新型重组活化凝血因子Ⅶa候选药物GEN-0828定量检测方法的建立及其在血友病模型小鼠中的药动学特征
刘雨璐1,朱晓霞2,甘 慧2,吴卓娜2,李 俭2,冯素香1,3,窦桂芳2,顾若兰2,孟志云2
(河南中医药大学1.药学院,3.呼吸疾病中医药防治省部共建协同创新中心,河南 郑州450046;2.军事科学院军事医学研究院辐射医学研究所,北京100850)
Hemophilia is the most serious congenital hemorrhagic disease known. It is an inherited (Xlinked) bleeding disorder characterized by the lack of clotting factor Ⅷ(FⅧ, hemophilia A) or factor Ⅸ(FⅨ, hemophilia B)[1-3]. Clinically, hemo⁃philia patients usually suffer bleeding and swelling in some part of the body, leading to joint disease,brain hemorrhage, organ damage, and pain, or even death[4-5]. Without proper treatment, the life expectancy of patients with severe hemophilia is only about 16 years[6].
Hemophilia is usually treated with an "alter⁃native therapy". However, patients with deficiency of coagulation factors develop inhibitors, leading to treatment failure and serious complications[7-8].The "bypass therapy" for hemophilia has attracted much attention. The mechanism of this approach is an intravenous infusion of recombinant activated factor Ⅶa (rFⅦa) in patients who developed anti⁃bodies of therapeutic FⅧor FⅨ. At pharmaco⁃logic doses,rFⅦa can directly activate factor X(FX)on the surface of activated platelets, forming a stable haemostatic plug that controls bleeding[9].rFⅦa products have been widely used clinically,not only for the treatment of hemophilia A or B patients present with FⅧand FⅨinhibitors anti⁃bodies, but also for the treatment of acquired hemophilia, congenital FⅦdeficiency, and Glanzmann thrombocytopenia[10-12]. In addition, they have good hemostatic effects in some traumatic surgeries and liver diseases[13-14]. Therefore, rFⅦa occupies a large share in the world market.Due to the compli⁃cated process of rFⅦa production and its extreme instability,to date,NovoSeven®(rFⅦa,NovoNor⁃disk, Bagsværd, Denmark) produced in 1996 is still the only rFⅦa product on the global market,though major international companies (CSL Behring,OPKO, Anunix, Syntonix, Novo Nordisk, Bayer,etc)have conducted investigations about rFⅦa[15-18].
GEN-0828 (rFⅦa) is a new type of rFⅦa candidate drug with high activity.It is mainly produced from Chinese hamster ovary (CHO) cells without adding any human or animal ingredients in its production. Compared with primitive rFⅦa (NovoS⁃even®) expressed by baby hamster kidney (BHK)cells[19], rFⅦa expressed by CHO cells is safer,easier for fermentation control, more suitable for serum-free suspension culture, and has higher protein expression. GEN-0828 has a stable and high FⅦ-expression system in industrial production,and the expression of rFⅦa exceeds 75 mg·L-1in each batch, with high purity and aggregation levels.Therefore, GEN-0828 is a promising rFⅦa drug with reliable safety and good property. However,the research about GEN-0828 is not full-fledged for lack of sensitive quantitative and preclinical pharmacokinetic analysis.
In this paper, we developed a rapid and convenient quantification method by activating FⅦa activity for the determination of GEN-0828 in plasma from mice. This method was specific for the detection of the activity of FⅦa, avoiding the influence of FⅦzymogen, and more suitable for the evaluation of low-level F Ⅶactivity. The method was validated and applied successfully to characterization of the pharmacokinetic prop⁃erties of GEN-0828 in genetically engineered mice with FⅨknock-out (B-F9 KO) model mice(hemophilia B model mice). We expect that this method can be applied to evaluation of clinical pharmacokinetic profiles of this new type of rFⅦa,providing reference for subsequent clinical stud⁃ies and clinical dosing regimens.
