Preparation of Alumina Binder-Added PtSnNa/AlSBA-15 Catalyst for Propane Dehydrogenation
Zhang Peixin; Zhou Yuming; Duan Yongzheng; Zhang Yiwei; Sheng Xiaoli
(1. School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189; 2. Geological Survey of Jiangsu Province, Nanjing 210018)
Preparation of Alumina Binder-Added PtSnNa/AlSBA-15 Catalyst for Propane Dehydrogenation
Zhang Peixin1,2; Zhou Yuming1; Duan Yongzheng1; Zhang Yiwei1; Sheng Xiaoli1
(1. School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189; 2. Geological Survey of Jiangsu Province, Nanjing 210018)
The present article compares the propane dehydrogenation performance of alumina binder-added PtSnNa/ AlSBA-15 catalysts prepared via three different procedures in comparison with the performance of a binder-free PtSnNa/ AlSBA-15 catalyst. All these catalysts have been investigated by reaction tests and some physico-chemical characterizations such as BET, H2chemisorption, catalytic grain crushing strength, NH3-TPD and TPO analyses. Test results showed that the addition of alumina binder could enhance the mechanical strength of catalyst evidently. Moreover, the different preparation procedures not only modified the characteristics of both acid and metal functions but also affected the coke deposition on the catalysts. Among these catalysts studied, the catalyst prepared by impregnation followed by the agglomeration of alumina binder had exhibited the highest catalytic activity and stability compared with other catalyst samples undergoing different preparation procedures. The possible reason may be attributed to the highest metallic dispersion and the strong interactions among Pt, Sn and the support.
AlSBA-15; binder; preparation procedure; propane dehydrogenation; alumina.
1 Introduction
The catalytic dehydrogenation of propane is of great commercial interest due to the growing demand of propene as an important basic chemical intermediate[1-2]. The PtSn/ Al2O3catalyst is a commonly used catalyst in this field, but it easily suffers from coke deposition, leading to poor catalytic stability. Therefore, many efforts have been made to develop catalysts with high catalytic properties. Over the last decade, the AlSBA-15 zeolite has been widely used as a catalytic support[3-5]or catalyst[6-8]for its large surface area and high thermal stability. But the use of AlSBA-15 zeolite as a catalyst for propane dehydrogenation has been seldom reported. Results from our previous work indicate that the PtSnNa/AlSBA-15 catalyst exhibited higher catalytic performance than the PtSn/SBA-15 catalyst in the propane dehydrogenation reaction[9].
However, the AlSBA-15 supported catalysts are in the powder form, and cannot be used directly for industrial applications. With the addition of binder, the powdered catalysts can be manufactured into extrudates, which can enhance the mechanical strength and avoid quick pressure drop in the reactor[10-12]. Generally speaking, the industrially used binders for zeolite or porous materials cover alumina, silica, and mixed silica and alumina. Using alumina or silica as the binder, however, may change the density of acid sites on the catalyst. In the case of using alumina as the binder, Al species from alumina-based binder can be incorporated into the zeolite framework, thereby forming additional acid sites[11,13]. Devadas, et al. and Wu, et al. found that the addition of silica binder could decrease the amount of acidity for H-gallosilicate (MFI) and zeolite Y[14-15]. In our previous work, the effect of different binder on the Pt and Sn metals supported on zeolite was discussed, and the catalyst agglomerated with alumina showed the highest catalytic performance among those catalysts studied[16].
As mentioned above, the addition of binder has an important influence on the physicochemical property and catalytic performance of the catalyst. However, there is still insufficient systematic research on the preparation procedures of binder added catalysts[12,17], especially forthe supported PtSn ones, mainly due to the preconception that such information is regarded as technical or trade secrets. This article compares the propane dehydrogenation performance between the alumina binder-added PtSnNa/AlSBA-15 catalysts prepared via three different procedures and the binder-free PtSnNa/AlSBA-15 catalyst. These preparation procedures studied covered: (a) impregnation of Na, Pt and Sn on AlSBA-15 zeolite followed by agglomeration of alumina binder, (b) agglomeration of AlSBA-15 zeolite with alumina binder followed by impregnation of Na, Pt and Sn over the above mixture, and (c) besides using those preparation procedures mentioned in (a), an additional calcination is accomplished subsequent to the impregnation process. The aim of this work is to investigate the modifications with which the preparation procedure can impose on the catalyst, and how these factors can affect the catalytic performance as far as propane dehydrogenation is concerned
2 Experimental
2.1 Preparation of catalysts
The mesoporous material AlSBA-15 (with a Si/Al molar ratio of 20) was prepared by a method described in the literature[18]. Alumina (gamma phase) was obtained from the Shanghai Super Industrial Co., Ltd.
