二氟沙星激发态氧化损伤氨基酸和脱氧鸟苷酸的激光光解研究
李海霞 刘艳成 唐睿智 张 鹏 马六逵 魏驰原 王文锋,*
(1南京工业大学浦江学院,南京211132;2中国科学院上海应用物理研究所,上海201800)
二氟沙星激发态氧化损伤氨基酸和脱氧鸟苷酸的激光光解研究
李海霞1刘艳成2唐睿智2张 鹏2马六逵1魏驰原1王文锋2,*
(1南京工业大学浦江学院,南京211132;2中国科学院上海应用物理研究所,上海201800)
本文利用激光光解法研究了二氟沙星激发态氧化损伤氨基酸和脱氧鸟苷酸等生物小分子。实验结果显示二氟沙星激发态水溶液氧化损伤色氨酸,酪氨酸,半胱氨酸和脱氧鸟苷酸的速率常数分别为1.97×108, 1.48×108,1.72×108,6.92×107dm3·mol-1·s-1。通过光谱分析和实验数据分析可以得出激发态二氟沙星氧化损伤色氨酸,酪氨酸,半胱氨酸和脱氧鸟苷酸是通过电子转移的方式进行的。
激光光解;二氟沙星;激发态;氨基酸
1 Introduction
It is now well established that UVA light has cytotoxic and mutagenic features.Because only a smallamountof UVAlightcan be absorbed by DNA or protein molecules,endogenous and exogenous photo-sensitizers are believed to play an importantrole in UVAcarcinogenesis1,2.UVAlight absorbed by photo-sensitizers may induce the damage of bio-molecules via photo-oxidation reactions3.
Approximately 68%of the dry weight of cells and tissues is composed of proteins4,so they are the major targets for potential photo-oxidation.Oxidative modifications of proteins have been suggested to play a key role in a number of human diseases and aging5,6.Two major types of processes can occur with protein photo-oxidation7.One process involves the formation of free radicals via the transfer of hydrogen atoms or electrons(Type I mechanism).The other process involves the generation of singlet oxygen(1O2)by energy transfer from the triplet state of the sensitizer to the ground triplet state of molecular oxygen(Type II mechanism).The crucial role of free and protein-bound tryptophan,tyrosine,cysteine in the lightsensitivity of biologicalsys-tems,either following the directabsorption of lightin the UVB range or promoted by compounds acting as photo-sensitizers,has received considerable attention8,9.
Itis wellknown thatguanine is the mosteasily oxidized base, and photosensitization of DNA primarily occurs at guanine residues,which demonstrates thatguanine lesions may be the major cause of mutations10-12.Guanine,possessing the lowest oxidation potential among the DNA bases,provides the most thermodynamically accessible source of electrons10,13,14.Therefore,intense attention has been focused on understanding the photosensitization of guanine as a DNA modelcompound to elucidate the mechanisms of photooxidative DNA degradation.For the currentstudy, dGMP was used because of its good solubility in water and the factthatitrepresents the bestmodelof the DNAmonomer unit.
Fluoroquinolones antibiotics(FQs)are one of the most successfulclasses of drugs15and are considered to be well-tolerated drugs with relatively limited adverse effects16,17;therefore,the use of several FQs in therapy is increasing.A number of reports have shown that FQs may be very efficientphoto-sensitizers18and have revealed that DNAis one of the most relevant biological targets for this effect19.Indeed,FQs are one of the few families of drugs thathave been found to be photo-carcinogenic in vivo.Thus,the photo-carcinogenic activity of lomefloxacin(LOM),fleroxacin (FLE),ofloxacin(OFL)and ciprofloxacin(CIP)has been tested in mice exposed to UVA after oral administration of the drugs. Under these conditions,radiation enhances tumor prevalence and drastically shortens the median latentperiod in comparison with UVAalone20.
In the present study,properties of the triplet states of DFX (Scheme 1)and the oxidation of amino acids photosensitized by DFX were studied using 355 nm laser flash photolysis(LFP).A possible mechanism was identified,and the related rate constants for electron transfer from amino acids and dGMP to3DFX*were obtained.Irradiation of protein with UVAlightin the presence of DFX was performed under aerobic and anaerobic conditions to study3DFX*-induced protein damage.The results suggest thatthe anti-tumor mechanism of DFX involves both Type Iand Type II processes and thatthe major mechanism in biologicalsystems is Type Iphotochemistry.

