Preparation of Bi2WO6and Its Ultra-Deep Oxidative Desulfurization Performance in Ionic Liquids
Xing Pengfei; Zhao Rongxiang; Li Xiuping; Gao Xiaohan
( College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001 )
Preparation of Bi2WO6and Its Ultra-Deep Oxidative Desulfurization Performance in Ionic Liquids
Xing Pengfei; Zhao Rongxiang; Li Xiuping; Gao Xiaohan
( College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001 )
The micro-spheric Bi2WO6was synthesized by a simple one-step hydrothermal method. Bi2WO6crystals were characterized using XRD, SEМ, EDS and BET techniques. Bi2WO6, H2O2and [HMIM][BF4] served as catalyst, oxidant and extracting agent in the oxidative desulfurization system (ODS), respectively. The infuence of extraction agent type, oxidant usage, catalyst dosage, temperature, sulfur compound type and other factors on the oxidative desulfurization was studied in the present work. The experimental results demonstrate that Bi2WO6possesses high activity for desulfurization of dibenzothiophene (DBT) and benzothiophene (BT). The desulfurization rate of DBT and BT in model oil could reach 98.1% in 80 minutes and 96.2% in 120 min at 70oC, respectively. Мoreover, the desulfurization performance of catalyst for DBT hardly changed after being recycled for 10 times.
Bi2WO6; catalyst; oxidative desulfurization; [HMIM][BF4]; dibenzothiophene
1 Introduction
SOxemission from fuel oil pollutes the environment and endangers human health[1-2]. Therefore, many countries in the world have formulated stringent environmental regulations to limit sulfur content in the fuel (S content <10 μg/g)[3]. Traditional hydrodesulfurization (HDS) can only be used to remove some of the sulfur compounds in fuel, such as thiols, sulfides, disulfides, etc. However, these sulfur compounds, such as DBT and its derivatives, can hardly be removed from fuel. For removing these sulfur compounds, some operating conditions (high temperature, high pressure and high hydrogen consumption) and large capital cost are needed[4-5]for implementing the HDS process. Thus, non-hydrodesulfurization technologies have been developed[6-12], such as oxidative desulfurization, biological desulfurization, absorptive desulfurization, extractive desulfurization, etc. Among these techniques, catalytic oxidative desulfurization is the most attractive technologies due to its mild reaction conditions and high sulfur removal rate.
In the ODS process, sulfur compounds are oxidized into the corresponding sulfoxide and sulfone, which can be readily removed by extraction methods[13]. A lot of oxidation agents have been used, such as oil-soluble oxidants[14], H2O2[15], and solid oxidizing agents[16]. Among these oxidants, H2O2is the most attractive because the by-product is H2O, so it does not pose a new threat of pollutant in the reaction system. Thus, H2O2is considered as a green oxidant.
The catalyst also play an important role in ODS process. Sodium tungstate as an efficient catalyst is used in the oxidative desulfurization process. For example, Liu Dan, et al.[17-18]reported that the desulfurization rate could reach up to 100% using sodium tungstate as catalyst in the ODS process. Collins, et al.[19]indicated that the desulfurization rate can reach 96% using sodium tungstate as catalyst in the presence of H2O2. However, it is difficult to recycle and reuse the catalyst due to the fact that sodium tungstate can easily dissolve in water.
In this study, Bi2WO6was synthesized by the simple onestep hydrothermal method. The oxidative desulfurization process was studied using Bi2WO6as the catalyst, H2O2as the oxidant and [HMIM][BF4] as extracting agent.Bi2WO6was highly effcient on the removal of DBT and could be easily separated for the recycling mixture under mild conditions. In addition, the reaction mechanism of the catalyst was discussed in detail.
