Infuence of Modifed Medical Stone on Behavior of Sulfur during Pyrolysis of Longma Coal
Li Jingkuan; Zhao Shuguang; Wang Baofeng,; Jin Yan; Zhang Jinjun; Cheng Fangqin
(1. College of Electrical and Power Engineering, Taiyuan University of Technology, Taiyuan 030024; 2. School of Chemistry & Material Science, Shanxi Normal University, Linfen 041004; 3. Research Institute of Resources and Environment Engineering, Shanxi University, State Environmental Protection Key Laboratory of Eff i cient Utilization of Waste Coal Resources, Taiyuan 030006)
Infuence of Modifed Medical Stone on Behavior of Sulfur during Pyrolysis of Longma Coal
Li Jingkuan1,3; Zhao Shuguang2; Wang Baofeng2,3; Jin Yan1; Zhang Jinjun2; Cheng Fangqin3
(1. College of Electrical and Power Engineering, Taiyuan University of Technology, Taiyuan 030024; 2. School of Chemistry & Material Science, Shanxi Normal University, Linfen 041004; 3. Research Institute of Resources and Environment Engineering, Shanxi University, State Environmental Protection Key Laboratory of Eff i cient Utilization of Waste Coal Resources, Taiyuan 030006)
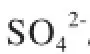
coal; modifed medical stone; sulfur behavior; pyrolysis; kinetics
1 Introduction
Coal is one of the main energy sources in the world, especially in China. As we know, coal contains variable amount of sulfur which exists in inorganic and organic forms. The inorganic sulfur occurs as sulfdes or sulfates while the organic sulfur is associated with organic structures existing in coal[1]. As one of the major pollution sources, sulfur in coal can inhibit the effective utilization of coal[2-3]. So, how to remove sulfur before utilization of coal is important. To grapple with this problem, one should know the mode of occurrence and the transformation of sulfur species in coal during its pyrolysis, gasification and combustion. Fixation of sulfur in the char during pyrolysis of coal is an effcient method to reduce the emission of sulfur. There are many studies regarding the transformation of sulfur during coal pyrolysis[4-11]. Our previous study showed that application of additives such as tourmaline, medical stone and sodium bentonite could influence the behavior of sulfur[12]. Мoreover, literature reports[13-17]showed that some catalysts such as modifed minerals could infuence the product distribution and the behavior of sulfur during coal treatment. A previous study[15]also showed that the Co-Mo/Al2O3catalyst and SO42–/ZrO2catalyst had an obvious catalytic effect on the treatment of coal; and the previous study[18]showed that CoMoP/Al2O3also had good catalytic activity.
Furthermore, as it has been known, the medical stone is a porous material and has strong adsorption capacity[19], which is 2000 times the adsorption capability of activated carbon. So, in our study, we used medical stone as a support, and loaded CoМoP and SO42–onto it. In this work, the effect of modified medical stone, viz.: the CoMoP/medical stone and the SO42–/medical stone, on sulfur behavior during coal pyrolysis was studied. Мoreover, the kinetics were also studied to furtherunderstand the influence mechanism related with the natural minerals.
2 Material and Methods
2.1 Samples
The Longma (LM) coal is excavated from Linfen City in Shanxi Province of China. The lump coal was first airdried and then crushed, ground and sieved to obtain the coal sample with a grain size ranging from 0.15 mm to 0.25 mm. Мedical stone (М) used in the experiments was gathered from Linshou in Hebei Province of China. The medical stone was ground and sieved to a grain size of less than 75 μm before use. The proximate and ultimate analysis of LM coal and the forms of sulfur species in LM coal were referred to in our previous study[12]. Мoreover, in order to know the mechanism related with the infuence of the medical stone on sulfur behavior, we also chose several simple minerals, which were contained in the medical stone, and mixed them uniformly according to a specifed ratio.
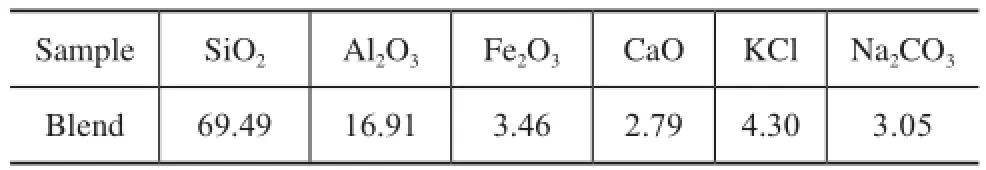