All binder-added PtSnNa/AlSBA-15 catalysts used in this study were prepared via one of the following procedures: (a) AlSBA-15 powder was initially impregnated in an aqueous solution of 0.427 mol/L NaCl at 80 ℃ for 4 h, then dried at 80 ℃ for 3 h, impregnated for a second time in a mixture of 0.033 mol/L H2PtCl6and 0.153 mol/L SnCl4solution, followed by drying. The nominal composition of each sample contained 0.5%Pt, 0.7%Sn, and 1.0%Na. Then, the prepared sample was fully agglomerated with 20% alumina and finally extruded. A catalyst prepared according to this procedure is defined as CBI (where C, B, and I stand for catalyst, binder, and impregnation, respectively). (b) AlSBA-15 powder was agglomerated with 20% of alumina binder, and then extruded. After drying, the strip-shaped carrier was calcined at 500 ℃for 5 h. Then, the elements Na, Sn, and Pt were incorporated on the strip-shaped carrier according to the method mentioned above. A catalyst defined as CBA (where C, B, and A stands for catalyst, binder, and agglomeration, respectively) was prepared according to this procedure. (c) Compared with (a), an additional calcination is accomplished after the impregnation process. The calcination is performed at 500 ℃ for 5 h. And the catalyst prepared according to this procedure is defined as CBC (where C, B, and C stand for catalyst, binder, and calcination, respectively). Hence, a catalyst prepared without binder is defined as CW (where C stands for catalyst, and W means binder free) was also provided according to the procedure (a).
All of these catalysts were crushed to the size of between 12—18 mesh, activated at 500 ℃ for 4 h, then dechlorinated at 500 ℃ for 4 h in air containing steam, and finally reduced under H2at 500 ℃ for 8 h.
2.2 Catalyst characterization
The nitrogen adsorption-desorption isotherms were measured at -196 ℃ on a Micromeritics ASAP 2000 apparatus. Before measurements, the catalyst samples were degassed at 300 ℃ and 1×10-3torr. The pore structural data were analyzed by the BJH (Barrett-Joyner-Halenda) method using the Halsey equation for multilayer thickness.
The platinum dispersion was determined by the chemisorption measurements. This experiment was carried out using the dynamic-pulse technique with an argon (99.99%) flow rate of 50 mL/min coupled with pulses of hydrogen. The experimental process was the same as reported by Dorado, et al.[19]except that the sample reduction temperature was 500 ℃ and the temperature of the argon gas for purging the hydrogen was 40 ℃ higher than the reduction temperature.
The experiments for measuring the mechanical strength of catalyst particle were carried out in a ZQJ-II mechanical strength tester. The catalyst samples were pretreated at 150 ℃ for 2 h and then measured after cooling to room temperature. A 5 mm length of sample was selected for every catalyst and each catalyst was measured 10 times. The value of the particle mechanical strength was acquired from the average of ten measurements.
The surface acidity was measured by NH3-TPD analysis in the TP-5000 apparatus at ambient pressure. The sample (150 mg) was preheated at 500 ℃ for 1 h, and then cooled to room temperature in flowing He. At this temperature, sufficient pulses of NH3were injected until the adsorptionsaturation. The TPD analysis was carried out in a temperature range from 100 ℃ to 500 ℃ at a heating rate of 10 ℃/min using helium (30 mL/min) as the carrier gas.
The TPO analysis was also measured with the same apparatus which was used for conducting NH3-TPD analysis. About 0.15 g of coked sample was placed in a quartz reactor at room temperature, and was then heated to 800 ℃at a rate of 10 ℃ /min in a 5% O2/He mixed gas stream (at a flow rate of 30 mL/min).
2.3 Catalytic performance tests
The catalytic dehydrogenation reaction was performed in a stainless-steel fixed bed tubular reactor at 590 ℃and atmospheric pressure using a catalyst dosage of 1.0 g. The propane weight hourly space velocity (WHSV) was 3 h-1, and the mole ratio of H2/C3H8was 0.25. The reaction products were analyzed with an online GC-14C gas chromatograph equipped with an activated alumina packed column and a flame ionization detector (FID). The conversion of propane (X) is defined as the percentage of propane converted to all different products. The selectivity to propene (S) is defined as the amount of obtained propene divided by the amount of reactant converted to all products.