1 MATERIALS AND METHODS
1.1 Reagents and instruments
GEN-0828 (1.096 g·L-1, 61429 kU·L-1, Talen Biophama Co., Ltd., Shanghai, China); FⅦdefi⁃cient plasma, FⅦa Cof-Plps and HEPEs BSA buffer (HYPHEN BioMed, France); CaCl2solu⁃tion (SIEMENS, Germany ); RAC-30 (automatic coagulation analyzer, Rayto Co., Ltd., Shenzhen,China); deionized water with a resistivity of 18.2 MΩ·cm-1was prepared with a Milli-Q®Advan⁃tage A10(Millipore Corp,Billerica,MA,USA).
1.2 Experimental animal
Eight SPF grade B-F9 KO model mice (half males and half females, body mass 22-32 g)were obtained from Biocytogen Jiangsu Gene Biotechnology Co., Ltd.. (Jiangsu, China). All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals(National Research Council of the USA, 1996),and approved by the Association for Assessment and Accreditation of Laboratory Animal Care.The license for experimental animal production was SCXK (Su)2016-0004.
1.3 Preparation of calibration standards and quality control samples
The calibration curve in plasma was prepared by adding GEN-0828 working solutions into diluted mixed plasma of blank model mice. The final concentrations of GEN-0828 in calibration curve were 62.5, 125, 250, 500,1000 and 2000 U·L-1,respectively. Quality control (QC) samples were additionally prepared on the day of analysis in the same way as calibration standard samples at 100 U·L-1(low quality control, LQC), 750 U·L-1(middle quality control, MQC) and 1500 U·L-1(high quality control,HQC).
1.4 Method development
The clotting assay for the quantitative deter⁃mination of FⅦa activity was performed as follows:firstly, calibration curve samples, QC samples and samples to be tested were all diluted at a 1:10 ratio in HEPES BSA buffer. Then 50 μL of blank plasma, standard samples, QC samples and unknown samples were added into the coagula⁃tion circular reaction cup, respectively. Each sam⁃ple was established with a pair of duplicate holes. Next, 50 μL of FⅦdeficient plasma (prein⁃cubated at 37℃) was added to each reaction cup and incubated at 37℃for 1 min before 50 μL of FⅦa Cof-Plps (human recombinant truncated TF and phospholipias, preincubated at 37℃)was added and incubated at the same tempera⁃ture for 2 min. Finally, 50 μL of 0.025 mol·L-1CaCl2solution (preincubated at 37℃) was added to each reaction cup, stirred evenly, and the clotting time (CT/s) was recorded by Automatic Coagula⁃tion Analyzer RAC-30.
1.5 Method validation
1.5.1 Linearity and sensitivity
The linearity was evaluated by processing calibration curves ranging from 62.5 to 2000 U·L-1for six consecutive days. Taking the FⅦa activity of GEN-0828 in the plasma as abscissa and the CT as ordinate, the calibration curve was fitted by a bi-logarithmic fitting. The linearity of the cali⁃bration curve was evaluated by the value of the correlation coefficient (R2). Blank plasma samples were used to confirm the absence of interfer⁃ence. The lower limit of quantification (LLOQ)was used to determine the sensitivity of the method.
1.5.2 Selectivity
Selectivity was examined by analyzing blank plasma for ten different B-F9 KO model mice.The mixed blank plasma of these model mice was used to prepare the upper limit of quantification(2000 U·L-1, ULOQ), the lower limit of quantification(62.5 U·L-1, LLOQ) and blank matrix samples. All analyses were accompanied by a standard curve to calculate the concentration of the sample.
1.5.3 Accuracy and precision
Accuracy and precision were evaluated at five QC levels (LLOQ, LQC, MQC, HQC and ULOQ) in three replicates within six days. The concentration of QC samples was estimated according to the standard curve of that day. Preci⁃sion was the expression of the relative standard deviation (RSD) and accuracy was expressed as the relative error (RE) respectively. The intra-and inter-day precision and accuracy were required to be within ± 20%, except for the LLOQ and ULOQ which should be less than±25%[20].
1.5.4 Dilution linearity
Dilution linearity was assessed to ensure ac⁃curate determination of samples with concentra⁃tions above the ULOQ. GEN-0828 working solution was diluted with mixed blank plasma to 7500 U·L-1and 4000 U·L-1and then further diluted 5-fold and 40-fold to 1500 U·L-1(HQC) and 100 U·L-1(LQC), respectively. Six samples were analyzed for each concentration, and the QC concentra⁃tion was calculated with the standard curve.
