Experimental Study on Preparation of Natural Gas Hydrate by Crystallization
Ma Shihui; Pan Zhen; Li Ping; Wu Yuguo; Li Bingfan; Kang Jinke; Zhang Zhien
(1. College of Petroleum Engineering, Liaoning Shihua University, Fushun 113001; 2. College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001; 3. College of Mechanical Engineering, Liaoning Shihua University, Fushun 113001; 4. School of Chemistry and Chemical Engineering, Chongqing University of Technology, Chongqing 400054)
Experimental Study on Preparation of Natural Gas Hydrate by Crystallization
Ma Shihui1; Pan Zhen1; Li Ping2; Wu Yuguo1; Li Bingfan1; Kang Jinke3; Zhang Zhien4
(1. College of Petroleum Engineering, Liaoning Shihua University, Fushun 113001; 2. College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001; 3. College of Mechanical Engineering, Liaoning Shihua University, Fushun 113001; 4. School of Chemistry and Chemical Engineering, Chongqing University of Technology, Chongqing 400054)
In this paper, the saturated solution crystallization method is proposed to promote the formation of hydrate by means of the known similarities between the hydrate formation process and the crystallization process. In this method, adding the second phase crystals was used to replace the spontaneous formation of hydrate crystal nuclei to form hydrate. The effects of saturated Na2SO4, МgSO4, NH4HCO3and CuSO4solutions on the formation rates of natural gas hydrate and gas storage capacity were investigated. The results showed that the saturated solution had an infuence on the hydrate formation process. Under the given experimental conditions, the saturated Na2SO4solution showed a highest increase in the hydrate formation rate, and the average hydrate formation rate in its presence was 11.8 times higher than that obtained in the deionized water. Мoreover, the largest formation rate of gas hydrates observed in the saturated Na2SO4solution was 386 times bigger than that in the deionized water, and the gas storage capacity increased by 10 times. In addition, the average hydrate formation rate in the saturated MgSO4solution was faster than that in water by 20 times. The largest formation rate of gas hydrates in the saturated MgSO4solution was 165 times faster than that obtained in the deionized water, and the gas storage capacity increased by 6.2 times. The saturated NH4HCO3and saturated CuSO4solutions also infuenced the formation process of hydrate. Therefore, the crystallization method of saturated solution can be used to achieve a higheffciency preparation of natural gas hydrates, which provides theoretical guidance for the storage of natural gas in the form of hydrate.
natural gas hydrate; saturated solution; crystal seeds; formation rate; gas storage capacity; phase equilibrium
1 Introduction
Clathrate hydrates or gas hydrates, are normally stabilized by the guest molecules enclathrated in hydrogen-bonded water cages such as CO2and CH4[1-2]. Generally, in terms of hydrate size[3], gas hydrates consist of three crystalline structures : viz.: structure I, structure II and structure H. Gas hydrates are an alternative method of transporting natural gas because of their advantages of high energy storage density, satisfactory storage safety, and relatively low cost, especially in comparison with the liquefied natural gas (LNG)[4-5]However, there are some limitations for their further development. For example, the amount of natural gas dissolved in water is small, and the induction period is extremely unstable. Therefore, it is important to make gas hydrates safer and more effcient. In order to increase the gas hydrate formation rate and gas storage capacity, Fan, et al. found that the porous medium can signifcantly shorten the required natural gas hydrate formation time, and the diameter of the porous medium affected the formation process of hydrate[6]. The effect of porosity on gas hydrate was studied by Asheesh, et al.[7]The microstructures of natural gas hydrate in porous media were observed by Zhao, et al. using a high precision micro CT system[8]. Gas hydrate formation from two types of dissolved gases (methane and mixed gas) was studied by Zang, et al. in a novel apparatus containing two different natural media from the SouthChina Sea under various thermodynamic conditions[9]. The mechanism of methane hydrate formation in light weight hollow silica was studied by Veluswamy, et al.[10]In addition, Babu, et al. illuminated the influence of propane as a co-guest during hydrate formation in silica sand[11]. Liu, et al. analyzed the effects of fine particle diameters on the hydrate phase equilibrium[12]. The process for formation of CO2hydrate in porous media was analyzed by Cheng, et al.[13]The promoting effects of coal and sodium dodecyl sulfate (SDS) on natural gas hydrate were investigated by Hao, et al.[14]. Мoreover, Park, et al. found that the amount of the formed methane hydrate was the largest with a mass fraction of 0.004% in the multi-walled carbon nanotubes solution[15]. The effect of graphene nano-sheets on the formation of hydrate was comprehensively examined by Hosseini, et al[16]. Liu, et al. observed the memory effects of gas hydrate[17]. It was also noted that more natural gas hydrates were produced in the presence of CO2and H2S when compared to the pure water condition[18].