2 Experimental
2.1 Chemical reagents
30% hydrogen peroxide (H2O2, A.R.), bismuth (III) nitrate pentahydrate (Bi(NO3)3·5H2O, A.R.) andn-octane (A.R.), were purchased from the Sinopharm Shanghai Chemical Reagent Co. Ltd. Dibenzothiophene (DBT, 98%), benzothiophene (BT, 99%), and thiophene (TH, 99.8%) were purchased from the Aladdin Industrial Corporation. Sodium tungstate (Na2WO4·2H2O, 99%) was purchased from the No. 2 Chemical Reagent Factory. 1-n-Hexyl-3-methylimidazolium tetrafluoroborate ([HMIM][BF4]) and 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM] [EtSO4]) were purchased from the Shanghai Chengjie Chemical Co. Ltd. All reagents were directly used without further purifcation.
2.2 Characterization
X-Ray powder diffraction (XRD) analysis of the samples was carried out at room temperature on a BRUKER D8 ADVANCE X-ray powder diffractometer (Bruker Corp.) with Cu-Kα radiation (λ=0.154 06 nm) and a scanning speed of 10(°)/min, with the accelerating voltage and emission current equating to 40 kV and 40 mA, respectively. Scanning electron microscopy (SEМ) and energy dispersive spectroscopy (EDS) were performed on a FEI Sirion200 scanning electron microscope (USA), using a scanning voltage of 5.00 kV for studying the morphology and elements of samples. The N2adsorptiondesorption measurement was tested using a TriStar II 3020 surface area and porosity analyzer (Micromeritics Instrument Corp.). The infrared spectroscopic analysis was implemented on a WQF-520 Fourier-transform infrared spectrometer (Beifen Ruili Analytical Instrument Company).
2.3 Preparation of the catalysts
The micro-spheric Bi2WO6was synthesized by the onestep hydrothermal method. Bi(NO3)3·5H2O (4.85 g) was dissolved in 40 mL of water prior to staying overnight to get a transparent solution (labeled as solution A); Na2WO4·2H2O (1.645 g) was dissolved in 40 mL of water to get a transparent solution (labeled as solution B). The solution B was added into the solution A under stirring, with the transparent mixture solution entering into reaction for 15 minutes. The transparent solution was placed into an 80-mL Tefon-lined autoclave at 160oC for 24 h. Then, the suspension solution was separated by centrifugation, washed with water for three times and dried in an oven at 100oC for 24 h.
2.4 Oxidative desulfurization procedure of model fuelThe model oil was obtained by dissolving 1.437 g of DBT in 500 mL ofn-octane, with its initial sulfur content reaching 500 μg/g. The catalyst, model oil, ionic liquids and H2O2(30%) were successively added into an 100-mL Erlenmeyer flask equipped with a condenser. Then the Erlenmeyer fask was heated in a water bath provided with a magnetic stirrer. After a specifed time, the reaction system was naturally cooled down to room temperature before separation into two layers. The sulfur content in the upper oil phase was tested with a WK-2D micro computer coulomb comprehensive analyzer.
3 Results and Discussion
3.1 Phase analysis of samples
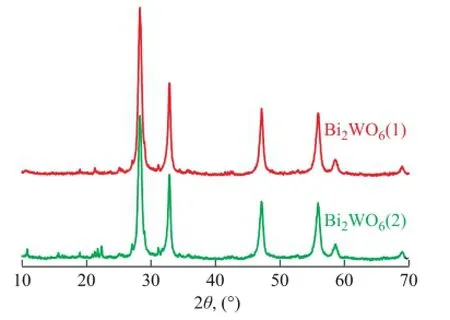
Figure 1 The XRD patterns of the samples (1)—The fresh Bi2WO6sample; (2)—Used Bi2WO6sample after 10 cycles
The crystal phase and structure of Bi2WO6were analyzed by XRD technique. The results are shown in Figure 1. According to XRD patterns, the peaks at 28.3°, 32.8°, 47.2°, 55.8°, 58.5° and 68.7° are attributed to the plane of (113), (024), (220), (313), (226) and (400) of Bi2WO6, which are consistent with the standard crystal card ofBi2WO6(JCPDS No. 39-0256). The Bi2WO6sample exhibits higher intensity and narrower diffraction peaks in Figure 1, the results indicate that the crystallinity and purity of Bi2WO6are high. The pattern peaks of the used and the fresh catalyst samples hardly change, which demonstrates that the catalyst is preeminently stabilized during the ODS reaction.
