Second Polytechnic University, Shanghai 201209, P. R. China)
This article describes one approach based upon the molecular self-assembly of lipids (N-acyl amino acid) to form various mesoporous microstructured silicas, together with a general approach for the structural analysis by electron microscopy. It has been found that,(1) the microstructure of the mesoporous silicas has been changed from helical ribbon (HR) analogous to folded proteins (Protein α/β helix), hollow sphere, disk-like and chiral hexagonal rod with increasing synthesis temperature from 0 to 10, 15 and to 20 °C; (2) the ammonium group functionalized disordered mesopores in the wall of HR and hollow sphere, and highly ordered straight and chiral 2d-hexagonal mesopores in the disk-like and helical rod have been observed, respectively; (3) the helical mesoporous ribbon silica was exclusively left-handed and the 2d-hexagonal chiral mesoporous silica was right-handed excess when l form N-acyl aminoacid has been used as lipid template. Findings could lead to a new insight into the formation of chiral materials at the molecular level and would facilitate a more efficient and systematic approach to generating rationalized chiral libraries.
helical ribbon; vesicle; chiral; mesoporous silica; lamellar; 2d-hexagonal
0 Introduction
It is well known that self-assembly is used to construct microstructure for functioning of living organisms. Living systems are constructed through the hierarchal assembly of these microstructures on many different size scales. It is essential to mimic biomolecular self-assembly techniques to design, engineer, and fabricate inorganic nano- and microstructures for the development of advanced materials such as sensors, catalysts and separation media[1].
Carbohydrate amphiphiles and phospholipids are basic building blocks of biomembranes in living systems. In the same fundamentals, in aqueous media, the lipid molecules self-assemble into diverse aggregate microstructured morphologies, depending on the molecular shape and solution condition such as lipid concentration, electrolyte concentration, pH, and temperature[2]. Among these morpholopies, vesicles[3], ultra-thin memberanes[4], nano-tubes[5], helical ribbons[5], helical tubes[6]and others[7]have been utilized as template to create nano-sized and microstructured inorganic materials. More advanced materials, techniques and fabrication mechanism must be developed to render these self-assembled nano- and microstructure inorganics.
Recently, we discovered a templating route for preparing well-ordered mesoporous silica based on the self-assembly of anionic surfactants and inorganic precursors in the presence of aminosilane or quaternary aminosilane as a co-structure-directing agent (CSDA)[8], which opened up a new possibility for the synthesis of mesoporous materials using lipid anionic amphiphilic molecules. In our previous work, we have synthesized 2d-hexagonally ordered chiral mesoporous silica by using N-miristoyl-L-alanine sodium salt (C14-L-AlaS) partially neutralized in HCl/C14-L-AlaS=0.08-0.10 at room temperature and 0oC, with an aminosilane or a quaternized aminosilane as a CSDA[10-11]. Here, we approached to synthesize the various microstructured mesoporous silicas by controlling the N-acyl aminoacid (N-AAA) packing parameters upon varying synthesis temperature. It is well knownthat many amphiphilic molecules spontaneously self-assemble to produce vesicles, ribbons and tubes with bi-/multi-layerd structures upon cooling, as reported by Clark, Schnur and Shimizu et al.[1,2,10]Surfactants such as amino acids derived amphiphilic lipid molecules self-organize into micelles of various shapes at the equilibrium state. The shape of the micelle has been quantified by the packing parameter, g = V/a0l, where V is the volume of the hydrophobic chain, a0the effective head group area per hydrophilic head group, and l the critical hydrophobic chain length. If g <1/3, the amphiphile has a tendency to form spherical micelles; if 1/3< g <1/2, cylindrical micelles will be favored; if 1/2< g <1, bilayers with a spontaneous curvature (vesicles) are produced; if g =1, planar bilayers will be favored[2]. In particular, it has been known that the packing parameter of the surfactant is increased when the temperature decreased, which leads to the formation of aggregate morphologies including globules, multi-, and single-walled vesicles, rods, tubes and disks with curved and planner bilayers[11-12]. On cooling, many types helical tubes and rods and