3 Results and Discussion
3.1 Catalysts characterization
Nitrogen adsorption-desorption isotherms of different catalysts are shown in Figure 1.
The catalyst without binder (CW) exhibits the type IV adsorption-desorption isotherm with H1 hysteresis, which is characteristic of ordered mesoporous material. On the other hand, the binder-added catalysts prepared by different procedures have similar isotherms, which suggests that the catalysts still maintain the mesoporous structure of AlSBA-15. And a decrease in the capillary condensation is observed for the catalyst with binder added, indicating that the pores of support are filled with some alumina binder molecules[10].
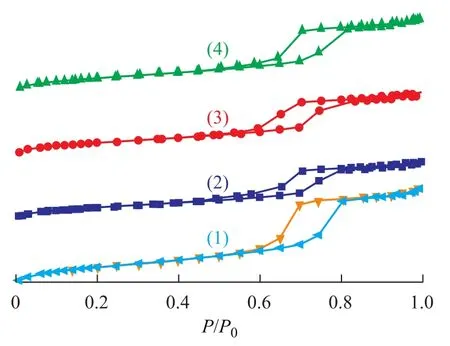
Figure 1 Nitrogen adsorption-desorption isotherms of different catalysts
Table 1 shows the data for characterization of different catalysts. It can be found that compared with the CW catalyst, the surface area of binder-added catalyst clearly decreases, which further indicates the inclusion of alumina molecules inside the porous structure of the support. It is quite interesting to observe that the surface area of CBC is higher than that of CBI, and much higher than that of CBA. This means that preparation procedure affects the structure of support evidently. When the agglomeration is followed by the impregnation, the entry of alumina binder to AlSBA-15 channel is hindered by the substrates (Pt, Sn and Na species) dispersed on both internal and external surface of AlSBA-15 zeolite[17], especially after the calcinations process, thus decreasing the influence of alumina binder on the zeolite surface area.
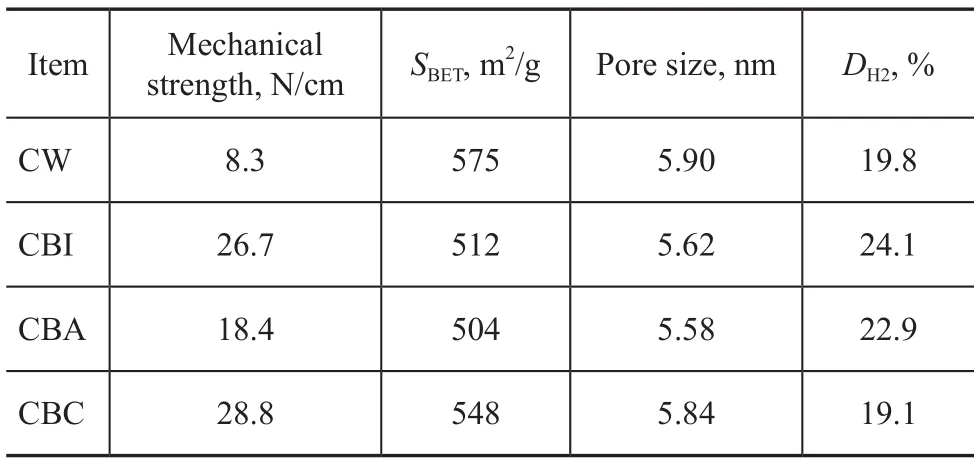
Table 1 Characterization data for different catalysts
To investigate the effect of preparation procedure on the metal dispersion in the catalyst, the hydrogenation chemisorption experiment was performed. As shown in Table 1, the metallic dispersion of CBC decreases by 0.7% in comparison with CW. In this case, some Pt particles can be covered by a definite amount of alumina binder, which leads to lower Pt dispersion on the CBC catalyst. Similar phenomenon has been observed by Choudhary, et al.[20]However, opposite results can be observed for CBI and CBA. As mentioned above, compared with CBC, more alumina molecules can enter the support channels, which can effectively reduce the opportunities for coverage of Ptspecies. Moreover, subsequent calcination can contribute to the migration of Pt species to the surface of alumina binder, resulting in an increase in the metallic dispersion. And the metallic dispersion of CBI is higher than that of CBA. It may occur because the powdered AlSBA-15 can be mixed more homogeneously with the impregnated solution as compared with the strip support[17].