Table 1 Main composition of blended minerals(w, %)
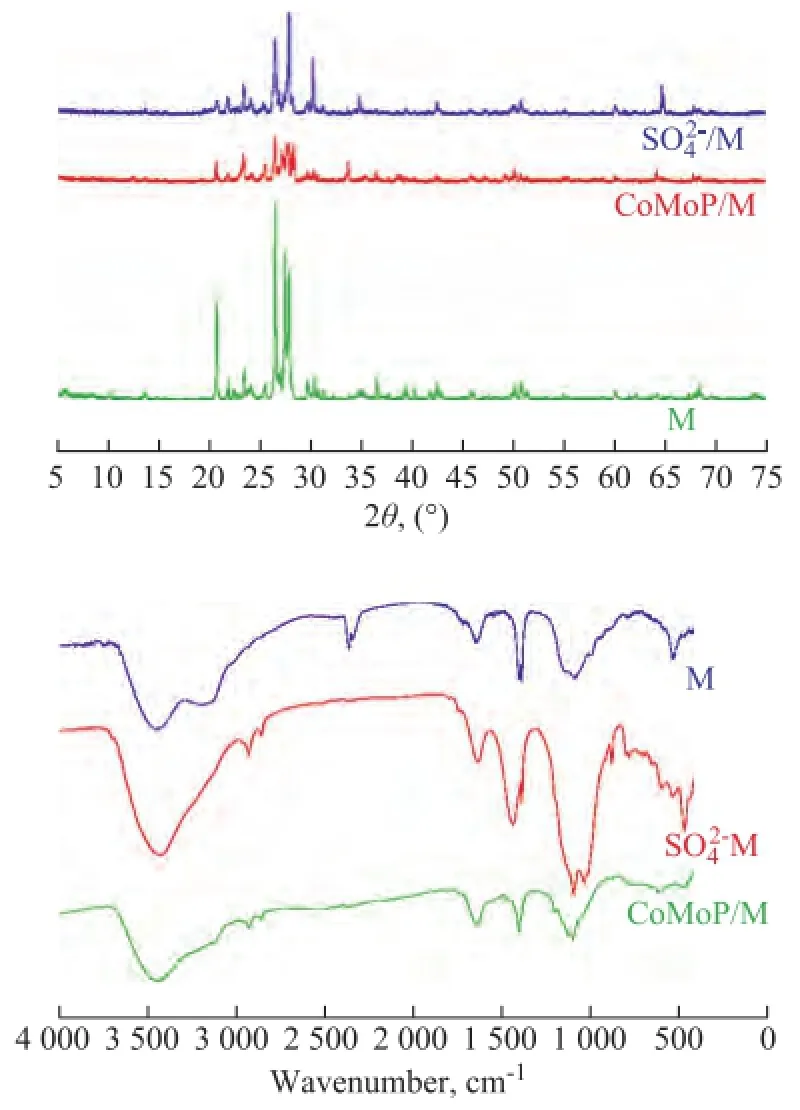
Figure 1 XRD patterns and FT-IR spectra of the medical stone and its modified minerals
Table 1 shows the composition of the blends. The modifed medical stone, viz.: CoМoP/ medical stone and SO4-IR spectra of the medical stone and its minerals-containing modifed forms. Figure 2 is the SEМEDX micrographs of the medical stone and its mineralscontaining modifed forms. It can be seen from Figure 1 and Figure 2 that the medical stone has been modifed veritably by the solution dipping method.

Figure 2 SEM-EDX micrographs of the medical stone and its minerals modified forms
2.2 Pyrolysis experiment
The pyrolysis experiments were performed in a fxed-bed reactor. The reactor and the experimental procedure were all the same as those referred to in our previous study[12].
2.3 Analysis and characterization methods
The amount of total sulfur in the solid residue was analyzed according to the Chinese Standard GB/T 214—2007. The content of pyritic sulfur and sulfate sulfur in coal and the solid residue obtained from pyrolysis were determined according to the Chinese standard GB/T 215—2003, and the organic sulfur content was obtained by difference.
2.4 Calculation methods
The yield of the solid residue and the retention of each type of sulfur in the solid residue (including total sulfur, organic sulfur and inorganic sulfur) were calculated by the equation mentioned in the previous literature[12].
3 Results and Discussion
3.1 Influence of modified medical stone on total sulfur retention during coal pyrolysis
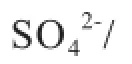
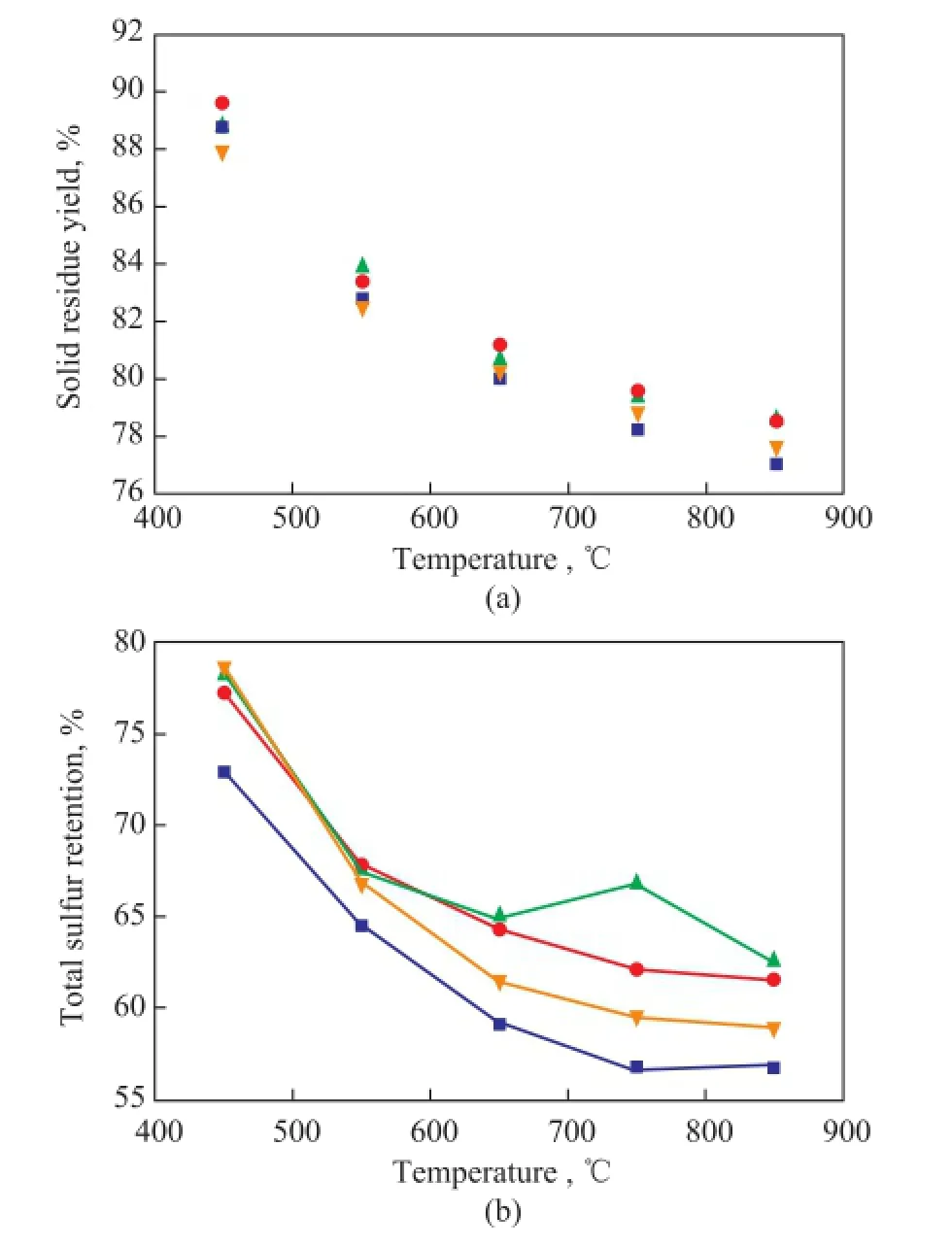
Figure 3 Solid residue yield (a) and sulfur retention (b) during LM coal pyrolysis at different temperatures with addition of the minerals modified medical stone
3.2 Infuence of minerals modifed medical stone on the retention of different forms of sulfur during coal pyrolysis
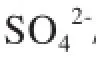
3.3 Influence of modified medical stone on the emission of H2S and COS during coal pyrolysis

Figure 4 Sulfur retention of: (a) sulfate sulfur, (b) pyritic sulfur, and (c) organic sulfur during LM coal pyrolysis at different temperatures with addition of the minerals modifed medical stone
Figure 5 illustrates the emission of H2S and COS during LМ coal pyrolysis. It can be seen from Figure 5(a) that for the raw LМ coal, the maximum emission of H2S was identifed at 450oC, while in the presence of medical stone or the minerals modified medical stone, the maximum emission of H2S was detected at 500oC. Мoreover, it can also be noticed that when the temperature was lower than 450oC, adding the modifed medical stone had no obvious effect on H2S emission, and when the temperature increased from 450oC to 750oC, adding CoМoP/medical stone and SO42-/medical stone both could increase the emission of H2S. When the temperature was higher than 750oC, adding the two kinds of modifed medical stone samples could all inhibit the emission of H2S. According to the literature report, the peak of H2S emission isattributed to the decomposition of pyrite, the shoulder at lower temperatures (<400oC) corresponds to the decomposition of aliphatic sulfur (such as thiols, dialkyl and aryl-alkyl sulfdes, etc.), and the right shoulder (>600oC) is related to the decomposition of aromatic sulfdes[21]. Then it is easy to infer that adding the modifed medical stone samples can intensify the decomposition of pyrite. This result can just coincide with the results of Figure 4(b), showing that when the temperature was lower than 650oC, the pyritic sulfur retention with addition of the modified medical stone was lower than that of raw LM coal. It also can be noticed from Figure 5(a) that when the temperature increased from 600oC to 700oC, addition of the modifed medical stone also made the emission of H2S higher, so that one could infer that adding the modified medical stone also was beneficial to the decomposition of aromatic sulfdes. It can be seen from