twisted ribbons and rolled-up sheets have been obtained in aqueous gels by self organization of appropriate spherical or sheet-like bilayers made of lipid molecules[2,13]. Therefore, our approach is based on controlling self-assembled microstructures formed from partially neutralized carboxylate surfactants upon cooling the synthesis system from higher temperature. When partially neutralized anionic N-AAA, CSDA (3-aminopropyltriethoxysilane (APES)) and silica source tetraethoxylsilane (TEOS) are mixed, the mesoporous silica can be formed through electrostatic interaction between the headgroup of surfactant and amino group of APES, and the co-condensation of silane side of APES and TEOS[10]. The temperature of the reaction system is an important factor in determining the shape of micelle because the structure of surfactants are strongly dependent on temperature and the kinetics of the hydrolysis of TEOS followed by condensation/polymerization is also highly dependent on temperature. As the mixture gel was cooled, the stereo-repulsion between the head-groups decreases, which leads to a decrease in the effective head-group area and results in increasing g parameter of micelle and resulted in a transition from cylindrical to bilayered micelles. The HR with bilayer structures have been expected to be formed under lower temperature, and other highly ordered mesoporous sililcas have been expected to be formed at higher temperature, respectively. The consequent formation of mesostructure by re-self-assembly of lipid microstructures, CSDA and silica source, and by the cooperatively liquid crystal templating route can be estimated, respectively.
Here we have investigated that the temperature produces a dominating effect on the mesoporous microstructures prepared with N-AAA in detail. To observe the structural evolution of the mesophases during synthesis, scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM) images and X-ray diffraction (XRD) patterns of products were measured as a function of the reaction time, which presented visible evidence for the mesostructural transformation. The temperature and reaction time dependences of microstructures and mesostructures were analyzed by XRD patterns and SEM images to discuss the possible synthesis process in various micro and mesostructure formations. L- and D-forms antipodal chiral lipids, racemic and achiral lipids have been used for comparing the difference of microstructural formation behavior.
1 Experimental Section
Materials: C14-D-AlaS were synthesized according to the previous reports[16-17]. NaOH and HCl were purchased from Shanghai Chemical Company. Tetraethoxylsilane (TEOS) were purchased from TCI and 3-aminopropyltriethoxysilane (APES, 98 %) from Azmax. All chemicals were used as received without further purification.
Synthesis: Different microstructured mesoporous silica was prepared by means of chiral anionic amphiphilic lipid molecule C14- D-AlaS as template and APES as co-structure directing agent. In a typical synthesis, C14-D-AlaS (0.32 g, 1mmol) was dissolved in deionized water (17.1 g) with stirring at room temperature. 0.01 M HCl (10 ml) was added to the above solution under vigorous stirring at room temperature to partially neutralize the salt to produce amino acid. Then, a mixture of TEOS (1.46 g, 7 mmol) and APES was added to the mixture with stirring at 0oC, 10oC, 15oC and 20oC. After 10 more min stirring, the resulted material was aged at 0oC, 10oC, 15oC and 20oC fordesired times to get the final products. The products were recovered by centrifugal separation and dried at different temperature. lipid molecule was removed by exhaustive solid-liquid extraction overnight using HCl (1M) in ethanol or calcination under 550oC.
Characterization: Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku X-ray diffractometer D/MAX-2 200/PC equipped with Cu Kα radiation (40 kV, 20 mA) at the rate of 0.1 deg/min over the range of 1o~6o(2θ). High-resolution transmission electron microscopy (HRTEM) was performed with a JEOL JEM-3 010 microscope operating at 300 kV (Cs = 0.6 mm, resolution 1.7 nm). The microscopic features of the sample were observed with SEM (JEOL JSM-7 401F). Brunauer-Emmett-Teller (BET) method and the pore size was obtained from the maxima of the pore size distribution curve calculated by the Barrett-Joyner-Halenda (BJH) method using the adsorption branch of the isotherm.13C CP/MAS NMR spectrum was measured on a MERCURYplus 400 spectrometer at 100 MHz and a sample spinning frequency of 3 kHz.