It can be seen from Table 1 that all of the binder added–catalysts exhibit good mechanical strength owing to the presence of binder. Generally speaking, the mechanical strength evolves through adhesive forces and crosslinking of terminal hydroxyl groups between neighboring alumina binder particles[21]. Among these alumina-added catalysts, CBA shows the worst mechanical strength, suggesting that immersion of the support in the impregnating solution for a long time can reduce the strength of resulted catalyst markedly. Moreover,CBC catalyst has higher mechanical strength than that of CBI, and this effect might be caused by the presence of more alumina binder on the external surface of support.
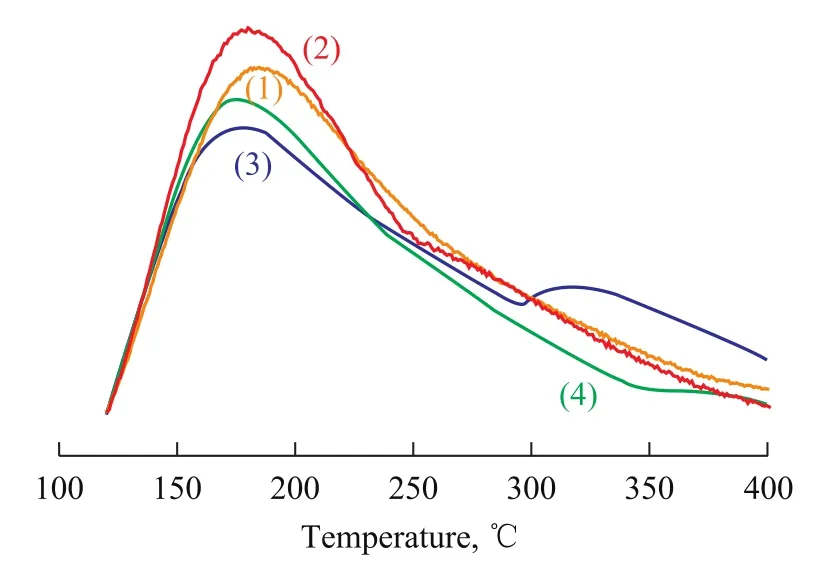
Figure 2 NH3-TPD profiles of different catalysts
The dehydrogenation of paraffins is intimately associated with the acidity of catalyst[22-23]. Figure 2 shows the NH3-TPD profiles of different catalysts. A desorption peak at about 180 ℃ can be observed in the profile of CW catalyst, which can be related to mild acid sites[18]. As regards the CBC catalyst, a similar peak appears, but its area is lower than that of CW, which may be caused by dealumination of support during the calcination process prior to the agglomeration of binder[24], leading to a decrease in the amount of acid sites, despite a subsequent addition of alumina binder. In comparison with CW, the peak area of CBI increases obviously, showing that the amount of acid sites is increased. Generally, an increase in the amount of acid sites of alumina binder-added catalyst is attributed to the migration of Al species to the framework of support, however, the metal component dispersed on the support could to some extent impede this migration[17], so a slight increase in the number of acid sites is identified for CBI. In addition to the peak at about 280 ℃ that is designated to the catalyst sample CBA, a small NH3desorption peak is found at 320 ℃, which is assigned to strong acid sites. This might be caused by the migration of Al species into the support framework during the agglomeration process in the absence of metal species; even though in the next step the presence of sodium could neutralize the strong acidic sites of the support preferentially, and a small amount of strong acid sites is still retained on the catalyst sample CBA.

Figure 3 TPO profiles of different catalysts
Coke deposition on the catalyst is one of the important factors which can influence catalytic deactivation. As we know that the coke deposits are mainly formed through polymerization of olefins that are generated during the dehydrogenation process, which is always catalyzed on the acid and metal sites. Generally, a typical TPO profile has two successive combustion peaks. The lower temperature peak corresponds to the combustion of a poorly polymerized coke located on the metal; while the higher temperature one corresponds to the combustion of highly polymerized coke located on the support[25-26]. The amount of coke formed over different catalysts (after 7 h of reaction) is analyzed by TPO and the results are shown in Figure 3. The coked CW catalyst shows one combustion peak located at about 570 ℃, assigned to the coke deposited on the support[27]. With respect to the CBI catalyst, a peak appears at about 460 ℃, which corresponds to the cokedeposited on the metallic Pt particles. There are two combustion peaks in the TPO pro file of CBA. The first peak temperature on CBA catalyst is higher and the corresponding area is larger in comparison with that of CBI, and the second one is also higher and larger compared with that of CW. This indicates that large amounts of carbon deposits are located not only on the support but also on the metallic Pt surface. As mentioned above, some strong acid sites are located on the CBA catalyst surface, making extra amount of coke be inevitably formed over this catalyst. No combustion peak can be observed in the TPO pro file of CBC catalyst, indicating that small amount of coke is deposited on the catalyst. Obviously, this can be attributed to small amount of acid sites that are present on its surface.