3.2 The morphology and energy dispersive spectroscopy
Figure 2 shows SEM pictures of the Bi2WO6sample. It can be seen that micro-sphere Bi2WO6is assembled with hundreds of nanosheets. The diameter of microspheres is about 3—5 μm. The enlarged image of the sample is shown in Figure 2 (b), where the micro-spheric Bi2WO6is composed of thin sheets according to a certain rule. It can be observed that the as-prepared samples only contain O, W and Bi elements, and no other impurity peaks appear in Figure 3, which demonstrates that Bi2WO6samples exhibit a high purity.
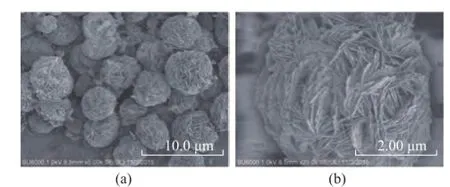
Figure 2 SEM pictures of the Bi2WO6samples
3.3 N2adsorption-desorption analysis
In order to study the specifc surface area and pore size distribution, N2adsorption-desorption analysis was carried out for the as-synthesized Bi2WO6. The isotherms can be observed in Figure 4 at a relative pressure (P/P0) of 0.5—1.0 with a hysteresis loop, showing the characteristics of the IV types of curves. The pore size distribution curve in the illustration confirms that the structure of the microspheric Bi2WO6is comprised of 2—50 nm mesopores, with the pore size being centrally located at about 18.131 3 nm. There is a large mesopore sized up to about 50 nm, which is formed due to overlap between fower-like Bi2WO6, and smaller pores are formed due to crossed arrangement between nanosheets. The specific surface area and pore volume of the sample are 28.554 0 m2/g and 0.127 806 cm3/g, respectively.

Figure 3 EDS pictures of the Bi2WO6sample
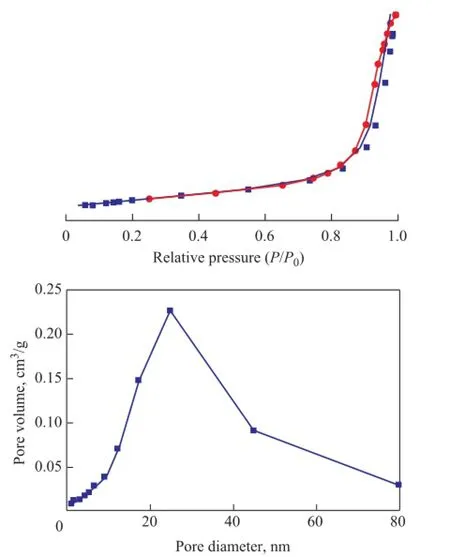
Figure 4 N2adsorption-desorption and pore size distribution of the fower-like Bi2WO6
3.4 Optimizing the reaction conditions of model fuel
3.4.1 Influence of different desulfurization systems on removal rate of DBT
Four different oxidation desulfurization systems, [EMIM][EtSO4]/H2O2, [EМIМ][EtSO4]/ H2O2/Bi2WO6, [HMIM][BF4]/H2O2and [HMIM][BF4]/H2O2/Bi2WO6were chosen for studying their effect on removal of DBTunder same conditions. Experimental results are shown in Table 1. The desulfurization rate of DBT in model oil only reached 10% in the system of [EMIM]EtSO4/ H2O2; however, the desulfurization rate increased to 24.2% with the addition of Bi2WO6in [EMIM][EtSO4]/ H2O2. When [HМIМ][BF4]/H2O2was used as a system to remove DBT in model oil, the desulfurization rate of DBT increased to 46.1%. In the system of [HМIМ] [BF4]/H2O2/Bi2WO6, the desulfurization rate of DBT increased sharply to 98.7%. These results showed that the type of ionic liquid and Bi2WO6had important influence on the desulfurization efficiency. Compared with two kinds of ionic liquids, [HМIМ][BF4] exhibited higher desulfurization activity due to its stronger extraction ability[20]. However, the desulfurization rate of the system still significantly increased by the addition of Bi2WO6. Obviously, the desulfurization system containing Bi2WO6, [HМIМ][BF4] and H2O2was evidently superior to other desulfurization systems studied in the present work.