2 Results and discussion
Mesoporous microstructure formation at different temperatures: Among the N-AAAs, here, C14-D-AlaS has been chosen as an example to show the experimental results in detail. The mesoporous silicas were synthesized at temperature range of 0 °C~20 °C for 1 day (see experimental section for details). SEM images, HRTEM images, XRD patterns and nitrogen adsorption-desorption results of the samples synthesized at 0 °C, 10 °C, 15 °C and 20 °C are shown in Fig. 1, Fig. 2, Fig. 3 and Fig. 4, respectively. All the samples were composed of particles uniformly in shape: the particles have well-defined external morphologies, depending on the synthesis temperature.
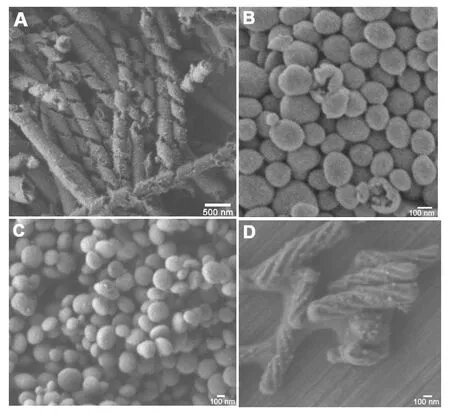
Fig. 1 SEM images of extracted mesoporous silicas synthesized at different temperature 0 °C (A), 10 °C (B), 15 °C (C) and 22 °C (D). Synthesis molar composition is: C14-L-AlaS:APES:TEOS:HCl:H2O =1:1:7:0.1:1780.
The sample synthesized at 0 °C (Fig. 1A) consisted exclusively of HR structure with uniform width of 200 nm ~250 nm and thickness of 30 nm~35 nm, which formed the tubular structure with a constant pitch length of 500 nm ~600 nm and the outer diameter of 150 nm~200 nm. This HR microstructure analogous to folded proteins (Proteinα/β helix). From the magnified SEM image (not shown in here) of cross section of ribbon, it can be observed that two parallel porous systems are existing in it without penetrating through the wall, which has also been observed from TEM images (vide post). A few rolled-up sheets have also been observed in this sample. As far as can be observed, all the tubular ribbons show a left-handed (P) helical motif regardless of the HR and rolled-up sheets. The existence of mesopores within the tubular ribbon wall was confirmed by the higher magnified high-resolution TEM images (Fig. 2A), nitrogen adsorption-desorption isotherms (Fig. 4). From TEM images, it can be seen that the ribbon consists of double adhered layers with the same thickness of ~15 nm, which possesses disordered mesoporous channels perpendicular to central axis of the tube distributed uniformly in the wall. No resolved peaks were observed from the XRD patterns (Fig. 3A) largely because of the presence of the disordered pore in the thin wall. To our best knowledge, this is for the first time to synthesize mesoporous HR.
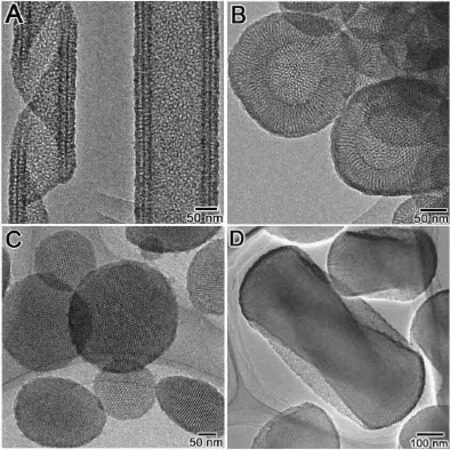
Fig. 2 TEM images of the samples shown in Fig. 1

Fig. 3 XRD patterns of the samples shown in Fig. 1
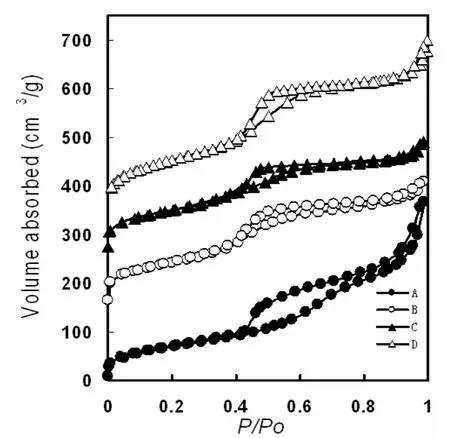
Fig.4 Nitrogen adsorption-desorption isotherms and pore size distributions of samples shown in Fig. 1.
SEM image shows that the irregular spherical particles, has been formed at 10 °C as shown in Fig. 1B. Thesespheres are small (100 nm~400 nm) in diameter and with broad size distributions. From the cracked spheres, the hollows can be observed clearly. TEM image (Fig. 2B) shows that these spheres possesses hollow with inner diameter ranging from 100 nm to 150 nm and mesoporous architecture in the wall. The wall thickness of the vesicles are in the range of 50 nm~60 nm that much thicker than the HR wall. It is found that the disordered channels are penetrating the wall from inner to outside. XRD pattern (Fig. 3B) of this sample show only one broad peak in 2θ range of 1°~2°, indicating the existence of the disordered pore system.
The samples synthesized at 15 °C consisted exclusively of very clear disk-like shaped crystals spindly-like in side view, with diameter of ~250 nm and height of 100 nm ~150 nm (Fig. 1C and Fig.2 C). The HRTEM images taken with the electron beam parallel to and perpendicular to the channel axis show well ordered hexagonally arranged mesopores. The X-ray diffraction (XRD) patterns (Fig. 3C) of all extracted chiral mesoporous silicas revealed the three well-resolved peaks in the range of 2θ = 1.5 °~6 °, which were indexed as 10, 11 and 20 reflections based on the 2d-hexagonal p6mm structure (which was also confirmed by TEM observation) with similar a = 6.5 nm~6.7 nm.
As reported in our previous work[14], when the synthesis temperature increased to 20 °C, 2d-hexagonal chiral mesoporous silica (CMS) was formed as confirmed in Figures 1d, 2d and 3d. The material has a twisted hexagonal rodlike morphology, with diameter 150 nm–200 nm and length 0.35μm–0.8 μm. The XRD pattern and TEM images confirm the presence of hexagonally ordered chiral channels of 5.8 nm diameter winding around the central axis of the rods. Handedness of this chiral mesoporous hexagonal rod was estimated by counting characteristic morphologies from 500 randomly chosen crystals in the SEM images, and the maximum right-/left -handed ratios proved to be 7.5/2.5, i.e. the right-handed excess.
The HR synthesized at 0 °C show a capillary condensation steps in the relative pressure range of 0.40-0.70 (Fig. 4A), which correspond to the adsorption in uniform mesopores of ~3.6 nm. The BET (Brunauer-Emmett-Teller) surface area and pore volume are 349 m2/g and 600 mm3/g, respectively. Nitrogen adsorption-desorption isotherms of hollow sphere, disk-like and CMSs (Fig.4 B, Fig.4 C and Fig.4 D) show type IV feature with only capillary condensation steps with a significant hysteresis loops at ~0.4 in relative pressure, indicating the existence of uniform mesopores. These three type materials possess similar pore diameters of ~3.6 nm, and the BET surface areas and pore volumes are in the range of 300 m2/g ~400 m2/g and 300 m2/g~400 mm3/g, respectively.
The formation processes of helical mesoporous silica ribbon has been illustrated as shown in Fig. 5. Firstly, partially neutralized chiral amphiphilic carboxylate self-assemble into flat-tape with bilayer structure upon decreasing the temperature to 0 °C, in which the long chiral molecules do pack parallelly to their nearest neighbors at a zero twist angle with respect to their nearest neighbors (Fig.5a). Lipid molecules arranged bilayerlly by the hydrophobic interaction of hydrocarbon chains. The hydrogen bonding between amide groups strengthens such bilayer arrangements, and the chirality of the asymmetric carbon demands the helical structure of bilayer strands or coiled ribbons[15]. After adding APES and TEOS, the carboxylic acid group will be negatively charged by amino group of APES through neutralization reaction Thus the charge density of the micelle surface increases and consequently increase the electrostatic repulsion between the charged surfactant’s head groups and the effective head group area of surfactant; at the same time, which create the arrangement of chiral molecules tilted with respect to the local layer due to gouche geometry positioned by the interaction between amino groups and C=O, and the favored twist from neighbor to neighbor leads the whole ribbon to coil into tube.[16]Simultaneously, APES and TEOS can easily penetrate into the ribbon from its surface and convert the lipid wall into a mesoporous one through re-self-assembly. For the lipid molecules, APES and TEOS can re-self-assemble to form a rod-like micelle in the ribbon wall and finally to form a disordered silica mesostructure with a corresponding increase in the wall thickness from 6.5 nm and in the width from 100 nm of lipid tape to those ~200 nm and ~250 nm of helical mesoporous ribbon, respectively. The re-self-assembly process of lipid tape with APES and TEOS with maintaining the ribbonmorphology occurred only at temperature below 0 °C. The driving force for shortening pitch length of the ribbon has been attempted by measuring the changing hydrogen bonding interaction and ionization degree increased by the polymerization of the silica species during the synthesis, however, we have not obtained any available data.
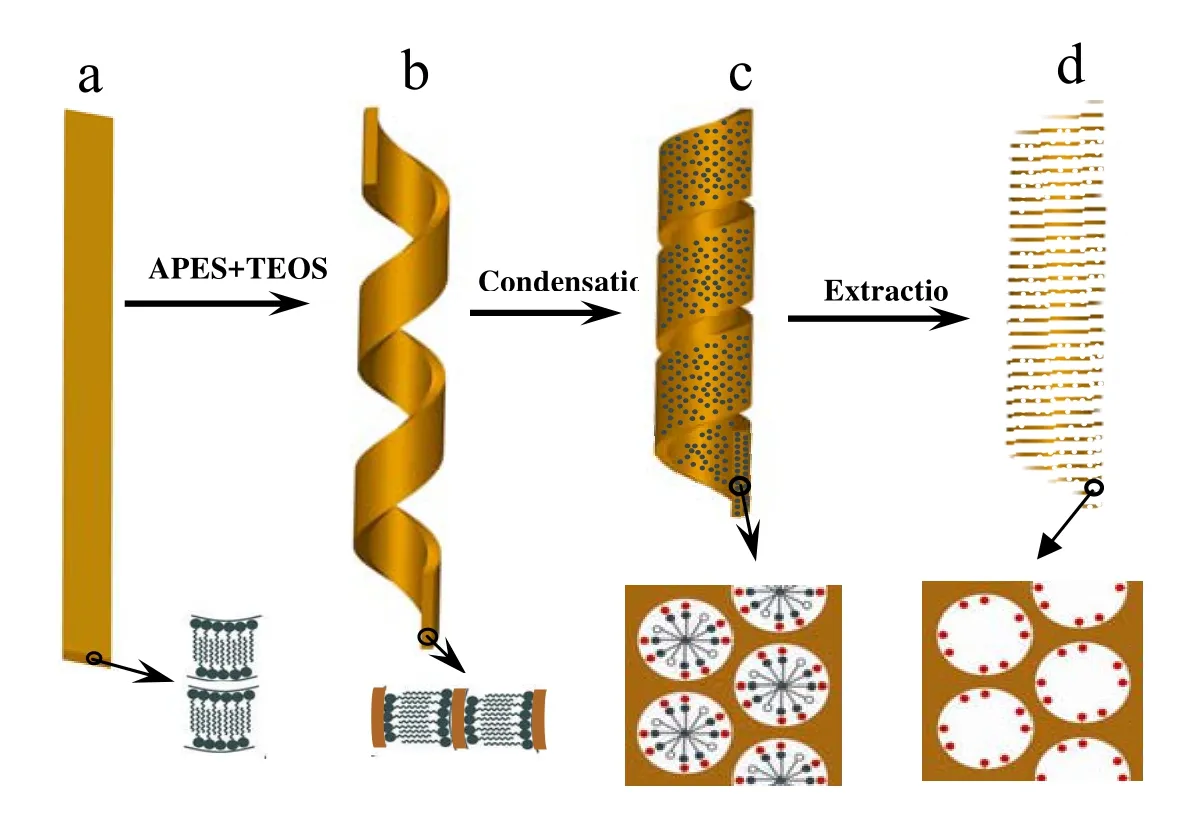
Fig. 5 Schematic illustration of the mesoporous silica helical and ribbon formation process
13C CP/MAS NMR spectrum of extracted mesoporous silica microstructures: After removing N-AAA, all of these four types mesoporous silicas can be amino group-functionalized in the pore surface. The absence of surfactant molecules after extraction was proved by13C CP/MAS NMR spectrum (Fig. 6). No resonance signal was detected between 20 ppm and 40 ppm, which would be attributable to the alkyl of C14- D-AlaS, whereas the resonance signals at 10 ppm, 21.7 ppm and 42.6 ppm assignable to C1, C2 and C3 of APES were clearly observed. These spectra demonstrate that the surfactant molecules were removed whilst quaternary amine groups remained on the surface of the mesopores. Elemental analysis results showed that the loading amount of the organic group –NH2in sample A, B, C and D are all 1.2 mmol/g SiO2.