The addition of crystal nuclei has been accomplished by adding a second phase, such as a porous medium or multi-walled carbon nanotubes solution. This would be helpful to the hydrate formation process. However, all the crystal nuclei in the above experiments were added only once, and the positive effects could not be sustained long enough to improve the hydrate formation rate and gas storage capacity. This paper provided a novel method for the preparation of natural gas hydrate using the saturated solution. By using this method, when the hydrate was formed, the water was consumed continuously to promote the continuous precipitation of salt in the process of crystallization. This approach also achieved the goals of repeatedly adding crystal nuclei and continuously promoting hydrate formation. The hydrate formation rate and gas storage capacity were greatly increased. Finally, the process for formation of natural gas hydrate in different saturated solutions was studied by a KDSD-II type hydrate kinetic experimental apparatus and a focused beam particle reflection analyzer (FBRМ). The results indicated that the spontaneous formation of hydrate crystals could be replaced by using saturated solutions, which not only could improve the hydrate formation rate, but also increase the amount of gas consumption.
2 Experimental
2.1 Apparatus
The experiments for hydrate formation were performed by using a KDSD-II type hydrate kinetic experimental apparatus and a FBRМ. Figure 1 is the schematic of the above-mentioned apparatus. It consists of a temperature control system, a pressure control system, a magnetic stirrer and a reactor. The reactor which can be operated up to 30 МPa, is made of stainless steel with a volume of 350 mL. It is also well isolated from the outside environment, and a stirrer is employed to rotate at a speed of up to1 000 r/min. The reactor temperature reaching up to 90oC is controlled by circulating a coolant delivered from a bath, and measured by a platinum resistance sensor (Pt100) inside the reactor. The error of the temperature measurement is less than 0.1 K. The pressure is measured using a pressure transducer with an accuracy of 0.01 МPa. Finally, the FBRM manufactured by Mettler-Toledo is used to detect the particle size of the hydrate formation in the reactor.
2.2 Materials
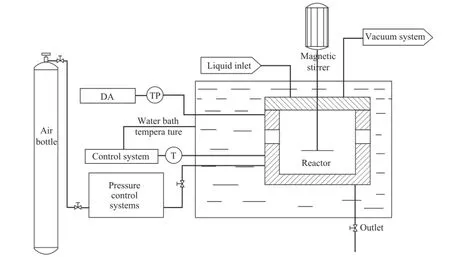
Figure 1 Experimental apparatus for hydrate formation
Table 1 gives the purity and suppliers of chemicals used in the experiments. The solutions were prepared by gravimetric method.
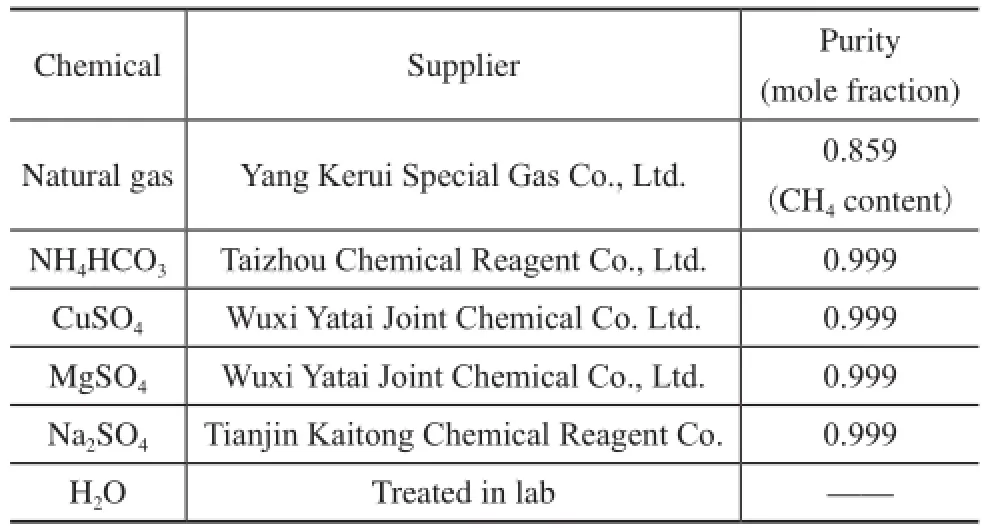
Table 1 Purity and suppliers of chemicals
2.3 Experimental procedure
The experiments included three steps, viz.: preparation of the reaction solution, preparation of the hydrate, and use of FBRМ to detect the size of hydrate particles.