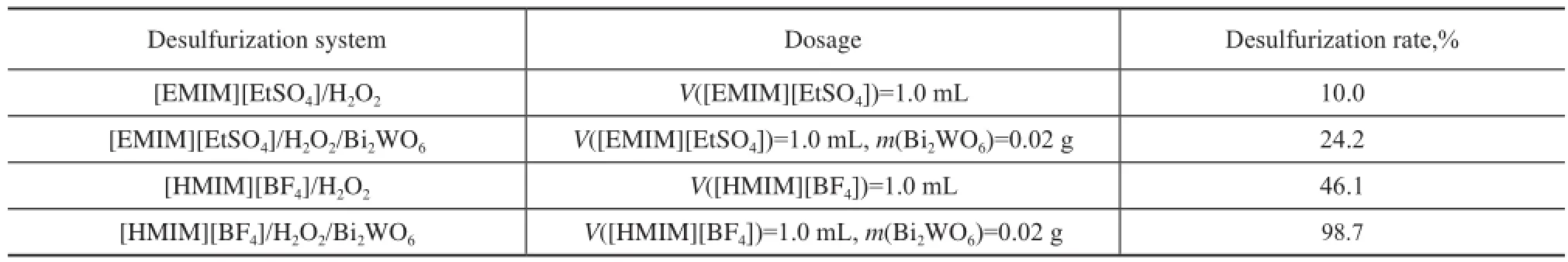
Table 1 Infuence of different desulfurization system on sulfur removal of DBT
3.4.2 Investigation of conditions adopted in the ODS process
Bi2WO6was used as the catalyst, H2O2as the oxidation agent, and [HМIМ][BF4] as the extraction agent in the oxidative desulfurization process. The reaction conditions were studied for obtaining the optimal desulfurization effect, as shown in Figure 5. The desulfurization rate could reach 98.7% under the optimal conditions (V(DBT, oil)=5 mL,w(DBT)=500 μg/g,T=70oC,V([HMIM] [BF4])=1.0 mL,m(Bi2WO6)=0.04 g,n(O)/n(S)=15,t=180 min). The desulfurization rate was enhanced by increasing the amount of Bi2WO6,n(O)/n(S), [HМIМ]BF4and raising the reaction temperature in the ODS process. However, excessive Bi2WO6,n(O)/n(S) and [HMIM] BF4for 5 mL of model oil could no longer accelerate the desulfurization rate. Besides, higher reaction temperature would lead to the decomposition of H2O2into H2O.
3.4.3 Influence of different sulfur compounds on sulfur removal rate
The catalyst for different sulfur compounds in model oil has different oxidation activity. The experimental data are shown in Figure 6, with the sulfur removal rate decreasing in the following order: DBT > BT > TH under the same reaction conditions. The desulfurization rate of DBT, BT and TH reached 98.7% in 80 min, 97.2% in 120 min and 51.3% in 140 min, respectively. The desulfurization rate relied on the electron density of the sulfur atoms contained in organic sulfides. Generally, lower electron density in sulfur atom has a lower desulfurization rate. For DBT, BT and TH, the electron density of the sulfur atom is 5.785, 5.739 and 5.696[21], respectively. Hence, the activity was related with the infuence of the electron density.
3.4.4 The recycling performance of the catalyst
The performance on recovery of the catalyst activity was researched during the ODS reaction. After each desulfurization reaction, the catalyst was separated by centrifugation. The separated catalyst was dried in a vacuum oven at 100oC. Subsequently, the recovered catalyst, fresh model oil, ionic liquids and H2O2were placed in the Erlenmeyer fask under the same reaction conditions. The experimental results are shown in Figure 7. It was found that the Bi2WO6after being recycled for ten times did not show a decrease in its activity. Thereby, the XRD pattern of recycled Bi2WO6was determined. It can be observed that the diffraction peaks of the used Bi2WO6were consistent with those of the fresh catalyst shown in Figure 2. The results demonstrate that the catalyst is quite stable during the ODS reaction.