Figure 5(b) that only a small amount of COS was emitted at 450—600oC during LМ coal pyrolysis, and this result was similar to that reported in the previous study[19]. When the temperature was lower than 400oC, adding CoМoP/ medical stone made the COS emission a little higher, while at a temperature exceeding 400oC, adding CoМoP/ medical stone made the COS emission lower. As regards the SO42-/medical stone, when the temperature was lower than 500oC or higher than 650oC, adding SO42-/medical stone made the COS emission a little higher, and when the temperature increased from 500oC to 650oC, adding SO42-/medical stone made the COS emission lower.
According to what we have known, carbonyl sulfide (COS) is mainly derived from pyrite and organic sulfur through secondary reactions[21]. Just as mentioned above, adding the modified medical stone is not beneficial to the removal of organic sulfur compounds from coal, so addition of CoMoP/medical stone can make the COS emission lower. Previous study[22]showed that CO2and CO contained in volatiles can react with the sulfur species to produce COS in line with the following reactions:
H2S + CO2= COS + H2O; and H2S + CO = COS + H2. Upon comparing Figure 5(a) and Figure 5(b), it can be noticed that the emission of H2S from pyrolysis of raw LM coal with or without addition of medical stone was all less than that the case with addition of the modified medical stone, while the emission of COS was all higher than the latter with addition of the modified medical stone. This phenomenon might be caused by the fact that H2S in the volatiles can react with CO2and CO, so that the higher the emission of COS is, the lower the emission of H2S would be.
3.4 Infuence of blends of simple minerals on sulfur retention during coal pyrolysis
3.4.1 Infuence of blends on total sulfur retention and solid residue yield
Figure 6 shows the solid residue yield and the total sulfur retention upon adding medical stone and the blends consisting of several simple minerals similar to the composition of the medical stone. Мoreover, in order to know the influence of the inherent minerals on the retention of sulfur[23]in Longma coal, the sulfur retention of the demineralized LM coal (LM-dem) during its pyrolysis was also studied. It can be seen from Figure 6(a) that adding medical stone or the blends all led to an increased solid residue yield, andupon comparing the solid residue yield obtained by adding medical stone only, addition of the blends of minerals increased the solid residue yield. The reason might be that although the composition of the blends was the same as that of the medical stone, however, the interaction between the constituents of medical stone was stronger, so the catalytic activity of medical stone was stronger too. Then the solid residue yield obtained upon adding medical stone was lower than the case with addition of the blends.
Figure 5 H2S and COS emission curve during LM coal pyrolysis at different temperatures with addition of the minerals modified medical stone
Furthermore, it can be seen from Figure 6(a) that the solid residue yield of LМ-dem was the lowest. It also can be seen from Figure 6(b) that the total sulfur retention of LM-dem was higher as compared to that of raw Longma coal, from which one could infer that removing the inherent minerals in Longma coal is benefcial to keeping sulfur in the residue, denoting that the existence of the inherent minerals is favorable to total sulfur removal. Unfortunately, this result does not agree well with the result obtained by Hu Haoquan[13]. The reason might be caused by the inherent minerals in the LM coal that are different from those in the Yanzhou coal as studied by Haoquan Hu. Мoreover, it can also be learned from Figure 6(b) that adding the blends also led to increased total sulfur retention, which just could coincide with the results mentioned above. It can be seen from the results that the influence of the blends on total sulfur retention is not exactly the same as that achieved by the medical stone.