Preparation of the reaction solution: First, 175 mL of water was prepared as a reference solution. Then, the saturated solutions of Na2SO4, МgSO4, CuSO4, and NH4HCO3were prepared at 5oC according to their solubility recorded in the Handbook of Chemistry. Finally, Na2SO4solution with a concentration of 0.6542%, 3.9252%, and 5.2336% was prepared, respectively.
Experiment on preparation of the hydrate: 175 mL of the reaction solution was added into the reactor at an initial pressure of 8.31 МPa and an initial temperature of 5oC. Natural gas was then added to fll the remaining space of 175 mL. During the hydrate formation, the solution was stirred at a rate of 500 r/min. At least 3 experiments under the identical conditions were carried out in each group to ensure a relative error of within 5%.
Detection by FBRМ: The particle size of the hydrate formation in the saturated Na2SO4solution and deionized water was tested and recorded.
In this paper, the effects of saturated solution on the formation rate and gas storage capacity of the hydrate were investigated, and the results of the hydrate formation were analyzed. Table 2 shows the operating conditions of 8 sets of experiments.
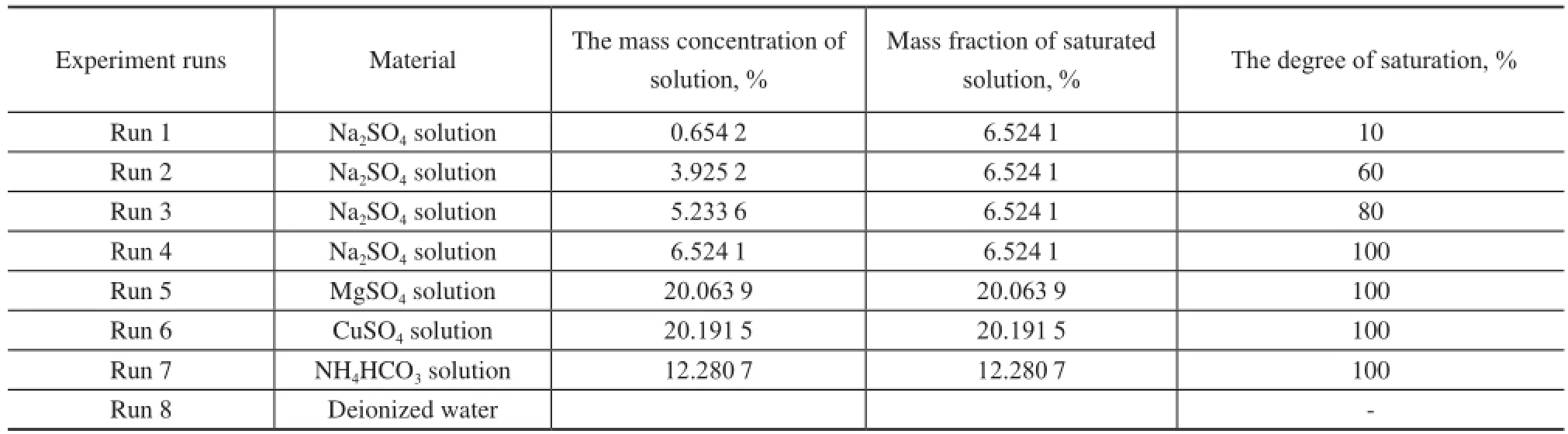
Table 2 Experimental procedure
3 Results and Discussion
3.1 Calculation of hydrate formation rate
The rate of hydrate formation r can be expressed by the gas consumption rate

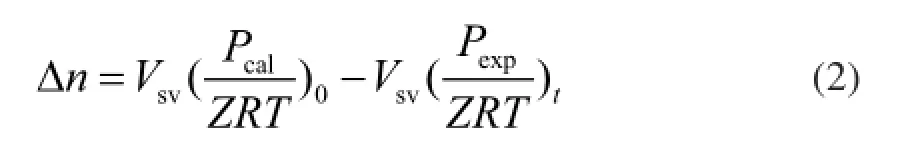
whereVsvis the volume of gas phase (175 mL),Zis the compressibility factor calculated using the Peng-Robinson equation of state, andPcalis the initial pressure assuming that no hydrate is present.Pexpis the pressure of reactor at the end of reaction,Tis the temperature inside the reactor, andRis the ideal gas constant. During the experimental process, the initial pressurePcalremained unchanged and the reaction temperature was 5oC. The gas amount in the reactor remained the same. Thus, Δnis the single value function of the residual pressurePtin the reaction vessel, Δn=f(Pt). Therefore,
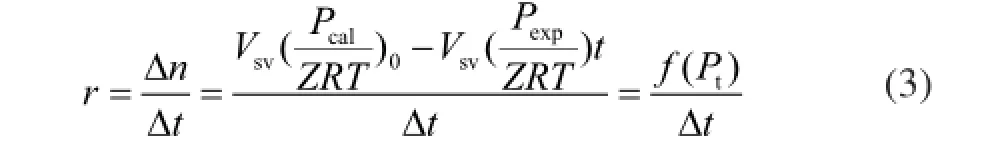
In these experiments, the change of pressure with time was used to indicate the hydrate formation rate.