Scheme 1 Structure of DFX
2 Materials and methods
2.1 Materials
High-purity(>99.8%)DFX,tryptophan,tyrosine,cysteine and dGMP were purchased from Sigma Chemicals and used as received.Phosphate salts were obtained from J&K Chemical Ltd. Allsolutions were freshly prepared with ultrapure water provided by a Millipore purification system.
2.2 Methods
Laser flash photolysis(LFP)experiments were carried outatthe Shanghai Institute of Applied Physics using an Nd:YAGlaser.The Nd:YAGlaser provided a 355 nm pulse with a duration of 5 ns and the maximum energy of 240 mJ per pulse was used as the pump lightsource.Axenon lamp was employed as the detecting light source.The laser and the analyzing lightbeam were passed perpendicularly through a quartz cell.The transmitted lightentered a monochromator equipped with an R955 photomultiplier.The output signal from the HP54510B digital oscillograph was transferred to a personal computer for further analysis.The LFP setup has been previously described21.All experiments were performed in water solutions.Samples were bubbled with highpurity(99.999%)N2,N2O,or O2(99.999%)for at least 20 min before photolysis experiments.
Pulse radiolysis experiments were performed utilizing a 10 MeV linear accelerator that delivers an electron pulse with duration of 10 ns.The dosimetry of the electron pulse was determined by a thiocyanate dosimeter using G[(SCN)2·-]=5.8(G: radiation chemical yield)in a 0.1 mmol·L-1KSCN solution saturated with N2O by takingε480nm=7600 dm3·m-1·cm-1for (SCN)2·-
.The details of the setup and operation conditions were given in a previous paper22.The dose perelectron pulse was 10 Gy.
3 Results and discussion
3.1 Production oftriplet states of DFX(3DFX*)
The transientabsorption spectra of a nitrogen-saturated 1×10-4mol·L-1aqueous solution of DFX atpH 7.17 after excitation by a 355 nm laser pulse showed absorption in both the shortand long wavelength regions(Fig.1).The long wavelength band(centered at720 nm)completely decayed within 3μs,with a first-order rate of 1.5×106s-1(inset of Fig.1).When a nitrous oxide-saturated solution was employed,the absorption band around 720 nm was no longer observed,while the shorterwavelength absorption bands remained unaffected.Based on this observation,the fast-decaying absorption at720 nm could reasonably be assigned to the hydrated electron,formed according to reaction(2).This electron could be scavenged by N2Oas described by reaction(3)23.

Fig.2 shows the transientabsorption spectra of DFX in nitrous oxide-saturated aqueous solution atdifferenttime after the laser pulse.The post-pulse spectrum showed a band centered at620 nm thatdecayed with a first-order rate of 7.69×105s-1;the decay ofthe transientspecies was found to increase to 3.32×106s-1in the presence ofoxygen.On the basis ofthis finding,the transientband at620 nm was tentatively assigned to the tripletstate of DFX.
3.2 Production ofa DFX anion radicaland neutral radical
The reaction of DFXwith the hydrated electron of the reducing species was investigated.Fig.3 shows the transient absorption spectra obtained by pulse radiolysis of the nitrogen-saturated solution containing 2.0×10-4mol·L-1DFX in the presence of 0.1 mol·L-1tert-butanolatdifferentpH values.Tert-butanolwas used to scavenge·OH produced by water radiolysis.The e-aqwas remained,after the complete decay of the e-aq(approximately 1μs), strong bleaching was observed below 350 nm,as well as two broad absorption bands,one band was around 380 nm with a shoulder atabout450 nm and the other band was between 550 and 750 nm in the transientspectra.