1.5.5 Stability
The stability of GEN-0828 was investigated by analyzing six replicates of two different QC levels under a variety of conditions. In brief, plasma from blank model mice was spiked with GEN-0828 to reach two concentrations of 100 and 1500 U·L-1with 6 parallel plasma samples at each concentration. The long-term stability was investi⁃gated by analyzing the samples stored at -80℃for 3 months and -20℃for 5 months, respectively.In addition, the short-time stability was deter⁃mined by keeping the QC samples at ambient temperature (25℃)for 3 h.
1.5.6 Parallelism
Six study samples with high concentrations(Cmax) were selected. Samples were diluted 10 times,20 times and 40 times to three different concen⁃trations with a blank plasma matrix and analyzed to determine the parallelism of the method.
1.6 Pharmacokinetic study design
To determine the pharmacokinetic character⁃istics of GEN-0828, genetically engineered B-F9 KO model mice were used. A single dose of GEN-0828 at 90.9 kU·kg-1(300 mg·L-1dissolved in sterilized water) was injected into the tail vein of the B-F9 KO model mice by intravenous bolus injection. Blood samples were collected from mice by jugular vein cannulation and about 50 μL of blood was collected from the jugular vein into sodium citrate anticoagulant tubes before dosing(0 h) and 0.0083, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12 h after dosing respectively. Plasma samples were collected by centrifugation at 1467×g for 10 min at 2-8℃and stored at-80℃until analysis.
1.7 statistical analysis
Microsoft Office Excel and Origin 8.0 were used for data process.The pharmacokinetic param⁃eters were calculated by non-compartment model analysis using WinNonlin software (version 6.4;Pharsight Corp.,MO,USA).
2 RESULTS
2.1 Method development
FⅦa is a serine esterase of an exogenous blood coagulation pathway, which combines with tissue factor (TF) to form a TF/FⅦa complex. In the presence of phospholipids and calcium ions,the TF/FⅦa complex can activate FX to FXa and eventually induce the formation of stable fibrin clots. In this method, the standard sub⁃stance or plasma sample diluted to 1∶10 in HEPES BSA buffer was added into the coagulation round reaction cup before FⅦdeficient plasma and FⅦa Cof-Plps were added. FⅦa and human recombi⁃nant truncated TF in FⅦa Cof-Plps form an enzyme complex, but the human recombinant truncated TF protein does not promote the activation of FⅦ.The clotting of plasma was induced by the addi⁃tion of calcium (Ca2+), performed at 37℃on the RAC-30 automatic coagulation analyzer in which the CT was recorded. And the linear relationship between FⅦa concentration and the correspond⁃ing CT was achieved.
2.2 Method validation
2.2.1 Linearity and sensitivity
The linearity (62.5-2000 U·L-1) and sensitivity(the concentration of F Ⅶa in blank plasma was less than 20% of LLOQ) were sufficient to allow reliable quantification of GEN-0828. The average regression equation and R2of six validation runs(n=6) were as follows: Y=(-0.2785±0.0073)X+(2.2233±0.0237), R2=0.9990±0.0006, where Y=lgy, y represents CT; X=lgx, x represents FⅦa activity. A typical standard curve was shown in Fig.1.
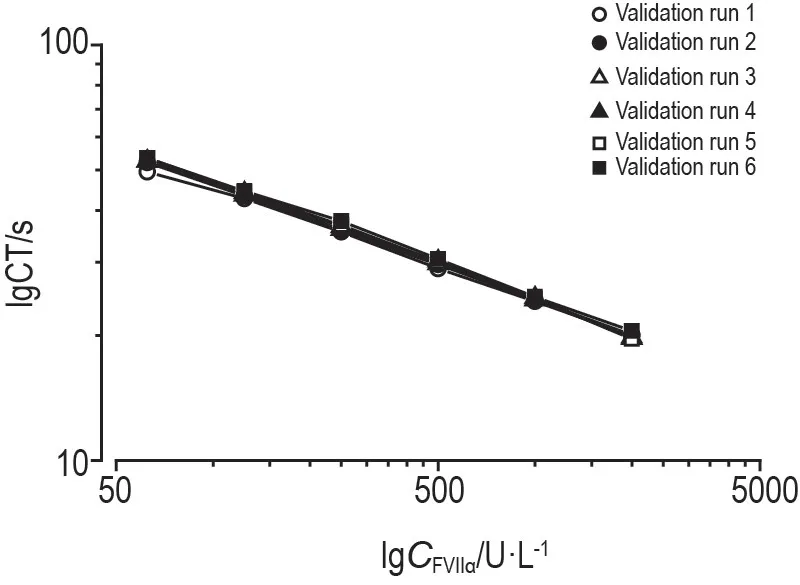
Fig.1 Typical standard curve of plasma FⅦa activity detection by clotting method. Six independent analysis batches were tested, and each line represents one analysis batch. CT:clotting time.