3.4.2 Infuence of the blends on retention of forms of sulfur
Figure 7 shows the sulfur retention at different temperatures. It can be seen from Figure 7(a) that when the temperature was lower than 550oC, the sulfate sulfur retention upon adding the blends was lower than the case with addition of the medical stone, and when the temperature was higher than 550oC, the sulfate sulfur retention with addition of the blends almost had no obvious difference with the addition of medical stone. It can be seen from Figure 7(b) that at the same temperature in comparison with the pyritic sulfur retention of raw Longma coal, the pyritic sulfur retention of LМ-dem was lower, denoting that the existence of inherent minerals was beneficial to retaining the pyritic sulfur in the residue. This result is similar to the result of the previous study made by Professor Hu[13], which has denoted that the inherent minerals in coal have little effect on the decomposition of pyrite. Мoreover, it can be learned from Figure 7(b) that in comparison with the pyritic sulfur retention with addition of medical stone, adding the blends could increase the pyritic sulfur retention when the temperature was increased from 450oC to 650oC, and when the temperature was higher than 650oC, the pyritic sulfur retention identifed with addition of the blends was just the same as the case of adding the medical stone. The reason might be ascribed to the temperature in excess of 650oC, at which most of the pyrite would be decomposed. It can be seen from Figure 7(c) that at the same temperature the organic sulfur retention of LM-dem was higher than that of LМ coal, denoting that the inherent minerals were conducive to the organic sulfur removal. Interestingly, these results could coincide with theresult of the previous study made by Professor Hu[13]. It can be learned from Figure 7(c) that the organic sulfur retention with the addition of the blends was higher than that achieved by addition of the medical stone. It can be known from all mentioned above that although the composition of the blends was similar to that of the medical stone, however, because of the difference in the combination mode, the retention of sulfate sulfur, pyritic sulfur and organic sulfur obtained with addition of the blends was totally different from that achieved by adding the medical stone.
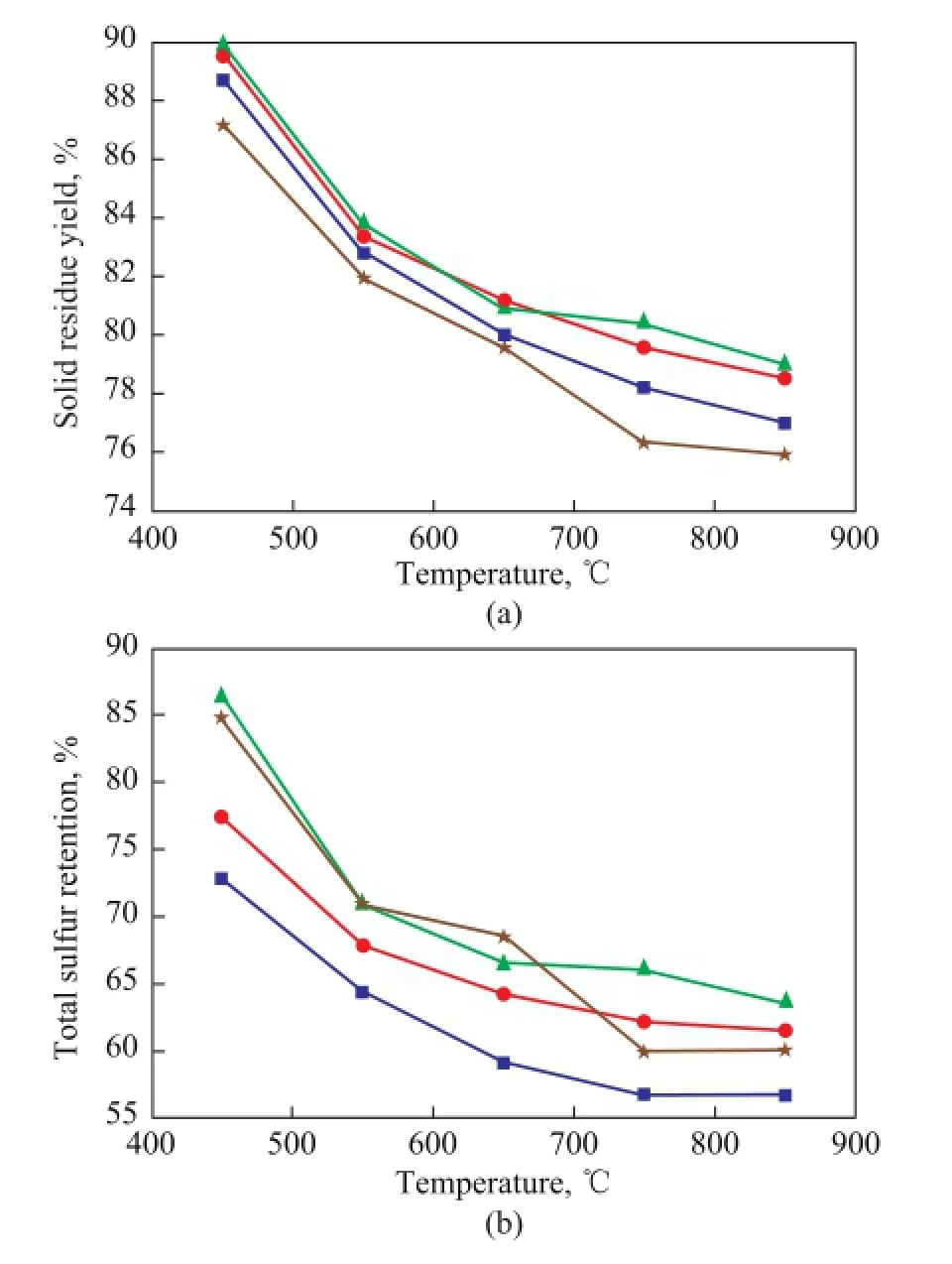
Figure 6 Solid residue yield (a) and the total sulfur retention (b) during LM coal pyrolysis with addition of blended minerals■—LM;●—LM+M;▲—LM+Blends;★—LM-dem
3.4.3 Infuence of the blends on emission of H2S and COS
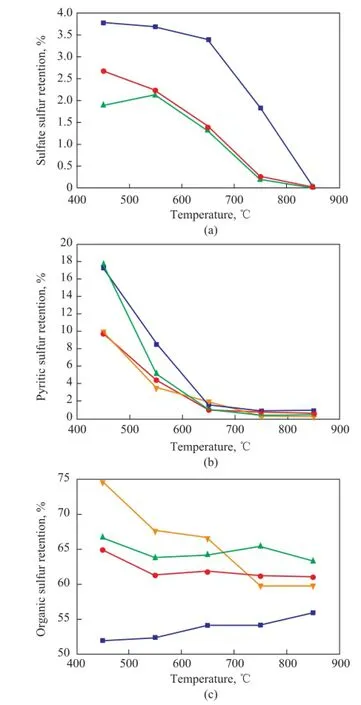
Figure 7 Sulfur retention at different temperatures in terms of: (a) sulfate sulfur, (b) pyritic sulfur, and (c) organic sulfur■—LM;●—LM+M;▲—LM+Blends;▼—LM-dem
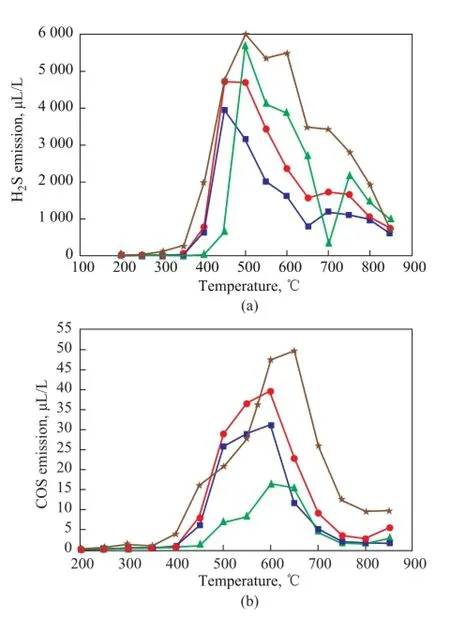
Figure 8 H2S and COS emission curve during LM coal pyrolysis upon adding blended minerals■—LM;●—LM+M;▲—LM+Blends;★—LM-dem