According to the dynamics at 380 and 650 nm,which follow first-order decay,the two bands were not ascribed to the same transient species.The decay rate at 390 nm was 6×105s-1. However,the decay rate at650 nm was 8×104s-1.The reaction of DFX with e-aqis a reduction reaction with the formation of a DFX radical anion(reaction(4)).Under neutralconditions,DFX exists as a zwitterion bearing a protonated 4′-N and a dissociated 3-carboxyl group in an aqueous solution(Scheme 2).Thus,the DFX radical anions might be protonated with the formation of neutral radicals or deprotonated with the formation of radical dianions(reactions(5)and(6)).To elucidate the assignmentof the two bands,we changed the pH.The absorption at 380 nm was evidently pH dependent(Fig.2).When the pH was decreased,the absorption at around 380 nm also decreased.However,the absorption at around 650 nm was enhanced,indicating that the absorption around 380 nm was attributed to the DFX radicalanion and thatthe absorption around 650 nm included the contribution of the neutralradicaland radicaldianions.
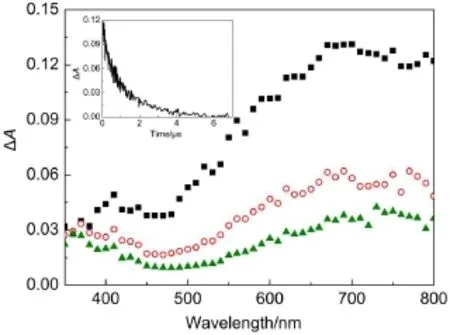
Fig.1 Transient difference absorption spectra observed at100 ns (-■-),1μs(-○-),and 2μs(-▲-)after subjecting a nitrogensaturated aqueous solution of 1×10-4mol·L-1FX in 2×10-3mol·L-1phosphate buffer(pH 7.17)to 8 mJ of 355 nm laser pulseInsetshows the decay profiles ofthe signalat720 nm obtained after laserexcitation of1×10-4mol·L-1aqueous DFXsolution.
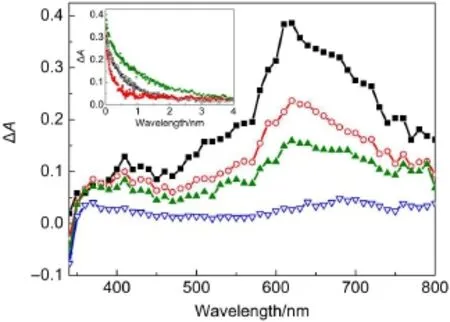
Fig.2 Transient difference absorption spectra observed at 100 ns (-■-),500 ns(-○-),1μs(-▲-),and 3μs(-▽-)after subjecting a nitrous oxide-saturated aqueous solution of 1.5×10-4mol·L-1DFX in 2×10-3mol·L-1phosphate buffer(pH 7.17)to 8 mJ of a 355 nm laser pulseInsetshows the decay profiles ofthe signalat620 nm obtained afterlaser excitation ofaqueous 1.5×10-4mol·L-1DFXsolutions saturated with N2(-■-),air(-○-)and O2(-▽-).
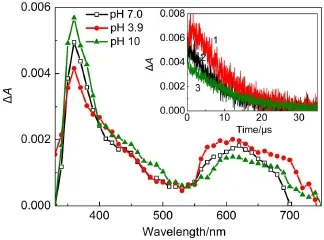
Fig.3 Transient difference absorption spectra observed at different pH conditions after pulse radiolysis ofan aqueous nitrogen-saturated 2×10-4mol·L-1DFX solution in 5×10-3mol·L-1phosphate buffer(pH 7.17)which contained 1%t-butanol and nitrogen saturated aqueousInsetshows the decay ofthe signalat380 nm atdifferentpHvalues (1:pH=10,2:pH=7.0,3:pH=3.9).

3.3 Reactions of3DFX*with amino acids
Further experiments were performed to investigate the reactivity of3DFX*and its ability to oxidize amino acids such as tryptophan,tyrosine and cysteine.These amino acids are common oxidation targets within proteins,and their oxidation could be one of the factors leading to photosensitized molecular damage.In the presence of amino acids,the decay of3DFX*was clearly accelerated,with rates proportionalto the concentrations of the amino acids present.These amino acids all have the accepted protons group―NH2and the provided protons group―COOH.In thisarticle,differentpH solution was used to dissolve the amino acids (Table 1).So in order to perform the differentform of the proton in differentamino acids,differentabbreviated formula was used in differentpHsolution.