Figure 5 The conditions of oxidative desulfurization(a)Infuence of the amount of H2O2on the desulfurization rate (V(DBT, oil)=5 mL,w(DBT)=500 μg/g,V([HMIM][BF4])=1.0 mL,T=70oC,m(Bi2WO6)=0.02 g,t=180 min); (b) Infuence of catalyst amount on the desulfurization rate (V(DBT, oil)=5 mL,w(DBT)=500 μg/g,V([HMIM][BF4])=1.0 mL,T=70oC,n(O)/n(S)=15,t=180 min); (c) Infuence of reaction temperature on the desulfurization rate (V(DBT, oil)=5 mL,w(DBT)=500 μg/g,V([HMIM][BF4])=1.0 mL,m(Bi2WO6)=0.04 g,n(O)/n(S)=15,t=180 min); (d) Infuence of the IL amount on the desulfurization rate (V(DBT, oil)=5 mL,w(DBT)=500 μg/g,T=70oC,m(Bi2WO6)=0.04 g,n(O)/n(S)=15,t=180 min)
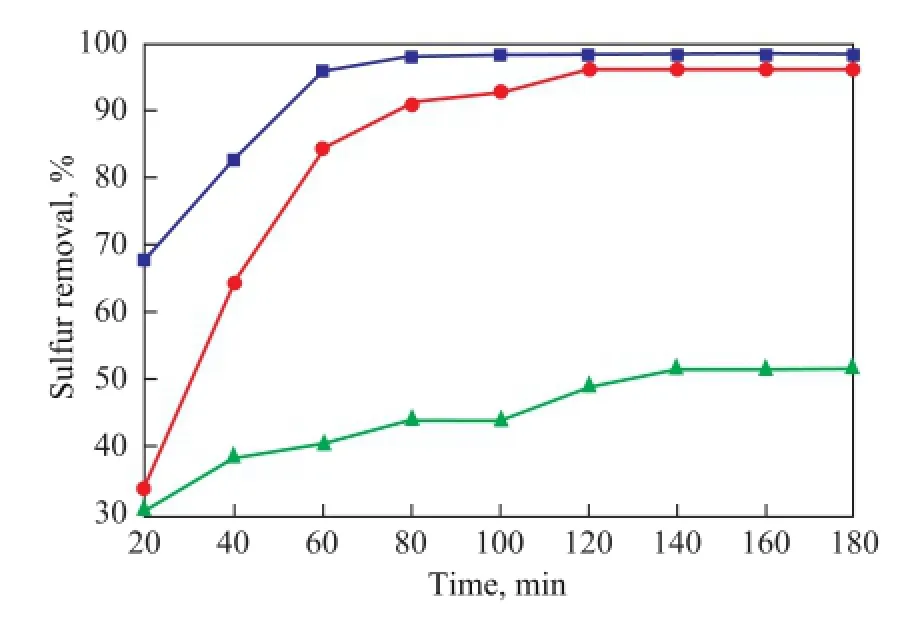
Figure 6 Sulfur removal activity of the reaction system for different sulfur compounds (V(Oil)=5 mL,w(DBT)=500 μg/g,w(BT)=500 μg/g,w(TH)=500 μg/g,T=70oC,m(Bi2WO6)=0.04 g,n(O)/n(S)=15,V([HMIM][BF4])=1.0 mL,t=180 min)■—DBT;●—BT;▲—TH
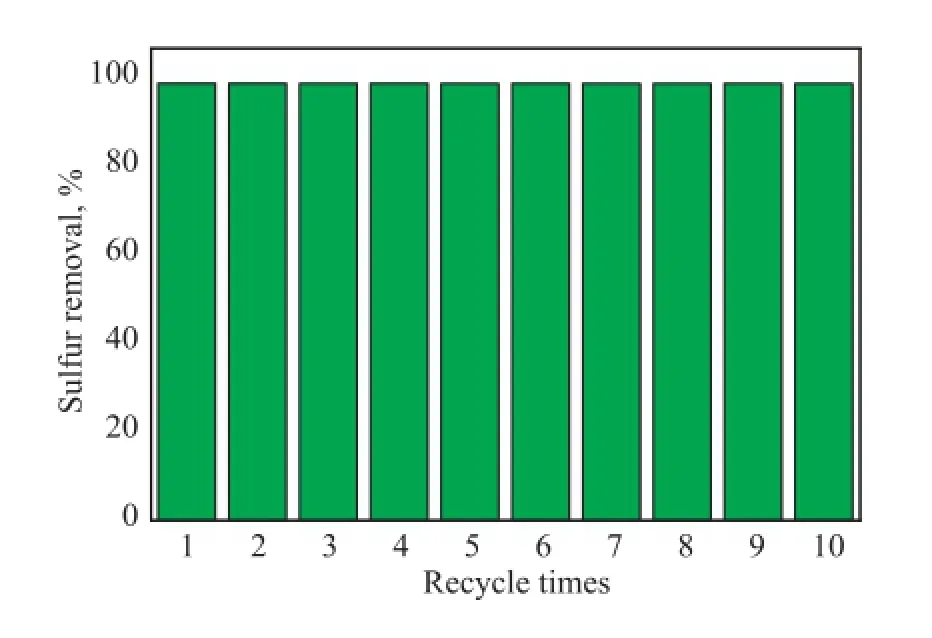
Figure 7 The recycling performance of the catalyst (V(DBT, oil)=5 mL,w(DBT)=500 μg/g,T=70oC,m(Bi2WO6)=0.04 g,n(O)/n(S)=15,V([HMIM][BF4])=1.0 mL,t=180 min.)
In order to ascertain the type of oxidation products derived from these sulfur compounds, the reverse extraction experiment was implemented using carbon tetrachloride as the solvent. Then, the carbon tetrachloride phase was removed by rotary evaporation and a white solid was obtained. The structure of the gained white sedimentwas measured by FT-IR spectroscopy, with the results shown in Figure 8. In comparison with the adsorption peaks for DBT, there were absorption peaks for sulfone at 128 6 cm-1and 116 8 cm-1[22], and also absorption peak for sulfoxide at 104 5 cm-1[22], demonstrating that DBT in model oil was oxidized to DBTO and DBTO2when Bi2WO6was employed as the catalyst.