Figure 8 shows the emission of H2S and COS achieved with adding the medical stone and the blends, respectively. It can be seen from Figure 8(a) that the emission of H2S from LM-dem was higher than that from the raw Longma coal. As we have known, most of sulfur species in pyrite can be transformed into volatile H2S. Since the LM-dem sample showed lower pyritic sulfur retention, it would have higher removal rate of pyritic sulfur, and the emission of H2S from pyrolysis of LM-dem sample was higher than that obtained from pyrolysis of raw Longma coal. It can be seen from Figure 8(a) that when the temperature was lower than 500oC, addition of the blends could prevent the emission of H2S, and when the temperature was higher than 500oC (except for 700oC), adding the blends resulted in the higher emission of H2S than that obtained by addition of the medical stone. It canbe learned from Figure 8(b) that the emission of COS from LM-dem sample was also higher than the case of raw Longma coal with the exception of the temperatures in the range from 500oC-550oC, denoting that removing inherent minerals also was beneficial to COS emission. This result also has been proved by Haokan Chen in previous study[24]. It can also be seen from Figure 8(b) that the addition of medical stone was beneficial to COS emission, while adding the blends could prevent the emission of COS. It has been known that during coal pyrolysis the COS is mainly produced from pyrite, organic sulfur or secondary reactions of organic sulfur among sulfur-containing compounds[23]. Just as it has been mentioned above, adding the blends can retain more organic sulfur in the residue, and on the other hand, about 78.3% of sulfur species in the LМ coal are composed of organic sulfur species, so adding the blends can inhibit the emission of COS.
3.5 Kinetic study of pyrolysis of LM coal upon adding natural minerals
3.5.1 TG/DTG curves of LM coal upon adding natural minerals
In order to delve in the mechanism of influence of the natural minerals on sulfur behavior, the kinetics of pyrolysis of LM coal upon adding natural minerals was studied. Figure 9 shows the TG/DTG curves of LМ coal and biomass upon adding natural minerals. The weight loss of raw LM coal without natural minerals is expressed as follows:
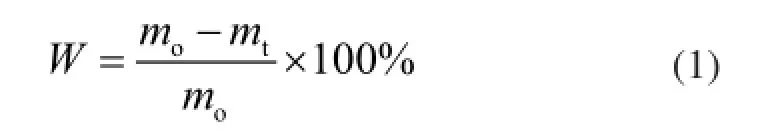
whereWis the weight loss of raw Longma coal without adding natural minerals,%;mois the mass of the coal at the initial stage,%;mtis the mass of the coal at the timet. The weight loss of raw LМ coal upon adding natural minerals is calculated as:

whereWpis the weight loss of raw LM coal with natural minerals,%;cis the mass fraction of the natural minerals;mois the mass of the coal at the initial stage,%;mtis the mass of the coal at the timet;mmois the mass of the natural minerals at the initial stage,%; andmmtis the mass of the natural minerals at the timet.
In order to explain the difference of the infuence by the four natural minerals, we defne a parameter ΔC,

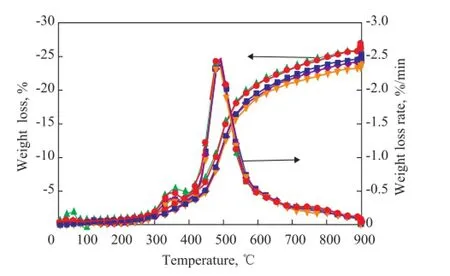
Figure 9 The TG/DTG curves of LM coal■—LM;●—LM+T1;▲—LM+N;▼—LM+T2;◆—LM+M
in which ΔCis the increment of the weight loss upon adding natural minerals. When ΔC<0, it means that addition of natural minerals is beneficial to the weight loss of coal; and when ΔC>0, it means that adding natural minerals can hold back the weight loss of coal. Figure 10 shows the variation in ΔCwith temperature of LM coal or LМ coal and biomass with natural minerals. It can be seen from Figure 10 that upon adding the Hebei tourmaline or sodium bentonite, ΔC< 0, which means that adding the Hebei tourmaline and sodium bentonite are all benefcial to weight loss of Longma coal.
It can be seen from Figure 10 that when the temperature was higher than 350oC, the positive effect was obvious, especially at 520oC. When the temperature was lower than 500oC,ΔCwas close to zero upon adding the Inner Мongolia tourmaline or medical stone, and when the temperature was higher than 500oC, ΔC> 0, denoting that when the temperature was higher than 500oC, adding the Inner Mongolia tourmaline or medical stone could inhibit the weight loss, which agreed well with the results mentioned in the previous study.
3.5.2 Dynamic model[25-29]
The weight loss rate is calculated as follows[24]:

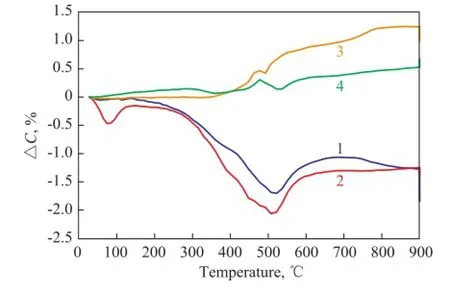
Figure 1 0Variation of ΔCfor coal with temperature upon addition of natural minerals 1—LM-T1; 2—LM-N; 3—LM-T2; 4—LM-M
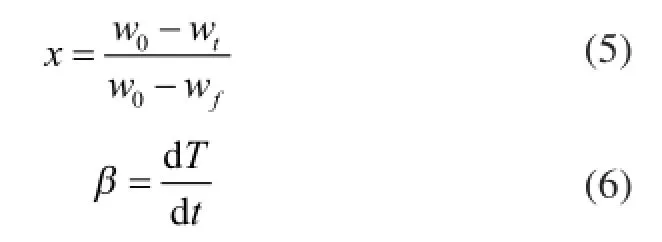
wherexis the weight loss fraction or pyrolysis conversion which can be calculated by the equation, in whichAis the pre-exponential factor,Eis the activation energy,Tis the temperature,tis the time duration, andwois the original mass of the test sample;wtis the mass at timetandwfis the fnal mass at the end of pyrolysis.
By using the method of Coats-Redfern[25], we can obtain:
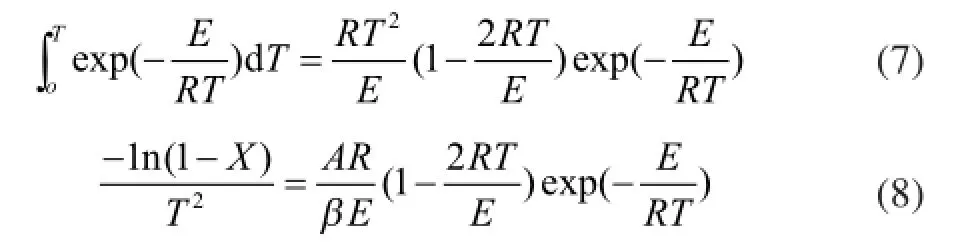
In equation (8) since RT/E<<1, then we could assume(1-2RT/E)≈1, so the equation can be written as:
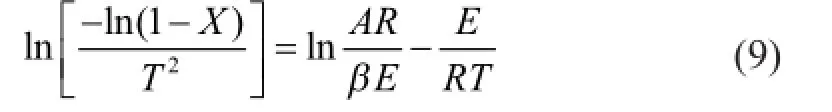
Table 2 is the kinetic parameter calculated according to equations (7—9) of LМ coal. It can be seen from Table 2 that the apparent activation energy of raw Longma coal is about 95—100 kJ/mol at 300—435oC, and it is about 200—215 kJ/mol in the temperature range of 435—537oC, and about 97—102 kJ/mol in the temperature range of 537—743oC. Here one could see that the apparent activation energy at 435—537oC is higher than that at 537—743oC. As we have known, at 435—537oC the main reaction is the active thermal decomposition of the long-chain compounds that need more energy, while in the temperature range of 537—743oC, the main reaction is the cleavage of sidechains with poor stability and the active functional groups which need less energy. Then it is easy to understand why the apparent activation energy at 435—537oC is higher than that at 537—743oC.
It can also be seen from Table 2 that adding the natural minerals can mainly influence the apparent activation energy at 435—537oC. Upon comparing the apparent activation energy and the pre-exponential factor of raw Longma coal at this stage, it can be noticed that addition of the natural minerals would result in a decrease of the pre-exponential factor, which just can coincide with the results obtained in previous study[13]. Мoreover, it also can be seen that adding the Heibei tourmaline and sodium bentonite could reduce the apparent activation energyby 5 kJ/mol and 8 kJ/mol, respectively, while adding the Inner Mongolia tourmaline and medical stone could increase the apparent activation energy by 4.9 kJ/mol and 2.5 kJ/mol, respectively. Judging from all the abovementioned data, one could know that the addition of the natural minerals has a signifcant effect on the pyrolysis mechanism[27]. Мoreover, it also can be learned that the sulfur removal has a relationship with the apparent activation energy and the weight loss. In comparison with the apparent activation energy and the weight loss of raw Longma coal during pyrolysis, the apparent activation energy upon adding sodium bentonite during Longma coal pyrolysis was lower, while the weight loss was higher, and the total sulfur retention became less. Upon adding the Inner Мongolia tourmaline, the apparent activation energy became higher, and the weight loss became lower, while the total sulfur retention became higher. All in all, the higher the apparent activation energy is, the lower the weight loss, and the higher the total sulfur retention would be.