The energy of the DFX triplet state(ET)is 263.5 kJ·mol-1(calculated by the energy transfer method),while the ETof tryptophan,tyrosine and cysteine are all at approximately 300 kJ· mol-1(for example,the ETof tryptophan is 297 kJ·mol-1and that of tyrosine is 342 kJ·mol-1)24.Consequently,energy transfer from the triplet states of FQs to tryptophan,tyrosine or cysteine is unlikely to occur.However,3FQs*are able to oxidize amino acids, forming FQradicalanions and oxidized radicals of amino acids.
In Fig.4,the transientabsorption spectra after 355 nm LFP of a N2-saturated aqueous solution with and without1.0×10-4mol· L-1tryptophan are shown.In the presence of tryptophan,a rapid decay of3DFX*at 620 nm and an increase of absorbance in the 380 and 480-560 nm region were observed.The absorption in the 480-560 nm region was ascribed to the contribution of the deprotonated radical cation of tryptophan7,24.Following the decay of triplet-state DFX,the spectrum with a maximum absorbance around 380 nm appeared(Fig.4).The location and the shape of the absorption bands shown were in good agreement with thatof the DFX radicalanion studied in the previous section by pulse radiolysis.The pH of the solution was 7.0,so TrpH was used as tryptophan abbreviated formula in the following article.Therefore, the transient absorption spectrum resulting from the photoexcitation of DFX in the presence of TrpHcould be explained in terms of electron transfer from TrpH to3DFX*producing a DFX anion radical(DFX·-)and a TrpH cation radical(TrpH·+).The formed TrpH·+(p Ka=4.3)became a neutralradicalTrp·by deprotonation under our experimentalconditions.
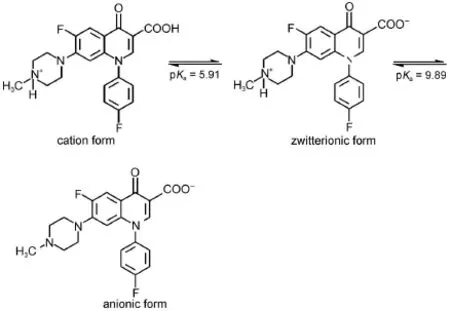
Scheme 2 Equilibrium between protonated forms of DFX

Table 1 Different amino acids abbreviated formula in different pH solution
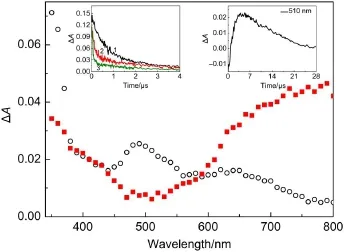
Fig.4 Transientabsorption spectra observed at5μs after 355 nm laser flash excitation of 1.0×10-4mol·L-1DFX in an aqueous N2-saturated 2.0×10-3mol·L-1phosphate buffer(pH=7.0)solution containing different concentrations of TrpH:in the absence ofTrpH(-■-)and in the presence of5.0×10-3mol·L-1TrpH(-○-) Inset(left)shows the plotof the decay in absorbance at620 nm of solutions containing differentconcentrations of TrpH(1:0 mmol·L-1,2:1 mmol·L-1, 3:2 mmol·L-1);the(right)insetshows the buildup trace at500 nm obtained by subtracting the trace of the 2.0×10-3mol·L-1TrpH at500 nm from the trace at500 nm of the solution without TrpH.

The quenching of3DFX*by TyrOH(The pHof the solution was 7.0,so TyrOH was used as tyrosine abbreviated formula in the following article)was similar to TrpH.In the presence of TyrOH, the decay of3DFX*was accelerated with rates roughly proportional to the concentration of TyrOH.Following the decay of triplet DFX,a prominent broad absorption around 400 nm also appeared,which was ascribed to TyrO·7,24(Fig.5).According tothese results,it was concluded that the reactions of3DFX*with TyrOHoccurred through electron transfer(reactions(9)and(10)).