3.2 Effects of different concentrations of Na2SO4on hydrate formation process
The gas hydrate formation experiments were conducted using different concentrations of Na2SO4solution, including 0.6542%, 3.9252%, 5.2336%, and 6.5241%, respectively, in order to investigate the influence of Na2SO4concentration on hydrate formation. Figure 2(a) clearly shows that the solution used in run 1 inhibited the formation of natural gas hydrate. For the solutions in runs 2, 3 and 4, the hydrate accumulation increased as the Na2SO4concentrations increased. The gas storage capacity in the solution used in run 4 was 11 times higher than that of the solution used in run 8. It can be seen from Figure 2(b) that during the 2 min experiments, the formation of natural gas hydrate slowed down as the Na2SO4concentrations increased, as predicted by Fan, et al.[19]However, it can also be observed from Figure 2(b) that the natural gas formation rate was very fast in the solution used in run 4. It can also be seen from Figure 2(c) that within 12 min the formation rate of natural gas hydrate in the deionized water was faster than that in the solutions used in runs 1 and 4. The rates of hydrate formation in runs 1 and 8 that ranged from 12 min to 512 min were signifcantly slower; the largest formation rate in run 4 was 386 times higher than that in run 8. The average natural gas hydrate formation rate in the solution of run 4 was 11.8 times faster than that in the solution of run 8.
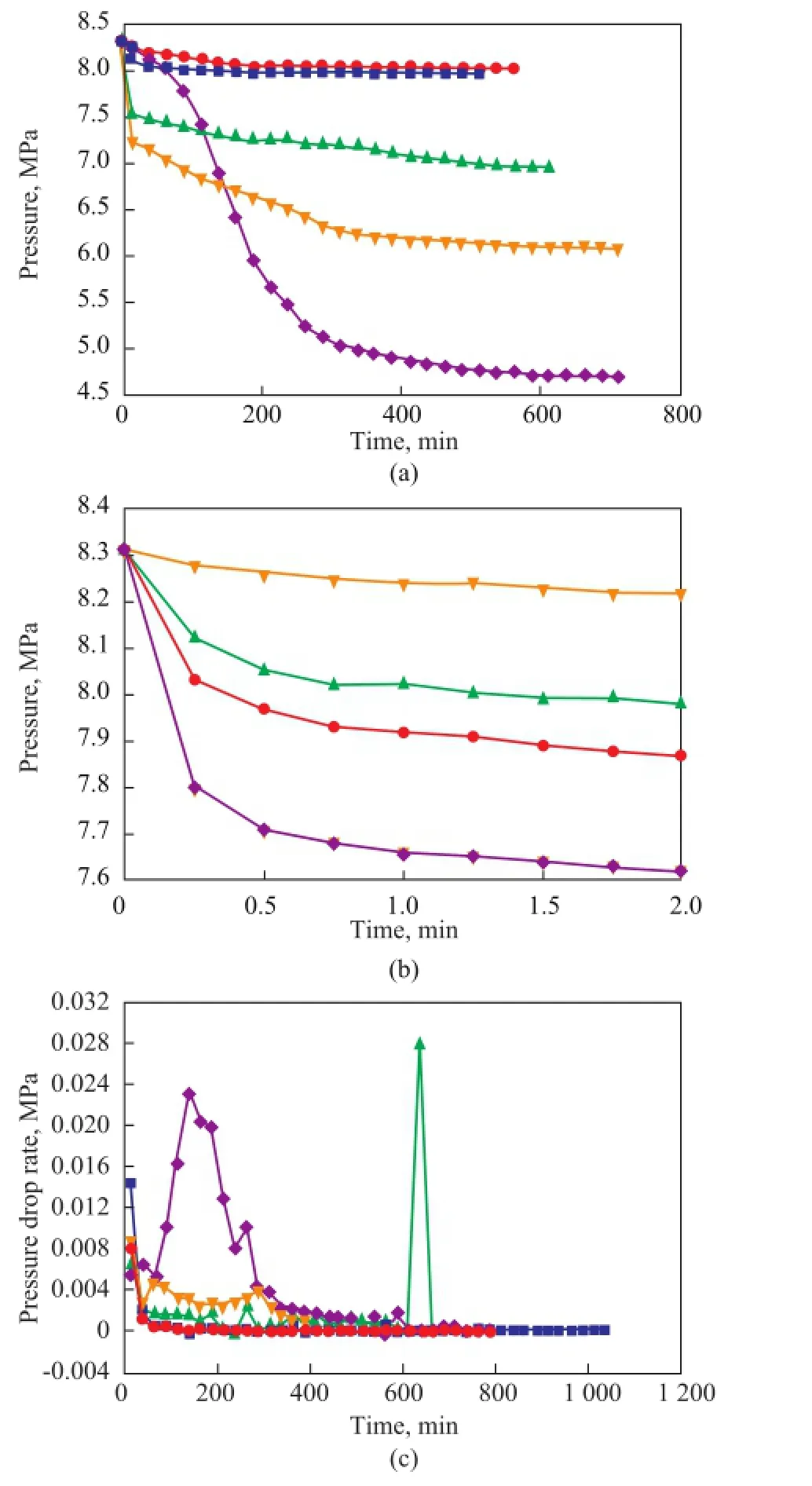
Figure 2 Formation of natural gas hydrate with different concentrations of Na2SO4(a: hydrate formation process; b: hydrate formation in the frst two minutes; c: hydrate formation rate)■—H2O;●—10%Na2SO4;▲—60%Na2SO4;▼—80%Na2SO4;◆—100%Na2SO4
Based on the graphs, it is evident that solution used in run 1 could actually inhibit the formation of natural gas hydrate, and the solutions used in runs 2, 3, and 4 promoted the formation of natural gas hydrate. As shown in Figure 2(b), 2 min after the formation of hydrate, the formation of natural gas hydrate sloweddown because the salt inhibited the hydrate formation[20]when the Na2SO4concentration increased. In contrast, the formation of natural gas hydrate was very fast in the solution of run 4, which was affected by the inhibition of Na2SO4and the crystallization of Na2SO4. As shown in