3.2 Catalytic performance
Catalytic performance of different catalysts during the dehydrogenation of propane were examined at 590 ℃, and the results are depicted in Figure 4. It can be seen from Figure 4a that the propane conversion on these catalysts declines with an increasing reaction time, because the coke deposition increases gradually as time going on and covers the metal surface slowly. Thus, the contact opportunity between propane and Pt particles is decreased, resulting in the reduction of propane conversion. Additionally, the sintering of Pt particles is a possible factor, notwithstanding a lower temperature is applied during this experiment.
It can be seen that the catalytic activity of samples for propane dehydrogenation increases in the following order: CBC<CW<CBA<CBI. To explain this, it should be noted that there are two active centers (metal and acid sites) for PtSn/NaAlSBA-15 catalyst. Generally speaking, platinum is the only active metal and propene is formed on it through dehydrogenation. The main cracking products (methane and ethene) are mainly formed via cracking reaction on the support, while the hydrogenolysis reaction product (ethane) is formed on the metal[28]. Moreover, the dehydrogenation and cracking of alkanes are assumed to proceed through carbonium-ion intermediates[29]. The higher concentration of acid sites favors the subsequent cracking reaction of the initially formedcarbenium ions. Therefore, Pt dispersion and support acidity can affect the catalytic properties effectively. The CW catalyst has an initial propane conversion of 20.7%, which is achieved thanks to its lower metallic dispersion. It is interesting to observe that the addition of alumina binder could change the catalytic performance effectively. Among these catalysts studied, the CBI catalyst has the highest initial propane conversion and catalytic stability, which are realized owing to the highest metallic dispersion on this catalyst. In comparison with the CBI catalyst, a slight decrease in initial activity is observed for the CBA catalyst although its metallic dispersion is much lower than that of the CBI catalyst. This is because some strong acid sites on this catalyst favor the cracking reaction, which enhances the initial activity. However, with an increasing reaction time, an enormous amount of coke covers the metal surface and acid sites on the support, leading to rapidly declining reaction activity. In our experiments, the performance of CBC catalyst is in the spotlight, because it exhibits the lowest catalytic activity although it has the same amount of alumina binder as the CBA and CBI catalysts do. Obviously, this phenomenon should be related to the worst metallic dispersion of this catalyst and the lowest amount of acid sites.
Figure 4b displays the propene selectivity versus reaction time for different catalysts. It can be found that the selectivity towards propene is enhanced over all catalysts with the exception of the CBC catalyst. This phenomenon can be interpreted as follows: The cracking and hydrogenolysis sites are densely placed on the surface of dehydrogenation catalyst, which results in the cleavage of C—C bonds in propane molecules to form methane, ethane, and ethene. These sites are prone to be poisoned by coke deposits, leading to decreased side reactions and increased selectivity to propene[30]. As for the CBC catalyst, the case is different. Generally, there are two kinds of active Pt species (M1sites and M2sites) on the surface of the PtSn based catalyst[31]. The M1sites are the ones in which Pt particles directly anchor on the support surface, while the M2sites correspond to the ones where Pt particles anchor on Sn oxide surface with a “sandwich structure”. Furthermore, the M1sites are mainly responsible for the hydrogenolysis and coking reactions, while the M2sites are in charge of dehydrogenation reactions. It has been reported that SnOxis anchored by the OH groups of Al in the support. As it has been mentionedabove, a serious dealumination reaction occurs during the calcination process for the CBC catalyst, thus decreasing the stability of SnOxand making the Pt species anchor on the support surface easily. This is to say that the proportion of M1sites increases, while the proportion of M2sites decreases, which can result in the decline of selectivity to propene.