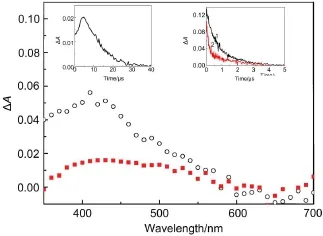
Fig.5 Transientabsorption spectra observed at6μs after 355 nm laser flash excitation of 1.0×10-4mol·L-1DFX in an aqueous N2-saturated 2.0×10-3mol·L-1phosphate buffer(pH=7.0) solution containing different concentrations of TyrOH:in the absence of TyrOH(-■-)and in the presence of 5.0×10-3mol·L-1TyrOH(-○-)Inset(right)shows the buildup trace at410 nm obtained by subtracting the trace at410 nm of the 5.0×10-3mol·L-1TyrOH solution from the trace at410 nm of the solution withoutTyrOH;the(left)insetshows the plotofthe decay in absorbance at620 nm of solutions containing differentconcentrations of TyrOH(1:0 mmol·L-1,2:5 mmol·L-1).

Fig.6 shows the reaction of3DFX*with cysteine.HCl was used to increase the solubility of cysteine in water;the pH was 4,so CysH was used as cysteine abbreviated formula in the following article.In the presence of CysH,the decay of3DFX*is accelerated; however,the absorption around 650 nm simultaneously indicated the generation of a long-life material(inset of Fig.6).This absorption was attributed to the neutral radical,which was verified in the previous section by pulse radiolysis(section 3.2).Another noticeably new broad absorption around 410 nm also appeared that was ascribed to(Cys)2·-25.According to these results,itwas concluded thatthe reactions of3DFX*with CysHoccurred through electron transfer(reactions(11)-(14)).


Fig.6 Transient absorption spectra observed at 6μs after 355 nm laser flash excitation of 1.0×10-4mol·L-1DFX in an aqueous N2-saturated 2.0×10-3mol·L-1phosphate buffer(pH=7.0)solution containing different concentrations of CysH:in the absence of CysH(-■-);in the presence of 3.0×10-3mol·L-1CysH(-○-)Inset(right)shows the buildup trace at420 nm obtained by subtracting the trace at420 nm of a solution with 5.0×10-3mol·L-1CysH from the trace at420 nm of a solution without CysH;the(left)insetshows the plotofdecay in absorbance at 620 nm ofsolutions containing differentconcentrations of CysH.

Table 2 Quenching rate constants(L·mol-1·s-1)ofDFX tripletstates by amino acids and dGMP
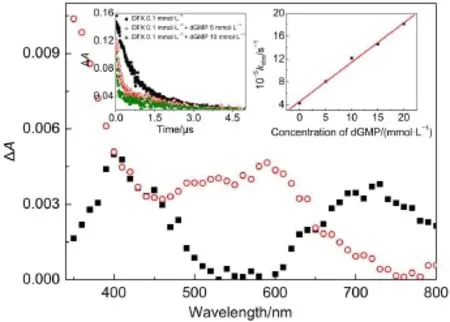
Fig.7 Transient absorption spectra observed at10μs after 355 nm laser flash excitation of 1.0×10-4mol·L-1DFX in an aqueous N2-saturated 2.0×10-3mol·L-1phosphate buffer(pH=7.0) solution containing different concentrations of dGMP:in the absence of dGMP(-■-);in the presence of 10 mmol·L-1dGMP(-○-)Inset(left)shows the plotof decay in absorbance at620 nm of solutions containing differentconcentrations of dGMP from 0 mol·L-1to 1×10-2mol·L-1; the(right)insetshows the correlation ofthe3DFX*decay rate constantat 620 nm,kobs,with the concentration of dGMP.
From the results obtained above,we concluded thatunder nitrogen conditions,the triplet state of DFX could be efficiently quenched by TrpH,TyrOH and CysH through electron transfer processes.The second-order rate constants for quenching of3DFX*by amino acids are shown in Table 2.