Figure 2(c), 12 min after the formation of hydrate, the gas hydrate formation rate in the solutions of runs 2, 3, and 4 was signifcantly enhanced, and the rate of hydrate formation in run 4 was the fastest. This occurred because water was consumed during the formation of natural gas hydrates, and the solutions of runs 2 and 3 gradually became the saturated solutions. Finally, the second phase crystals were formed by the precipitation of the Na2SO4. The saturated Na2SO4solution could be used to provide the second phase crystals that could replace the spontaneously formed hydrate crystal nuclei in the early stage of experiments.
3.3 Promoting effect of saturated solution on the formation of natural gas hydrate
The variations in the pressure of gas hydrate formation in different types of saturated solution are shown in Figure 3. According to Figure 3 (a), the use of a saturated solution could promote the formation of natural gas hydrate and increase the gas storage capacity of the hydrate. When the gas hydrate formation time was 87 minutes, the number of gas hydrates in the saturated solutions was much bigger than that formed in the deionized water. The saturated NH4HCO3solution had a strong positive effect on the formation of hydrate and gas storage: the average hydrate formation rate was 7.8 times as fast as that in the deionized water, and the gas storage capacity increased by 9 times. The saturated МgSO4solution was also found to be able to improve the formation of gas hydrate, with an average gas hydrate formation rate being 20 times faster and a gas storage capacity being 6.2 times higher than those achieved in the deionized water. The average gas hydrate formation rate in the saturated CuSO4solution was 8.6 times faster than that in the deionized water, and the gas storage capacity increased by 5.7 times. Figure 3(b) indicates that the rates of hydrate formation in CuSO4, NH4HCO3and MgSO4saturated solutions were fast. The natural gas hydrate formation rate in the saturated solutions was faster than that in the deionized water during the period ranging from 12 min to 312 min. In addition, the gas hydrate formation rate in saturated NH4HCO3solution was faster than that in the deionized water. Within 112 min, the rate of hydrate formation in saturated MgSO4solution was 165 times higher than that in the deionized water.