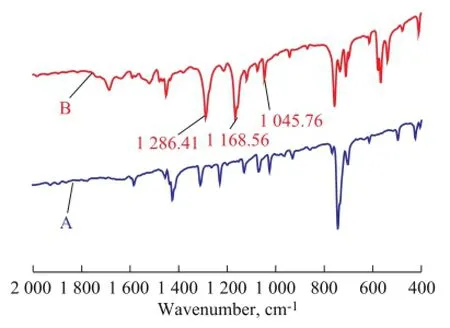
Figure 8 FT-IR spectra of DBT (A) and its oxidation products (B)
3.4.5 Reaction mechanism of oxidative desulfurization
Recently, sodium tungstate was chosen as the catalyst in the ODS process. The activity of the catalyst mainly derives from peroxotungstate, which is obtained through oxidation of sodium tungstate by H2O2. Мajid Vafaeezadeh, et al.[23]synthesized silver tungstate, which served as the catalyst for the photoxidative cleavage of cyclohexene to adipic acid. Research shows that silver tungstate can form peroxotungstate under the action of hydrogen peroxide. The peroxotungstate plays an important role in the formation of oxidation products. Sivasankara Rao Ede, et al.[24]found that SnWO4can be oxidized by H2O2to form Sn(WO(O2)2(OH)2). The Sn(WO(O2)2(OH)2) plays a crucial role for the oxidation of butanol to butanoic acid. Inspired by these facts, we believe that bismuth tungstate can also be oxidized by hydrogen peroxide into corresponding peroxide.
The oxidative desulfurization process of sulfur compounds contains two steps. One is the extract and other is the oxidation reaction[25]. Firstly, the DBT in model oil is extracted into IL phase. DBT is oxidized by Bi[WO(O2)2(OH)2] into the corresponding sulfoxide[24]. Then the oxidation reaction of sulfur compounds could lead to the reduction of the concentration of DBT in the ionic liquid to further realize the extraction process and oxide formation. Therefore, the ultra-deep removal of sulfur compounds could be attained. The mechanism of catalytic oxidation and extraction of products derived from for DBT is described in Figure 9.