3.4 Reaction of3DFX*with dGMP
Fig.7 shows the transient absorption spectra recorded 10μs after photolysis of 1.0×10-4mol·L-1DFX neutral aqueous solutions containing either 1×10-2mol·L-1dGMP or no dGMP. Under these conditions,DFX was excited to its triplet state (3DFX*),and the amountof lightdirectly absorbed by dGMPlikely was negligible,which was further confirmed by performing the experiment with a blank.After the laser pulse,the transient absorption of3DFX*appeared,and itdecayed faster in the presenceofdGMP.Therefore,3DFX*can be effectively quenched by dGMP, and the quenching rate was determined to be 6.9×107dm3·mol-1· s-1.After3DFX*was quenched,a new transient absorption maximum around 370 nm(attributed to the DFX radical anion)was observed.Simultaneously,a considerably strong absorption in the 500-600 nm region appeared,which was ascribed to the contribution of the transientabsorption spectra of the deprotonated radical cation of dGMP[dGMP(-H)·]after the complete decay of3DFX*.However,itshould be noted thatthe pH value of the solutions is 7.1,and in such solutions,dGMP·-(p Ka=3.9)26quickly switches to dGMP(-H)·by losing a hydrogen atom. Therefore,the reactions were estimated as follows:
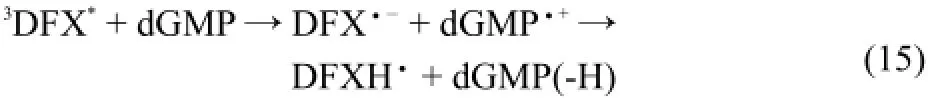
4 Conclusions
To the best of our knowledge,the oxidation of tryptophan, tyrosine,cysteine and dGMP by oxidized DFX radicals was observed with a kinetically significant rate for the first time.The reactions of oxidized radicals and excited states of DFX with similar rate constants and similar reaction products demonstrate thatboth the tripletstates and oxidized radicals of DFX mightbe involved in amino acid and protein photosensitization.The results clearly demonstrate the importance of oxidized DFX radicals in DFX photo-chemistry and photo-biology,which may suggest a new mechanistic modelfor photo-dynamic therapy.
(1)Cadet,J.;Ravanat,J.L.;Douki,T.J.Photochem.Photobiol.BBiol.2001,63,88.doi:10.1016/S1011-1344(01)00206-8
(2)Ali,D.;Verma,A.;Pathak,A.K.;Ali,H.;Hans,R.K.Chem. Ecol.2016,32,446.doi:10.1080/02757540.2016.1146711 (3)Pattison,D.I.;Rahmanto,A.S.;Davies,M.J.Photochem. Photobiol.Sci.2012,11,38.doi:10.1039/c1pp05164d
(4)Davies,M.J.Biochem.Biophys.Res.Commun.2003,305,761. doi:10.1016/S0006-291x(03)00817-9
(5)Levine,R.L.;Stadtman,E.R.Exp.Gerontol.2001,36,1495. doi:10.1016/S0531-5565(01)00135-8
(6)Anjum,N.A.;Sofo,A.;Scopa,A.;Roychoudhury,A.;Gill,S. S.;Iqbal,M.;Lukatkin,A.S.;Pereira,E.;Duarte,A.C.;Ahmad, I.Environ.Sci.Pollut.Res.2015,22,4099.doi:10.1007/ s11356-014-3917-1
(7)Lu,C.Y.;Liu,Y.Y.Biochimica Et Biophysica Acta-General Subjects 2002,1571,71.doi:10.1016/S0304-4165(02)00215-5
(8)Aubert,C.;Vos,M.H.;Mathis,P.;Eker,A.P.M.;Brettel,K. Nature 2000,407,926.doi:10.1038/35014644
(9)Heelis,P.F.;Okamura,T.;Sancar,A.Biochemistry 1990,29, 5694.doi:10.1021/bi00476a008
(10)Lu,C.Y.;Lin,W.Z.;Wang,W.F.;Han,Z.H.;Yao,S.;Lin,N. Y.Phys.Chem.Chem.Phys.2000,2,329.doi:10.1039/ A908492D
(11)Eshleman,J.R.;Norris,A.L.;Sadakari,Y.;Debeljak,M.; Borges,M.;Harrington,C.;Lin,E.;Brant,A.;Barkley,T.; Almario,J.A.;Topazian,M.;Farrell,J.;Syngal,S.;Lee,J.H.; Yu,J.;Hruban,R.H.;Kanda,M.;Canto,M.I.;Goggins,M. Clin.Gastroenterol.Hep.2015,13,963.doi:10.1016/j. cgh.2014.11.028
(12)Shafirovich,V.;Kropachev,K.;Anderson,T.;Liu,Z.; Kolbanovskiy,M.;Martin,B.D.;Sugden,K.;Shim,Y.;Chen, X.J.;Min,J.H.;Geacintov,N.E.J.Biol.Chem.2016,291, 5309.doi:10.1074/jbc.M115.693218
(13)Sugiyama,H.;Saito,I.J.Am.Chem.Soc.1996,118,7063. doi:10.1021/ja9609821
(14)Houk,K.N.;Prat,F.;Foote,C.S.J.Am.Chem.Soc.1998,120, 845.doi:S0002-7863(97)02331-7
(15)Gorityala,B.K.;Guchhait,G.;Fernando,D.M.;Deo,S.; McKenna,S.A.;Zhanel,G.G.;Kumar,A.;Schweizer,F. Angew.Chem.Int.Edit.2016,55,555.doi:10.1002/ anie.201508330
(17)Lipsky,B.A.;Baker,C.A.ClinicalInfectious Diseases 1999, 28,352.doi:10.1086/515104
(18)Karran,P.;Brem,R.DNA Repair 2016,44,178.doi:10.1016/j. dnarep.2016.05.024
(19)Perucca,P.;Savio,M.;Cazzalini,O.;Mocchi,R.;Maccario,C.; Sommatis,S.;Ferraro,D.;Pizzala,R.;Pretali,L.;Fasani,E.; Albini,A.;Stivala,L.A.J.Photochem.Photobiol.B-Biol.2014, 140,57.doi:10.1016/j.jphotobiol.2014.07.006
(20)Lhiaubet-Vallet,V.;Bosca,F.;Miranda,M.A.Photochem. Photobiol.2009,85,861.doi:10.1111/j.1751-1097.2009.00548.x
(21)Zuo,Z.H.;Yao,S.D.;Luo,J.A.;Wang,W.F.;Zhang,J.S.; Lin,N.Y.J.Photochem.Photobiol.B-Biol.1992,15,215. doi:10.1016/1011-1344(92)85125-E
(22)Yao,S.D.;Sheng,S.G.;Cai,J.H.;Zhang,J.S.;Lin,N.Y. Radiat.Phys.Chem.1995,46,105.doi:10.1016/0969-806X(94) 00120-9
(23)Buxton,G.V.;Greenstock,C.L.;Helman,W.P.;Ross,A.B.J. Phys.Chem.Ref.Data 1988,17,513.doi:10.1063/1.555805
(24)Bensasson,R.V.Bulletin Des Societes Chimiques Belges 1983, 92,615.doi:10.1002/bscb.19830920606
(25)Land,E.J.;Prutz,W.A.Int.J.Radiat.Biol.1979,36,75. doi:10.1080/09553007914550831
(26)Steenken,S.Chem.Rev.1989,89,503.doi:10.1021/ cr00093a003
Reactions of Triplet-State Difloxacin with Amino Acids and dGMP:ALaser Flash Photolysis Study
LIHai-Xia1LIU Yan-Cheng2TANG Rui-Zhi2ZHANGPeng2MA Liu-Kui1WEIChi-Yuan1WANG Wen-Feng2,*
(1Pujiang Institute,Nanjing Tech University,Nanjing 211132,P.R.China;2Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,P.R.China)
The reactions oftriplet-state difloxacin(DFX)with various amino acids and deoxyguanylic acid in aqueous media were studied using laser flash photolysis.Tryptophan,tyrosine,cysteine,and 2′-deoxyguanosine-5′-monophosphate(dGMP)were found to completely quench the tripletstate of DFX in aqueous solution,the corresponding second-order rate constants being 1.97×108,1.48×108,1.72×108,and 6.92×107dm3·mol-1· s-1.The quenching mechanism involves electron transfer to the photoexcited triplet state of DFX from the tryptophan,tyrosine,cysteine,and dGMP moieties,followed by fast protonation ofthe resulting DFX anion radical.
Laser flash photolysis;Difloxacin;Tripletstate;Amino acid
O644
Stahlmann,R.;Lode,H.Drugs 1999,58,37.
10.2165/ 00003495-199958002-00007
doi:10.3866/PKU.WHXB201702201
Received:November18,2016;Revised:February 20,2017;Published online:February 20,2017.
*Corresponding author.Email:wangwen@sinap.ac.cn;Tel:+86-21-39194953;Fax:+86-21-59553021. The projectwas supported by the NationalNatural Science Foundation of China(21173252).
国家自然科学基金(21173252)资助项目©Editorialoffice of Acta Physico-Chimica Sinica