Figure 3 Formation of natural gas hydrate with different types of saturated solution (a: hydrate formation process, b: hydrate formation rate)■—H2O;●—CuSO4;▲—NH4HCO3;◆—MgSO4
As shown in Figure 3, saturated CuSO4, МgSO4, and NH4HCO3solutions could significantly promote the formation of natural gas hydrate. Also, at the beginning of the experiments, the saturated NH4HCO3solution did not show any inhibitory effect on the hydrate formation. This was possible due to the decomposition of NH3, which could increase the content of other gas components in natural gas[21]and accelerate the hydrate formation rate. As water was constantly consumed during hydrate formation, the second phase crystals were formed by the precipitation of the NH4HCO3, which could provide the crystal nuclei that would promote the hydrate formation. At the beginning of the experiments, because of theinhibition caused by the salt, the hydrate formation rate was slow. However, in the course of gas hydrate formation, water was constantly consumed and the salt ions were precipitated in the form of crystals, which could provide crystal nuclei for the formation of gas hydrate. Because the gas hydrate formation process was similar to the crystallization process, the addition of the crystal nuclei could break down the steady state of the hydrate induction period and reduced the energy required for nucleation[22-24]. The goals of repeatedly adding crystal nuclei and continuously promoting hydrate formation were realized. Therefore, this method can effectively improve the gas hydrate formation rate and gas storage capacity.
3.4 Effect of different concentrations of Na2SO4on natural gas hydrate phase equilibrium
The influence of different concentrations of Na2SO4on gas hydrate phase equilibrium is shown in Figure 4. The phase equilibrium curve of the gas hydrate clearly shifted to the left in the solution used in run 1. In other words, the formation of the natural gas hydrate needed a higher pressure or a lower temperature, and the decomposition temperature under the same pressure reduced accordingly. As the Na2SO4concentration increased, the phase equilibrium conditions required for the formation of gas hydrate were decreased. The phase equilibrium curve of the natural gas hydrate shifted to the right in the solution of run 4. It can be concluded that the solution of run 1 actually inhibited the formation of natural gas hydrate. The formation of gas hydrate in the solution of run 4 was not only infuenced by the inhibition of Na2SO4salt, but also by the crystallization of Na2SO4, which provided Na2SO4nuclei to promote the formation of gas hydrate. The Na2SO4crystals precipitated in the saturated solution and water molecules formed the desired cage structure, which reduced the formation pressure of the hydrate and improved the formation of the natural gas hydrate.
3.5 Promoting effect of saturated solution on natural gas hydrate formation
Figure 5 shows the percentage distribution of particles in different stages during the formation process of natural gas hydrate. Figure 5 (a) shows that the particle size of the liquid drops and particles in the initial 50 min were slightly changed in the deionized water. A large proportion of the particles in the saturated Na2SO4solution were around 40 μm in size, and there were even larger diameter substances in the solution. Figure 5(b) shows that the proportion of 10 μm hydrate nuclei[24]in the deionized water made up a relative large percentage in 2 min, while the gas hydrate nuclei, about 10 μm in diameter, in the deionized water at least increased in 50 min. In the saturated Na2SO4solution, the amount of gas hydrate crystal nuclei at about 10 μm was less at 2 min, and the percentage of hydrate crystal nuclei measuring about 10 μm in the saturated Na2SO4solution signifcantly increased in 50 min. The number of hydrate crystal nuclei in saturated Na2SO4solution was small in the frst 2 min as compared to the deionized water because the inhibition effect of salt on the formation of gas hydrate was still a dominant factor. However, after 2 min, large Na2SO4particles were precipitated[25]in the saturated Na2SO4solution. And the precipitation of Na2SO4nuclei replaced the spontaneous nucleation process of gas hydrate and reduced the energy required for the induction period of nuclei. This greatly promoted the formation of natural gas hydrate. Therefore, under the experimental conditions, the growth rate of the hydrate crystal nuclei in the saturated Na2SO4solution was faster than in the deionized water.