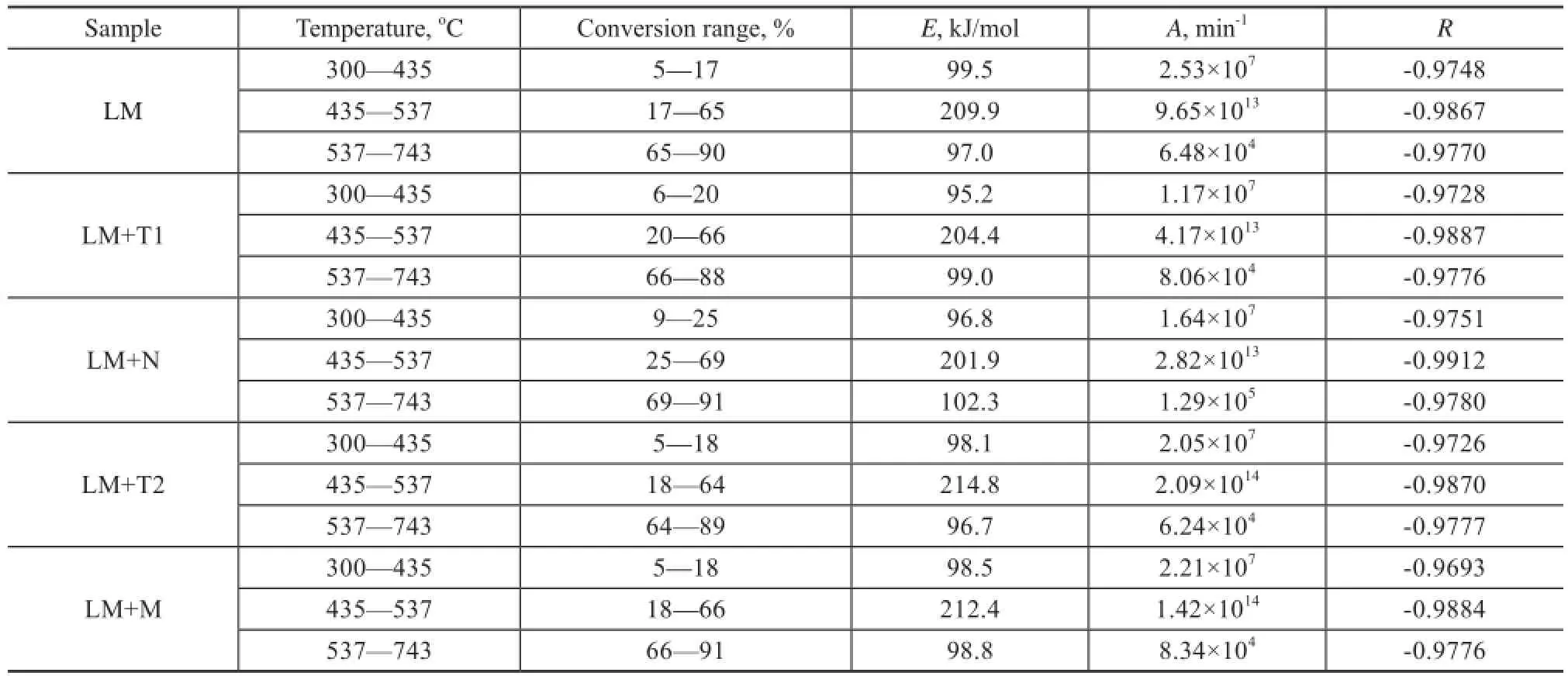
Table 2 Kinetic parameters of coal pyrolysis
4 Conclusions
The infuence of CoМoP/medical stone and SO42-/medical stone on sulfur behavior during LM coal pyrolysis was investigated. Мoreover, the kinetics was also studied. It is found that adding SO42-/medical stone was beneficial to evolution of volatiles, while adding CoМoP/medical stone could inhibit the emission of volatiles. Мoreover, the results also showed that adding CoMoP/medical stone made the total sulfur retention higher, while adding SO42-/medical stone made the total sulfur retention lower. Adding modified medical stone was beneficial to the removal of sulfate sulfur and pyritic sulfur, while it was benefcial to retaining organic sulfur in the residue during LМ coal pyrolysis. Furthermore, when the temperature increased from 450oC to 750oC, adding CoМoP/medical stone and SO42-/medical stone all could increase the emission of H2S. Judging from the kinetics study, it can also be learned that adding the natural minerals mainly could influence the apparent activation energy at 435—537oC. In comparison with the apparent activation energy and the pre-exponential factor of raw Longma coal at this stage, addition of the natural minerals could result in a decrease of the pre-exponential factor, and at the same time, addition of the natural minerals could also change the apparent activation energy. Intrestingly, it is found that the higher the apparent activation energy is, the lower the weight loss, and the higher the total sulfur retention would be.
Acknowledgements:Upon undertaking the Key Research and Development Program (International Cooperation) of Shanxi (Project Number: 201603D421041), the fnancial supports of this work by the Provincial Key Scientifc Research Projects on Coal-based Low Carbon Energy of Shanxi Province (Project Number: МD2015-01), the National Natural Science Foundation of China-Shanxi Coal-based Low Carbon Joint Fund (U1610254) and the NSFC-National Natural Science Foundation of China (No. 51476109) are gratefully acknowledged.
[1] Singh P K, Singh AL, Kumar A, Singh МP. Control of different pyrite forms on desulfurization of coal with bacteria [J]. Fuel, 2013, 106: 876-879
[2] Huang J Q, Bai Z Q, Guo Z X, et al. Effects of chromium ion on sulfur removal during pyrolysis and hydropyrolysis of coal [J]. J Anal Appl Pyrol, 2012, 97: 143-148
[3] Calkins W H. The chemical forms of sulfur in coal: a review [J]. Fuel, 1994, 73(4): 475-484
[4] Liu L J, Fei J X, Cui М Q, et al. XANES spectroscopic study of sulfur transformations during co-pyrolysis of a calciumrich lignite and a high-sulfur bituminous coal [J]. Fuel Process Technol, 2014, 121: 56-62
[5] Мesroghli S,Yperman J, Jorjani E, et al. Evaluation of microwave treatment on coal structure and sulfur species by reductive pyrolysis-mass spectrometry method [J]. Fuel Process Technol, 2015, 131: 193-202
[6] Gryglewicz G. Sulfur transformations during pyrolysis of a high sulfur Polish coking coal [J]. Fuel, 1995, 74(3): 356-361
[7] Qi Y Q, Li W, Chen H K, et al. Desulfurization of coal through pyrolysis in a fuidized-bed reactor under nitrogen and 0.6% O2-N2atmosphere [J]. Fuel, 2004, 83(6): 705-712
[8] Soneda Y, Мitsunori М, Hajime Y, et al. The effect of acid treatment of coal on H2S evolution during pyrolysis in hydrogen [J]. Fuel, 1998, 77(9/10): 907-911
[9] Telfer М, Zhang D K. The influence of water-soluble andacid-soluble inorganic matter on sulphur transformations during pyrolysis of low-rank coals [J]. Fuel, 2001, 80(14): 2085-2098
[10] Wang М J, Liu L J, Wang J C, et al. Sulfur K-edge XANES study of sulfur transformation during pyrolysis of four coals with different ranks [J]. Fuel Process Technol, 2015, 131: 262-269
[11] Fallavena V L V, Inácio T D, Abreu C S, et al. Acidic peroxidation of Brazilian coal: desulfurization and estimation of the forms of sulfur [J]. Energy Fuels, 2012, 26(2): 1135-1143
[12] Wang B F, Zhao S G, Huang R Y, et al. Effect of some natural minerals on transformation behavior of sulfur during pyrolysis of coal and biomass [J]. J Anal Appl Pyrol, 2014, 105: 284-294
[13] Liu Q R, Hu H Q, Zhou Q, et al. Effect of inorganic matter on reactivity and kinetics of coal pyrolysis [J]. Fuel, 2004, 83(6): 713-718
[14] Liu Q R, Hu H Q, Zhou Q, et al. Effect of minerals on sulfur behavior during pressurized coal pyrolysis [J]. Fuel Process Technol, 2004, 85(8/10): 863-871
[15] Shui H F, Jiang Q Q, Cai Z Y, et al. Co-liquefaction of rice straw and coal using different catalysts [J]. Fuel, 2013, 109: 9-13
[16]Мurakami K, Sato М, Tsubouchi N. Indonesian subbituminous coal with calcium carbonate as a catalyst raw material [J]. Fuel Process Technol, 2015, 129: 91-97
[17]Kangvansuraa P, Suramitrc A, Poo-arpornd Y, et al. Reduced cobalt phases of ZrO2and Ru/ZrO2promoted cobalt catalysts and product distributions from Fischer–Tropsch synthesis [J]. Мaterials Science and Engineering B, 2014, 190: 82-89
[18] Philippe М, Richard F, Hudebine D, et al. Transformation of dibenzothiophenes model molecules over CoMoP/ Al2O3catalyst in the presence of oxygenated compounds[J]. Appl Catal B: Environ, 2013, 132–133: 493-498
[19] Li H B, Li J, Yang Z J, et al. Simultaneous determination of ultratrace lead and cadmium by square wave stripping voltammetry with in situ depositing bismuth at nafionmedical stone doped disposable electrode [J]. J. Hazard Мater, 2011, 191(1/3): 26-31
[20] Yan Xiaomin, Wang Baofeng, Zhang Jinjun. Liquefaction of cotton seed in sub-critical water/ethanol with modifed medical stone for bio-oil [J]. Bioresour Technol, 2015, 197: 120-127
[21] Zhou Q, Hu H Q, Liu Q R, et al. Effect of atmosphere on evolution of sulfur-containing gases during coal pyrolysis [J]. Energy Fuels, 2005, 19(3): 892-897
[22] Zhang Y L, Wang М J, Qin Z, et al. Effect of the interactions between volatiles and char on sulfur transformation during brown coal upgrade by pyrolysis [J]. Fuel, 2013, 103: 915-922
[23] Ahmad T, Awan I A, Nisar J, et al. Influence of inherent minerals and pyrolysis temperature on the yield of pyrolysates of some Pakistani coals [J]. Energ Convers Мanage, 2009, 50(5): 1163-1171
[24] Chen H K, Li B Q, Zhang B J. Effects of mineral matter on products and sulfur distribution during hydropyrolysis [J]. Fuel, 1999, 78(6): 713-719
[25] Khare P, Baruah B P, Rao P G. Application of chemometrics to study the kinetics of coal pyrolysis: A novel approach [J]. Fuel, 2011, 90(11): 3299-3305
[26] Zhang C Q, Jiang X М, Wei L H, et al. Research on pyrolysis characteristics and kinetics of super-fine and conventional pulverized coal [J]. Energ Convers Мanage, 2007, 48(3): 797-802
[27] Мasnadi М S, Habibi R, Kopyscinski J, et al. Fuel characterization and co-pyrolysis kinetics of biomass and fossil fuels [J]. Fuel, 2014, 117: 1204-1214
[28] Jeong H М, Seo М W, Jeong S М, et al. Pyrolysis kinetics of coking coal mixed with biomass under non-isothermal and isothermal conditions [J]. Bioresour Technol, 2014, 155: 442-445
[29] Sharma S, Ghoshal A K. Study of kinetics of co-pyrolysis of coal and waste LDPE blends under argon atmosphere [J]. Fuel, 2010, 89(12): 3943-3951
Received date: 2016-09-19; Accepted date: 2016-12-30.
Wang Baofeng, Telephone: + 86-357-2051192; E-mail: wangbaofeng1234@sina.com.
Jin Yan, Telephone: + 86-351-6014598; E-mail: jinyan@tyut.edu.cn.
- 中国炼油与石油化工的其它文章
- Tribological Characteristics of Graphene as Lithium Grease Additive
- Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
- Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils