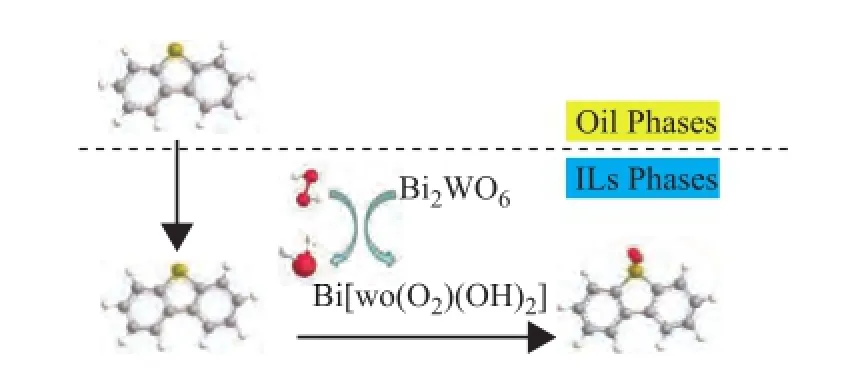
Figure 9 Extraction and catalytic oxidation course of deep oxidative desulfurization process
4 Conclusions
In conclusion, the micro-spheric Bi2WO6was synthesized by the simple one-step hydrothermal method. The Bi2WO6was used as the catalyst, H2O2as the oxidant and [HMIM]BF4as the extraction agent for the ultradeep desulfurization of model oil. Under the optimal experimental conditions (V(Oil)=5 mL,w(DBT)=500 μg/g,T=70oC,m(Bi2WO6)=0.04 g,n(O)/n(S)=15, V([HМIМ] [BF4])=1.0 mL), the sulfur removal of DBT and BT could reach 98.7% in 80 min and 97.2% in 120 min. The results demonstrate that the oxidative desulfurization process is highly effective for treating DBT and BT. The catalytic activity did not decline after 10 times of catalyst recycles, which demonstrated that the catalyst was stable because it did not lose its activity in the heterogeneous ODS reaction.
Acknowledgements:The authors also acknowledge the financial support of the Natural Science Foundation of China (Project No. 21003069) and the Doctoral Fund of Liaoning Province (201501105).
[1] Zhu W, Wang C, Li H, et al. One-pot extraction combined with metal-free photochemical aerobic oxidative desulfurization in deep eutectic solvent[J]. Green Chemistry, 2015, 17(4): 2464-2472
[2] Li F, Liu R, Wen J, et al. Desulfurization of dibenzothiophene by chemical oxidation and solvent extraction with Me3NCH2C6H5Cl· 2ZnCl2ionic liquid[J]. Green Chemistry, 2009, 11(6): 883-888
[3] Eßer J, Wasserscheid P, Jess A. Deep desulfurization of oil refinery streams by extraction with ionic liquids[J]. Green Chemistry, 2004, 6(7): 316-322
[4] Li H Y, Gao J B, Jiang Z X. Ultra-deep desulfurization of diesel by selective oxidation with [C18H37N(CH3)3]4[H2NaPW10O36] catalyst assembled in emulsion droplets[J]. J Catal, 2006, 239(2): 369
[5] Wang Z, Chen S L, Pei J, et al. Insight into the intraparticle diffusion of residue oil components in catalysts during hydrodesulfurization reaction[J]. AIChE Journal, 2014, 60(9): 3267-3275
[6] Campos-Мartin J М, Capel-Sanchez М C, Fierro J L G. Highly efficient deep desulfurization of fuels by chemical oxidation[J]. Green Chemistry, 2004, 6(11): 557-562
[7] Li C, Jiang Z, Gao J, et al. Ultra-deep desulfurization of diesel: Oxidation with a recoverable catalyst assembled in emulsion[J]. Chemistry-A European Journal, 2004, 10(9): 2277-2280
[8] Hernández‐Мaldonado A J, Yang R T. Desulfurization of transportation fuels by adsorption[J]. Catalysis Reviews, 2004, 46(2): 111-150
[9] Babich I V, Мoulijn J A. Science and technology of novel processes for deep desulfurization of oil refnery streams: A review[J]. Fuel, 2003, 82(6): 607-631
[10] Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catalysis Today, 2003, 86(1): 211-263
[11] Ito E, Van Veen J A R. On novel processes for removing sulphur from refinery streams[J]. Catalysis Today, 2006, 116(4): 446-460
[12] Rang H, Kann J, Oja V. Advances in desulfurization research of liquid fuel[J]. Oil Shale, 2006, 23(2): 164-176
[13] Anisimov A V, Fedorova E V, Lesnugin A Z, et al. Vanadium peroxocomplexes as oxidation catalysts of sulfur organic compounds by hydrogen peroxide in biphase systems[J]. Catalysis Today, 2003, 78(1): 319-325
[14] Venkateshwar Rao T, Sain B, Kafola S, et al. Oxidative desulfurization of HDS diesel using the aldehyde/ molecular oxygen oxidation system[J]. Energy & Fuels, 2007, 21(6): 3420-3424
[15] Jiang X, Li H, Zhu W, et al. Deep desulfurization of fuels catalyzed by surfactant-type decatungstates using H2O2as oxidant[J]. Fuel, 2009, 88(3): 431-436
[16] De Filippis P, Scarsella М. Functionalized hexagonal mesoporous silica as an oxidizing agent for the oxidative desulfurization of organosulfur compounds[J]. Industrial & Engineering Chemistry Research, 2008, 47(3): 973-975
[17] Liu D, Gui J, Ding J, et al. Oxidation of dibenzothiophene catalyzed by Na2WO4in a halogen-free ionic liquid[J]. Reaction Kinetics, Мechanisms and Catalysis, 2011, 104(1): 111-123
[18] Liu D, Gui J, Liu D, et al. Deep oxidative desulfurization of real diesel catalyzed by Na2WO4in ionic liquid[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2013, 35(1): 1-8
[19] Rakhmanov E V, Anisimov A V, Tarakanova A V, et al. Oxidative desulfurization of catalytically cracked gasoline with hydrogen peroxide[J]. Petroleum Chemistry, 2013, 53(3): 201-204
[20] Lu L, Cheng S, Gao J, et al. Deep oxidative desulfurization of fuels catalyzed by ionic liquid in the presence of H2O2[J]. Energy & Fuels, 2007, 21(1): 383-384
[21] Zhang J, Zhu W, Li H, et al. Deep oxidative desulfurization of fuels by Fenton-like reagent in ionic liquids[J]. Green Chemistry, 2009, 11(11): 1801-1807
[22] Zhang H, Gao J, Мeng H, et al. Removal of thiophenic sulfurs using an extractive oxidative desulfurization process with three new phosphotungstate catalysts[J]. Industrial & Engineering Chemistry Research, 2012, 51(19): 6658-6665
[23] Vafaeezadeh М, Hashemi М М. One pot oxidative cleavage of cyclohexene to adipic acid using silver tungstate nanorods in a Brønsted acidic ionic liquid[J]. RSC Advances, 2015, 5(40): 31298-31302
[24] Ede S R, Kundu S. Мicrowave synthesis of SnWO4nanoassemblies on DNA scaffold: A novel material for high performance supercapacitor and as catalyst for butanol oxidation[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(9): 2321-2336
[25] Zhao D, Wang J, Zhou E. Oxidative desulfurization of diesel fuel using a Brønsted acid room temperature ionic liquid in the presence of H2O2[J]. Green Chemistry, 2007, 9(11): 1219-1222
Received date: 2016-08-29; Accepted date: 2016-10-19.
Dr. Zhao Rongxiang, E-mail: zylhzrx@126.com.
- 中国炼油与石油化工的其它文章
- Tribological Characteristics of Graphene as Lithium Grease Additive
- Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
- Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils