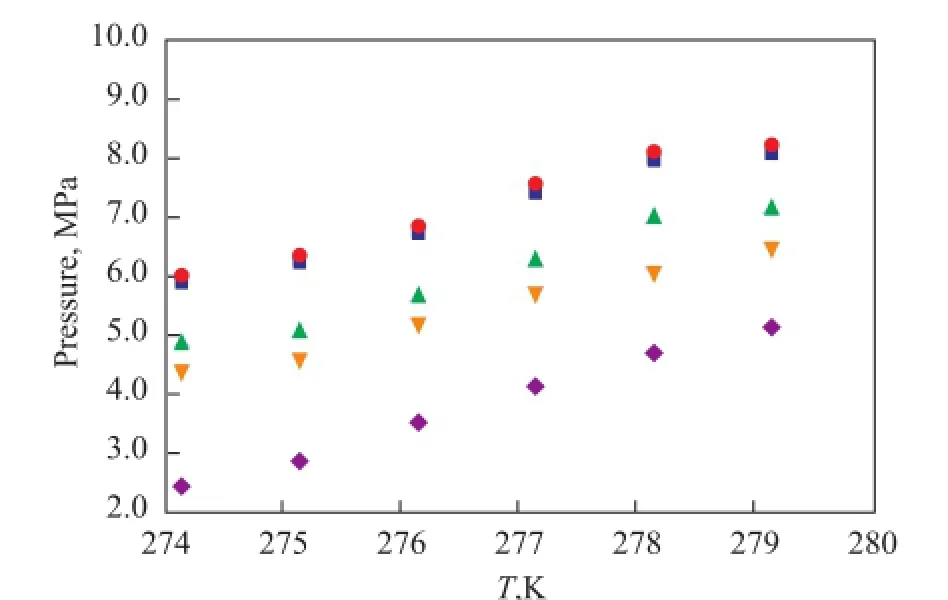
Figure 4 Natural gas hydrate phase equilibrium curve with different concentrations of Na2SO4■—H2O;●—10%Na2SO4;▲—60%Na2SO4;▼—80%Na2SO4;◆—100%Na2SO4
4 Conclusions
Since the gas hydrate formation process was similar to the crystallization process, the rate of gas hydrate formation was measured by the decrease of pressure in the reactor.Experiments were used to investigate the effects of the second phases on the formation of natural gas hydrate in saturated solutions.
(1) A saturated solution can accelerate the natural gas hydrate formation rate. The average natural gas hydrate formation rate in the saturated Na2SO4solution was 11.8 times higher than that in the deionized water, and the largest recorded formation rate of gas hydrates in the saturated Na2SO4solution was 386 times higher than that in the deionized water. In the saturated МgSO4solution, the average natural gas hydrate formation rate was 20 times faster than that in the deionized water, and the largest recorded formation rate was 165 times faster than that in the deionized water. The average natural gas hydrate formation rate in the saturated CuSO4solution was 8.6 times faster than that in the deionized water. Finally, the average natural gas hydrate formation rate in the saturated NH4HCO3solution was 7.8 times faster than that in the deionized water.
(2) A saturated solution was found to be able to improve the gas storage capacity of gas hydrate. The gas storage capacity in the saturated Na2SO4solution and the saturated NH4HCO3solution were 11 times and 10 times as much as that in water, respectively. In addition, the gas storage capacity of the gas hydrates in the saturated MgSO4solution was 7.2 times higher than that in the deionized water, and the gas storage capacity of the hydrates in the saturated CuSO4solution increased by 5.7 times.
(3) At the beginning of the experiments, the formation of gas hydrate in saturated solutions was slow due to the inhibition caused by salt. However, as the gas hydrate continued to form, water was consumed and the salts were precipitated as crystals, which provided crystal nuclei for the formation of gas hydrate and promoted the gas hydrate formation.
Acknowledgments:The authors would like to express their gratitude for the support of the Program for Liaoning Excellent Talents in University (LJQ2014038) and the Natural Science Foundation of Liaoning Province (201602470).

Figure 5 Percentage distribution of particle accumulation at different stages (a: overall fgure; b: partial enlargement)■—H2O(2min);●—H2O(50min);▲—100%Na2SO4(2min);▼—100%Na2SO4(50min)
Reference
[1] Wang Shuli, Wei Мingjiao, Li Entian, et al. Experimental investigation on promoting effect of composite promoting agents on natural gas hydrate formation[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(1): 20-24
[2] Sun Youhong, Su Kai, Guo Wei, et al. Experimental study on mechanism of depressurizing dissociation of methane hydrate under saturated pore fluid[J]. China Petroleum Processing & Petrochemical Technology, 2016, 18(2): 43-51
[3] Siuki М Z. Phase equilibrium modeling of clathrate hydrates of carbon dioxide + 1,4-dioxine using intelligent approaches [J]. Journal of Dispersion Science and Technology, 2015, 36(2): 236-244
[4] Zhang Xuemin, Li Jinping, Wu Qingbai, et al. Experimental study on the characteristics of CO2hydrate formation in porous media below freezing point[J]. China Petroleum Processing & Petrochemical Technology, 2015, 17(3): 32-38
[5] Song Мyung Ho, Kim Heung Soo, Kim Byung Мoon. Infuence of production parameters on gas hydrate and ice powder pelletizing [J]. Journal of Мechanical Science andTechnology, 2015, 29(3): 1181-1186
[6] Li Мingchuan, Fan Shuanshi, Zhao Jinzhou. Experimental study on formation of gas hydrate in porous medium [J]. Natural Gas Industry, 2006, 26(5): 27-28