2.2.2 Selectivity
The selectivity of an reliable method is the ability to unequivocally evaluate the substances under analysis in the presence of components that may interfere with their determination in a complex sample. The results showed that the accu⁃racy of all samples was in the range of -15.9%to 13.8%, and the content of F Ⅶa in the blank plasma matrix without GEN-0828 was far lower than the LLOQ, indicating that there was no inter⁃ference with endogenous substances in plasma samples.
2.2.3 Accuracy and precision
Accuracy and precision for GEN-0828 were evaluated by analysis of the LLOQ, LQC, MQC,HQC and ULOQ in three replicates within six days, respectively. The main results are summa⁃rized in Tab.1. The precisions of intra-and interday were 3.9%-5.2% and 5.2%-11.1%, respec⁃tively. The accuracy ranged from -3.5% to 1.8%,and the total error of the method was between 5.7% and 15.5%, demonstrating that the estab⁃lished method was precise and accurate.
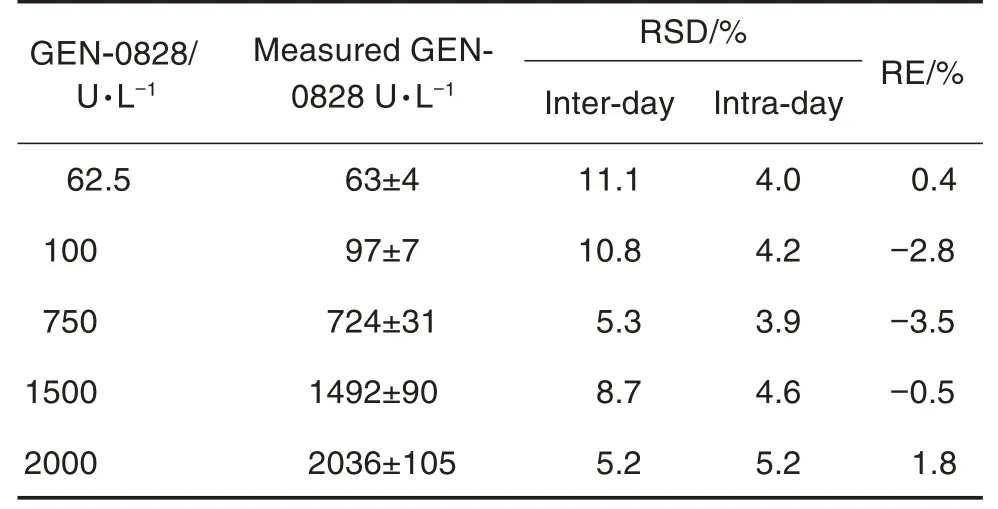
Tab.1 Inter- and intra-day precision and accuracy of clotting assay to determine GEN-0828 in plasma
2.2.4 Dilution linearity
In pharmacokinetics experiments, the concen⁃tration of some plasma samples in the high-dose group of GEN-0828 was higher than ULOQ and the dilution linearity needed to be investigated.The result showed that, compared with the labeled values, the reverse calculation accuracy RE (±%)of the concentration of GEN-0828 diluted 5 times and 40 times was 10.9% and 8.0%, respectively.In addition, the RSD (±%) of 5-fold and 10-fold dilu⁃tion was both 2.9%, suggesting that there was no dilution effect within GEN-0828 samples diluted by 40 times.
2.2.5 Stability
Stability data are displayed in Tab.2. The results showed that under different conditions, the RE(%) of each quality control plasma sample was within ±15%, indicating that GEN-0828 plasma samples could be kept stable for 3 h at room temper⁃ature, 141 d at -20℃, and 92 d at -80℃, which met the experimental requirements.
2.2.6 Parallelism
Considering the matrix effects, or differences in the affinity of metabolites, a parallel between serially diluted test samples was investigated when actual test samples were available. The results showed that the precision (RSD) between serial dilution samples was below 4.3%.
2.3 Pharmacokinetics of GEN-0828
GEN-0828 90.9 kU·kg-1was injected into the tail vein of eight B-F9 KO model mice. Plas⁃ma samples were collected and analyzed using the method developed and validated above. The plasma concentration-time profiles of GEN-0828 in B-F9 KO model mice were shown in Fig.2.The pharmacokinetic parameters were analyzed with WinNonlin 6.4 software for non-compartmental model analysis in Tab.3. The AUClastwas (115±23) h·kU·L-1, suggesting that GEN-0828 showed good absorption in vivo. And the Vzwas (3.7±0.7)L·kg-1;and the Cl was(0.69±0.12)L·h-1·kg-1.

Fig.2 Plasma concentration-time curves of GEN-0828 90.9 kU·kg-1 after intravenous administration by clot⁃ting assay in B-F9 KO model mice. 1#, 2#, 3# and 4#:female mice;5#,6#,7#and 8#:male mice.

Tab.2 Stability of GEN-0828 plasma samples placed for 3 h at 25℃,141 d at-20℃and 92 d at-80℃
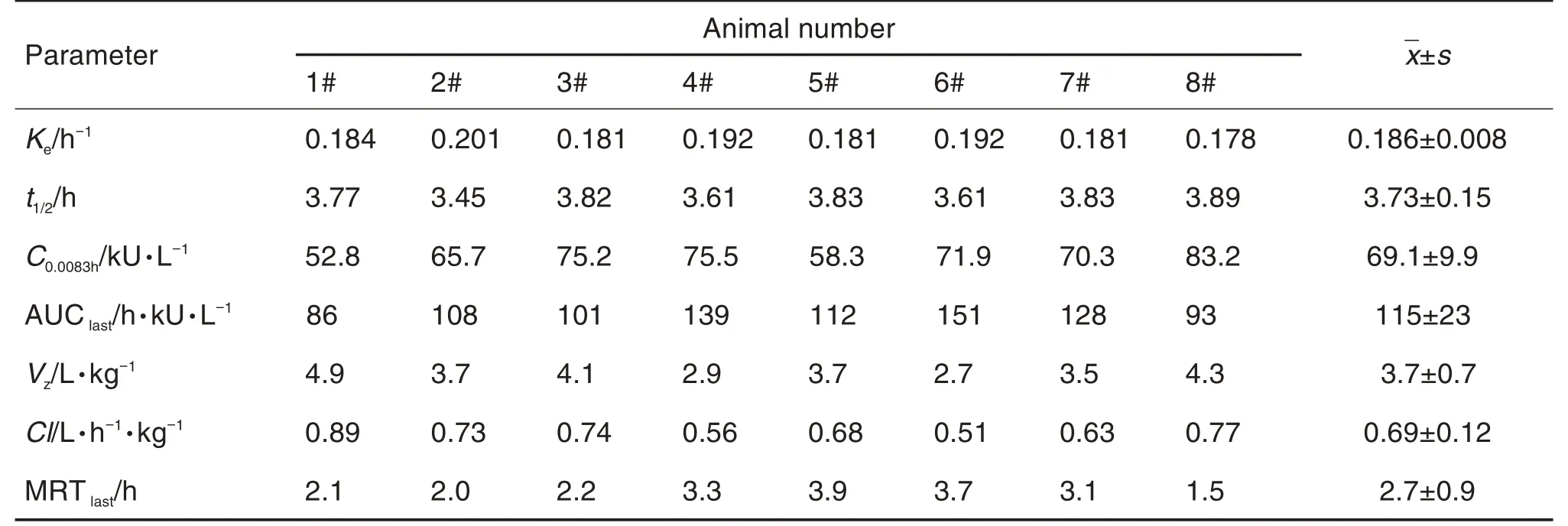
Tab.3 Main pharmacokinetic parameters of FⅦa determined by clotting assay following single tail intravenous injection of GEN-0828 90.9 kU·kg-1 to B-F9 KO model mice
3 DISCUSSION
For experimental animals, B-F9 KO model mice were chosen as the model animals for the preclinical pharmacokinetic study of the new rFⅦa drug GEN-0828. The model, derived from knocking out the FⅨgene of C57BL/6 mice, has proved to be a powerful tool for validating the effi⁃cacy of anticoagulants[21-23]. The deletion of the FⅨgene leads to insufficient FⅨwith coagula⁃tion disorders, which can be considered hemo⁃philia B with recessive inheritance of the X chro⁃mosome. However, due to the limited blood volume of B-F9 KO model mice, the routine method of blood collection could not obtain enough blood samples to draw a complete drug-time curve.Therefore, the jugular vein cannulation blood collection method was adopted to ensure that blood samples from a single mouse could meet the full requirements for pharmacokinetic analysis.In addition, the sample plasma was pretreated and the minimum required dilution was investigated before detection because of the interference of endogenous FⅦin mice. Consequently, when blank samples were diluted 50-fold, the concentration of rFⅦa was much lower than the LLOQ. The 50-fold minimum required dilution means that the blank mixed plasma used for the calibration curve and quality control samples in the experi⁃ment was diluted 50 times with HEPES-BSA buffer before analysis. Similarly, the samples to be tested were diluted 50 times with HEPES-BSA buffer before testing. This ensured that the matrix of the sample was consistent with that of the stan⁃dard curve. Morover, the blood sample collection and pretreatment which will directly affect the accu⁃racy of the plasma FⅦa determination for the FⅦare key factors in the exogenous coagulation pathway. Therefore, after collection of blood samples in 3.8% sodium citrate at 1∶9, sufficient mixing was required. If a clot was found in samples, the samples would be discarded. The two commonly used methods for rF Ⅶa activity determination are coagulant activity of FⅦ(FⅦ∶C) and FⅦa activity determination. Given the poor accuracy(especially for low activity levels) and poor intraassay variation of FⅦ∶C assay[24-25], FⅦa activity assay is a better choice in pharmacokinetic studies.This method could be applied to the detection of low-level activity of FⅦa, free of the effects on FⅦzymogen interference.
The pharmacokinetic results showed that GEN-0828 could be detected at a low concentra⁃tion of 62.5 U·L-1and at 12 h after administra⁃tion. In addition, the terminal elimination half-life(t1/2) of GEN-0828 was (3.73±0.15) h, the area under the plasma concentration-time curve from zero to the last sampling time (AUClast) was (115±23) h·kU·L-1, the maximum plasma concentra⁃tion (C0.0083h) was (69.1±9.9) kU·L-1, the volume of distribution (Vz) was (3714±720) L·kg-1, and the total body clearance(Cl)was(0.69±0.12)L·h-1·kg-1.The elimination of GEN-0828 in mice was slow,for its t1/2was significantly longer than that of NovoSeven®[t1/2=(1.94±0.57) h] whose unpub⁃lished pharmacokinetic study was carried out in our lab. And GEN-0828 had similar distribution ranges to NovoSeven®for the similar Vz. The C0.0083hof GEN-0828 was significantly higher than that of NovoSeven®; and the FⅦa activity of GEN-0828 at each blood collection time points was higher than that of NovoSeven®. Our analysis suggested that the differences in the terminal carbohydrate residues of N-linked glycans of these two rFⅦa resulted in a higher level of FⅦactivity of GEN-0828 expressed in CHO cells[26].
GEN-0828 is clinically intended to be used in the treatment of hemophilia. Our experiment is based on the B-F9 KO model mice (hemophilia B model), and the experiment data obtained is too single to be representative of all. In some reports in regard to other rFⅦa drugs[27-29], FⅧknockout model mice have been used as hemophilia A model. Therefore, our group will focus on studies on the metabolism of GEN-0828 by using hemo⁃philia A model animals in order to obtain the met⁃abolic process of the new rFⅦa candidate drug GEN-0828 in different hemophilia animals.
In conclusion, a new method has been devel⁃oped and validated to determine the content of the new rFⅦa candidate drug GEN-0828 in plasma.In addition, the pharmacokinetic profiles of GEN-0828 in hemophilia B model are revealed, which is useful for later clinical drug regimen design and conducive to subsequent studies.