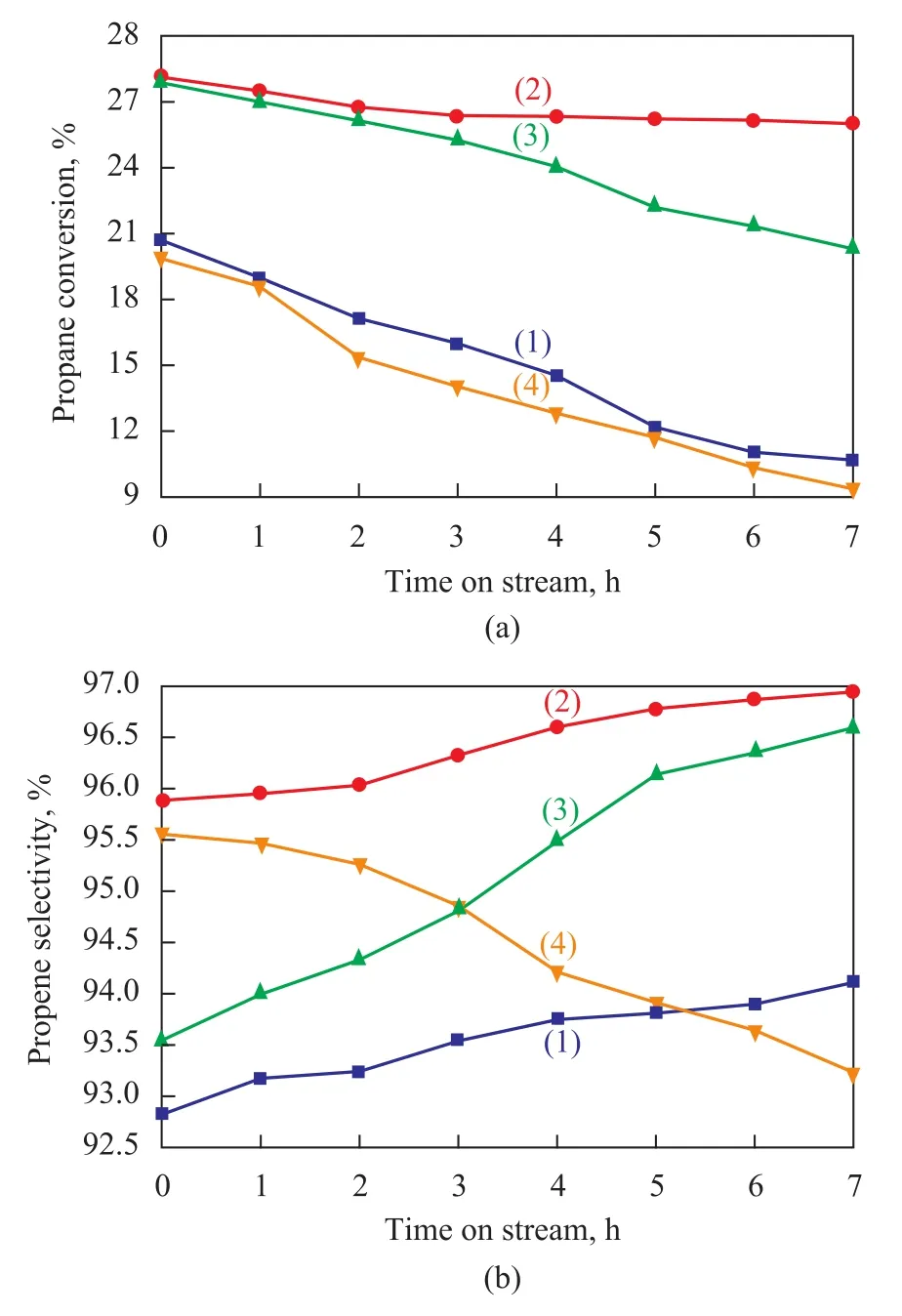
Figure 4 Propane conversion (a) and propene selectivity (b) versus reaction time of the different catalysts
4 Conclusions
The propane dehydrogenation reaction performance of the alumina binder-added PtSnNa/AlSBA-15 catalysts prepared with different procedures were compared with the performance of the binder-free PtSnNa/AlSBA-15 catalyst at 590 ℃. The different preparation procedures could affect not only the acidity and metallic dispersion on the surface of catalyst but also the coke deposition evidently. The NH3-TPD profiles have shown that some strong acid sites appear in the CBA catalyst, which leads to a signif icant amount of coke deposits. Thus, this catalyst suffered from deactivation caused by serious coke formation. For the CBC catalyst, dealumination of support occurs during the calcination process, which weakens the interaction between Pt and Sn species, and results in a worst catalytic performance. Among these catalysts studied, the CBI catalyst has the highest metallic dispersion and the best catalytic performance. After having taken part in propane dehydrogenation reaction for 7 h, the CBI catalyst has reached a propene selectivity in excess of 96.5% with a corresponding propane conversion rate of about 26.0%.