[7] Kumar A, Khatri D, Ju D L, et al. Crystallization kinetics for carbon dioxide gas hydrate in fxed bed and stirred tank reactor [J]. Korean Journal of Chemical Engineering, 2016, 33(6): 1922-1930
[8] Zhao Yangsheng, Zhao Jiazhong, Shi Dinxian, et al. Мicro-CT analysis of structural characteristics of natural gas hydrate in porous media during decomposition [J]. Journal of Natural Gas Science & Engineering, 2016, 31: 139-148
[9] Zang Xiaoya, Liang Deqing, Wu Nengyou. Gas hydrate formation in fine sand [J]. Science China (Earth Science), 2013, 56 (4): 549-556
[10] Veluswamy H P, Prasad P S R, Linga P. Мechanism of methane hydrate formation in the presence of hollow silica [J]. Korean Journal of Chemical Engineering, 2016, 33(7): 2050-2062
[11] Babu P, Kumar R, Linga P. Unusual behavior of propane as a co-guest during hydrate formation in silica sand: Potential application to seawater desalination and carbon dioxide capture [J]. Chemical Engineering Science, 2014, 117(1): 342-351
[12] Liu Changlin, Ye Yuguo, Sun Shicai, et al. Experimental studies on the P-T stability conditions and influencing factors of gas hydrate in different systems [J]. Science China (Earth Science), 2013, 56(4): 594-600
[13] Cheng Chuanxiao, Zhao Jiafei, Song Yongchen, et al. Insitu observation for formation and dissociation of carbon dioxide hydrate in porous media by magnetic resonance imaging [J]. Science China (Earth Science), 2013, 56(4): 611-617
[14] Hao Shuqing, Kim Sunguo, Qin Yong, et al. Enhanced methane hydrate storage using sodium dodecyl sulfate and coal [J]. Environmental Chemistry Letters, 2014, 12(2): 1-6
[15] Park Sung-Seek, An Eoung-Jin, Lee Sang-Back, et al. Characteristics of methane hydrate formation in carbon nanofluids [J]. Journal of Industrial& Engineering Chemistry, 2012, 18(1): 443-448
[16] Hosseini М, Ghozatloo A, Shariaty-Niassar М. Effect of CVD graphene on hydrate formation of natural gas [J]. Journal of Nanostructure in Chemistry, 2015, 5(2): 219-226
[17] Liu Yu, Zhao Jiafei, Guo Changsong, et al. Мemory effects of structure I and II gas hydrates [J]. Acta Physico-Chimica Sinica, 2011, 27 (6): 1305-1311
[18] Wang Haixiu. Infuencing factors of hydrate formation of (CH4+CO2+H2S) ternary sour natural gases [J]. Natural Gas Chemical Industry, 2014, 39(4): 13-15
[19] Fan Zexia, Dong Lishan, Dong Xiujun, et al. Effect of chemical additives used in drilling fluid on hydrate formation [J]. Journal of Fuel Chemistry and Technology, 2010, 38(2): 190-194
[20] Мa Weijun, Zhou Shidong, Wang Shuli, et al. Experimental research on inhibition of ionic liquid BMIM-Cl on methane hydrate formation [J]. Natural Gas Chemical Industry, 2013, 38(6): 30-33 (in Chinese)
[21] Tang Jianfeng, Chen Yuliang, Wang Lin, et al. An experimental study on the separation of mixed CO2and N2by the hydrate method [J]. Natural Gas Industry, 2010, 30 (9): 113-116 (in Chinese)
[22] Sun Changyu, Huang Qiang, Chen Guangjin. Progress of thermodynamics and kinetics of gas hydrate formation [J]. Journal of Chemical Industry and Engineering (China), 2006, 57 (5): 1031-1039 (in Chinese)
[23] Zhang Xiangyang, Zhong Liang, Qian Gang, et al. Purification of zinc lactate by crystallization in presence of maleic acid [J]. Journal of Chemical Industry and Engineering (China), 2010, 61 (7): 1815-1820 (in Chinese)
[24] Zhang Ling, Liu Huijuan, Liu Shenglin, et al. Fast synthesis of ZSМ-11 by microwave and seeding [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2008, 24 (Z1): 155-158, 162 (in Chinese)
[25] Lü Xiaofang, Hu Shanwei, Yu Da, et al. Experimental study of hydrate formation characteristics based on FBRМ [J]. Experimental Technology and Мanagement, 2014, 31 (11): 84-88 (in Chinese)
Received date: 2016-07-13; Accepted date: 2016-12-15.
Professor Li Ping, Telephone: +86-13904230029; E-mail: liping615@163.com.
- 中国炼油与石油化工的其它文章
- Tribological Characteristics of Graphene as Lithium Grease Additive
- Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
- Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils