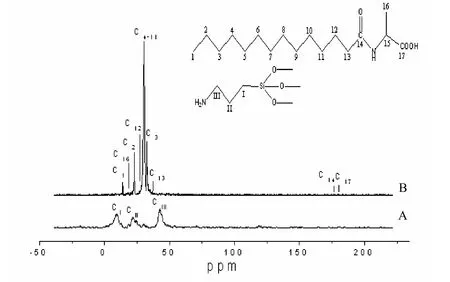
Fig. 6 C CP/MAS NMR spectra of extracted mesoporous silica HR (A) and C14-D-Ala (B)13
3 Conclusions
As mentioned above, the various microstructures such as HR, hollow sphere, disk-like and helical rod observedfrom lipid-based self-assembly have been obtained by varying the synthesis temperature. The disordered and 2d-hexagonally ordered mesopores in these microstructured silicas have been formed through re-self-assembly of lipid molecules, CSDA and silica source. These results demonstrated the effectiveness of the strategy of varying the temperature and using CSDA with pendant amino group to initiate silica condensation at the surface of self-organized lipid molecules. It has been interestingly found that, under lowest temperature, the HR has been formed through the morphological transition from the flat-tape ribbon; and under higher temperatures, the other microstructures have been formed by melting process with addition of CSDA and TEOS.
In order to seriously consider the use of molecularly assembled microstructures in a general sense, it is important to understand the relation between the actual structure of the individual molecules comprising the microstructure and the dimension or geometry of the microstructures. Unfortunately, the level of our understanding has not yet reached this detailed molecular level in the HR formation. HR and CMS with opposite handedness have been synthesized with the same surfactant at different reaction temperature, which has not been clear yet. Although the formation mechanism of this fascinating structure is still not completely understood, we believe that this will provide a new insight into the molecular factors governing inorganic-organic macro- and mesophase formation and technological utility of lipid molecules in most advanced materials applications.
Reference:
[1] SCHNUR J M.Lipid tubules: a paradigm for molecularly engineered structures[J]. Science, 1993, 262: 1669-1676.
[2] SHIMIZU T, MASUDA M, MINAMIKAWA H.Supramolecular nanotube architectures based on amphiphilic molecules[J]. Chem. Rev. ,2005, 105: 1401-1444.
[3] JUNG J H, LEE S H, YOO J S, et al. Creation of double silica nanotubes by using crown-appended cholesterol nanotubes[J]. Chem. Eur. J. ,2003, 9:5307-5313.
[4] LIN H P, MOU C Y."Tubules-Within-a-Tubule" hierarchical order of mesoporous molecular sieves in MCM-41[J]. Science ,1996, 273: 765-768.
[5] NAKASHIMA N, ASAKUMA S, KUNITAKE T.Optical microscopic study of helical superstructures of chiral bilayer membranes[J]. J. Am. Chem. Soc., 1985, 107: 509-510.