Acknowledgments:The authors are grateful to the National Nature Science Foundation of China (50873026, and 21106017), the Production and Research Prospective Joint Research Project (BY2009153), the Science and Technology Support Program (BE2008129) of Jiangsu Province of China and Specialized Research Fund for the Doctoral Program of Higher Education of China (20100092120047) for financial supports.
[1] Salmones J, Wang J A, Galicia J A, et al. H2reduction behaviors and catalytic performance of bimetallic tin-modified platinum catalysts for propane dehydrogenation [J]. J Mol Catal A,2002, 184(1/2): 203-213
[2] Duan Y, Zhou Y, Zhang Y, et al. Propane dehydrogenation on PtSnNa/AlSBA-15 catalyst: Influence of tin as a promoter [J]. China Petroleum Processing and Petrochemical Technology, 2012, 14 (1): 37-45
[3] Boutros M, Denicourt-Nowicki A, Roucoux A, et al. A surfactant-assisted preparation of well dispersed rhodium nanoparticles within the mesopores of AlSBA-15: Characterization and use in catalysis [J]. Chem Commun, 2008, 25(1): 2920-2922
[4] Kanda Y, Aizawa T, Kobayashi T, et al. Preparation of highly active AlSBA-15-supported platinum catalyst for thiophene hydrodesulfurization [J]. Appl Catal B, 2007, 77(1/2): 117-124
[5] Oh K S, Woo S I. Catalytic property of Pt/AlSBA-15 in selective catalytic reduction of NO[J]. Catal Lett, 2006, 110(3/4): 247-254
[6] Vinu A, Sawant D P, Ariga K. Benzylation of benzene and other aromatics by benzyl chloride over mesoporous AlSBA-15 catalysts [J]. Micropor Mesopor Mater, 2005,80(1/2/3): 195-203
[7] Xu B J, Hua W M, Yue Y H, et al. Alkylation of hydroquinone with tert-butanol over AlSBA-15 mesoporous molecular sieves[J]. Catal Lett, 2005, 100(1/2): 95-100
[8] Nishita L, Amol P, Amrute K, et al. Non-phosgene route for the synthesis of methyl phenyl carbamate using ordered AlSBA-15 catalyst [J]. J Mol Catal A, 2008, 295(1/2): 29-33
[9] Duan Y, Zhou Y, Zhang Y, et al. Effect of sodium addition to PtSn/AlSBA-15 on the catalytic properties in propane dehydrogenation[J]. Catal Lett, 2011, 141(1): 120-127
[10] Chandrasekar G, Hartmann M, Palanichamy M, et al. Extrusion of AlSBA-15 molecular sieves: An industrial point of view[J]. Catal Commun, 2007, 8(3): 457-461
[11] Zhang Y, Zhou Y, Qiu A, et al. Effect of alumina binder on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Ind Eng Chem Res, 2006, 45(7): 2213-2219
[12] Honda K, Chen X, Zhang Z. Preparation of highly active binder-added MoO3/HZSM-5 catalyst for the nonoxidative dehydroaromatization of methane[J]. Appl Catal A,2008, 351(1): 122-130
[13] Shibabi D S, Garwood W E, Chu P, et al. Aluminum insertion into high-silica zeolite frameworks: II. Binder activation of high-silica ZSM-5[J]. J Catal, 1985, 93(2): 471-474
[14] Devadas P, Kinage A K, Choudhary V R. Effect of silica binder on acidity, catalytic activity and deactivation due to coking in propane aromatization over H-gallosilicate (MFI) [J]. Stud Surf Sci Catal, 1998, 113: 425-432
[15] Wu X, Alkhawaldeh A, Anthony R G. Investigation on acidity of zeolites bound with silica and alumina[J]. Stud Surf Sci Catal, 2000, 143:217-225
[16] Liu H, Zhou Y, Zhang Y, et al. Influence of binder on the catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Ind Eng Chem Res, 2008, 47(21): 8142-8147
[17] Liu H, Zhou Y, Zhang Y, et al. Effect of preparation processes on catalytic performance of PtSnNa/ZSM-5 for propane dehydrogenation [J]. Ind Eng Chem Res, 2009, 48(12): 5598-5603
[18] Nie C, Huang L, Zhao D Y, et al. Performance of Pt/Al-SBA-15 catalysts in hydroisomerization ofn-dodecane [J]. Catal Lett,2001, 71(1/2): 117-125
[19] Dorado F, Romero R, Canizares P. Influence of clay binders on the performance of Pd/HZSM-5 catalysts for the hydroisomerization ofn-butane[J]. Ind Eng Chem Res, 2001, 40(16): 3428-3434
[20] Choudhary V R, Devadas P, Kinage A K, et al. In fluence of binder on the acidity and performance of H-gallosilicate (MFI) zeolite in propane aromatization[J]. Appl Catal A, 1997, 162(1/2): 223-233
[21] Freiding J, Patcas F C, Kraushaar-Czarnetzki B. Extrusion of zeolites: Properties of catalysts with a novel aluminium phosphate sintermatrix[J]. Appl Catal A,2007,328(2): 210-218
[22] Nawaz Z, Tang X P, Wang Y, et al. Parametric characterization and in fluence of tin on the performance of Pt-Sn/ SAPO-34 catalyst for selective propane dehydrogenation to propylene [J]. Ind Eng Chem Res, 2010, 49(3): 1274-1280
[23] Li Y X, Klabunde K J, Davis B H. Alloy formation in supported Pt-Sn catalysts - Mossbauer studies[J]. J Catal, 1991, 128(1): 1-12
[24] Zhang Y, Zhou Y, Li Y, et al. Effect of calcination temperature on catalytic properties of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Catal Commun, 2007, 8(7): 1009-1016
[25] Barbier J, Marecot P, Martin N, et al. Selective poisoning by coke formation on Pt/Al2O3[J]. Stud Surf Sci Catal, 1980, 6: 53-62
[26] Duan Y, Zhou Y, Zhang Y, et al. Effect of aluminum modification on catalytic properties of PtSn-based catalysts supported on SBA-15 for propane dehydrogenation [J]. J Nat Gas Chem, 2012, 21(2): 207-214
[27] Vu B K, Bok S M, Ahn I Y, et al. Oxidation of coke formed over Pt-Al2O3and Pt-SBA-15 in propane dehydrogenation [J]. Catal Lett, 2009, 133(3/4): 376-381
[28] Mikael L, Magnus H, Edd A B, et al. the effect of reaction conditions and time on stream on the coke formed during propane dehydrogenation[J]. J Catal, 1996, 164(1): 44-53
[29] Guisnet M, Gnep N S. Aromatization of propane over GaHMFI catalysts. Reaction scheme, nature of the dehydrogenating species and mode of coke formation[J]. Catal Today, 1996, 31(3/4): 275-292
[30] Duan Y, Zhou Y, Sheng X, et al. Influence of alumina binder content on catalytic properties of PtSnNa/AlSBA-15 catalysts [J]. Micropor Mesopor Mater,2012, 161(1): 33-39
[31] Lin L W, Yang W S, Jia J F, et al. Surface structure and reaction performance of highly dispersed and supported bimetallic catalysts[J]. Sci China Ser B, 1999, 42(6): 571-580 (in Chinese)
Recieved date: 2012-07-26; Accepted date: 2012-11-16.
Dr. Zhou Yuming, Tel: +86-25-52090617; Fax: +8625-52090617; E-mail: ymzhou@seu.edu.cn.
- 中国炼油与石油化工的其它文章
- Alumina Supported Vanadium Oxide Catalysts for Residue Hydrotreating
- Highlights on Planned Grassroots Styrene Units and Expansion of Existing Styrene Units in China
- Hydrocarbon Composition of Different VGO Feedstocks and Its Correlation with FCC Product Distribution
- Purification of Crude Glycerol from Waste Cooking Oil Based Biodiesel Production by Orthogonal Test Method
- Degradation of Nitrobenzene-Containing Wastewater with O3and H2O2by High Gravity Technology
- Et3NHCl-AlCl3Ionic Liquids as Catalyst for Alkylation of Toluene with 2-Chloro-2-methylpropane