[6] JUNG J H, ONO Y, SHINKAI S. Sol-Gel polycondensation of tetraethoxysilane in a cholesterol-based organogel system results in Chiral Spiral silica[J]. Angew. Chem. Int. Ed. ,2000, 39: 1862-1865.
[7] JUNG J H, ONO Y, SAKURAI K, et al.Novel vesicular aggregates of crown-appended cholesterol derivatives which Act as gelators of Organic solvents and as templates for silica transcription[J]. J. Am. Chem. Soc. ,2000,122:8648-8653.
[8] CHE SA, GARCIA-BENNETT A E, YOKOI T, et al. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure[J]. Nature Materials, 2003, 2: 801-805.
[9] CHE SA , LIU Z, OHSUNA T, et al. Synthesis and characterization of chiral mesoporous silica[J]. Nature ,2004, 429: 281-284.
[10] JIN HY, QIU HB, SAKAMOtO Y, et al. Mesoporous silicas by self-Assembly of lipid molecules : ribbon, hollow sphere, and chiral materials[J]. Chem. Eur. J., 2008 , 14:6413-6420.
[11] THOMAS B N, SAFINYA C R, PLANO R J, et al.Lipid tubule self-assembly: length dependence on cooling rate through a first-order phase transition[J]. Science ,1995, 267:1635-1638.
[12] DUBOIS J E, ALAOUI M E, TOULLEC J.Formation of stable bilayer assemblies in water from single-chain amphiphiles. Relationship between the amphiphile structure and the aggregate morphologyJ[J]. J. Am. Chem. Soc. ,1981, 103: 5401-5413.
[13] FUHRHOP J H, BOETTCHER C.Stereochemistry and curvature effects in supramolecular organization and separation processes of micellar N-alkylaldonamide mixtures[J]. J. Am. Chem. Soc. ,1990, 112: 1768-1776.
[14] JIN HY, LIU Z, OHSUNA T, et al. Control of morphology and helicity of chiral mesoporous silica[J]. Adv. Mater., 2006, 18: 593-596.
[15] TOYOKO I, YOSHIYUKI T, HIDETOSHI M.Formation of fibrous molecular assemblies by amino acid surfactants in water[J]. J. Am. Chem. Soc. ,1992, 114: 3414-3419.
[16] SCHNUR J M, RATNA B R, SELINGER J V, et al. Diacetylenic lipid Tubules: experimental evidence for a chiral molecular Architecture[J]. Science ,1994, 264: 945-947.
[17] TREWYN B G, WHITMAN C M, LIN V S Y.Morphological control of room-temperature ionic liquid templated mesoporous Silica nanoparticles for controlled release of antibacterial agents[J]. Nano Lett. ,2004, 4: 2139-2143.
脂质分子通过自组装合成不同形貌的介孔材料
靳海英
(上海第二工业大学城市建设与工程学院,上海 201209)
主要描述了通过氨基酸分子自组装来制备四种不同形貌的介孔硅材料,并通过常用的检测仪器:电子显微镜进行结构解析。已经发现:(1)通过改变反应温度从0, 10, 15到20 °C,可以制备得到不同形貌的介孔材料,即类似于蛋白质分子的螺旋飘带,空心球,飞碟状和手性棒状形貌的二氧化硅介孔材料;(2)在螺旋飘带和空心球形貌材料中介孔孔道排列是无序的,而在飞碟和手性棒状材料中介孔孔道是高度有序排列的;(3)螺旋飘带形貌的材料是纯左手的,而手性棒状形貌的材料是右手过剩的介孔材料。该研究将为在分子水平合成手性材料提供一种新的方法。
螺旋飘带;囊泡;手性;介孔硅;层状;二维六方
TB332
A
1001-4543(2010)01-0022-08
2009-12-15;
2010-01-14
靳海英(1976-),女,黑龙江人,博士,主要研究方向为多孔材料,电子邮件:hyjin@eed.sspu.cn。
上海市选拔培养优秀青年教师专项基金(No.RYQ308010),上海第二工业大学校基金(No.XQD208006)

