Selective catalytic reduction of NOx with NH3 assisted by non-thermal plasma over CeMnZrOx@TiO2 core-shell catalyst
Tao ZHU (竹涛), Xing ZHANG (张星), Zhenguo LI (李振国),Xiaoning REN (任晓宁), Baodong WANG (王宝冬),Xuyang CHONG (种旭阳) and Hongli MA (麻红利)
1 Institute of Atmospheric Environmental Management and Pollution Control,China University of Mining& Technology (Beijing), Beijing 100083, People’s Republic of China
2 State Key Laboratory of Petroleum Pollution Control, CNPC Research Institute of Safety and Environmental Technology, Beijing 102206, People’s Republic of China
3 National Engineering Laboratory for Mobile Source Emission Control Technology, China Automotive Technology & Research Center Co., Ltd, Tianjin 300300, People’s Republic of China
4 National Institute of Clean-and-Low-Carbon Energy, Beijing 102211, Peo ple’s Republic of China
Abstract The presented work reports the selective catalytic reduction(SCR)of NOx assisted by dielectric barrier discharge plasma via simulating marine diesel engine exhaust, and the experimental results demonstrate that the low-temperature activity of NH3-SCR assisted by non-thermal plasma is enhanced significantly,particularly in the presence of a C3H6 additive.Simultaneously,CeMnZrOx@TiO2 exhibits strong tolerance to SO2 poisoning and superior catalytic stability.It is worthwhile to explore a new approach to remove NOx from marine diesel engine exhaust,which is of vital significance for both academic research and practical applications.
Keywords: non-thermal plasma, selective catalytic reduction, marine diesel engine exhaust,CeMnZrOx@TiO2, NOx conversion
1.Introduction
With the rapid development of the transportation industry,the emission of high-power marine diesel engine exhaust has aroused widespread concern from all over the world.The marine diesel engine mainly uses heavy fuel oil with high sulfur as fuel, and its major pollutants are NOxand SO2,which not only destroy the ecological environment but also pose great threats to human beings.The selective catalytic reduction of NOxwith ammonia (NH3-SCR) is the most effective technology to remove NOxfrom marine diesel engine exhaust, and has been successfully applied in the field of mobile source exhaust for several years [1-4].V2O5-WO3/TiO2has been commercially used in the exhaust treatment of marine diesel engines, but several issues have severely impeded its further application.The main obstacles of V-based catalysts are the narrow operating temperature window, high SO2oxidation activity and biological toxicity of V species.In addition, the exhaust temperature of a marine diesel engine is usually between 150 °C and 200 °C [5, 6].Previous studies have reported that Ce-and Mn-based SCR catalysts boast remarkable lowtemperature activity on account of their superior performance in the store-release of O and excellent redox ability.However, they are easily deactivated in the presence of SO2because(NH4)2SO4,NH4HSO4and metal sulfates can block and destroy the active sites [7, 8].In these circumstances,constructing an SCR catalyst with core-shell structure could improve the SO2tolerance effectively [9-11].Gan et al synthesized a MnOx@CeO2core-shell catalyst using the chemical deposition method, and it exhibited excellent lowtemperature activity and strong SO2tolerance.Further research showed that the high activity could be attributed to the rich Mn4+/Ce3+species and highly crystalline α-MnO2,while the strong tolerance to SO2was ascribed to the CeO2shell that protected the active α-MnOxspecies from been poisoned [12].
Non-thermal plasma (NTP) has been confirmed to be a prospective technology for the removal of NOx, which offers an unconventional way to initiate chemical reactions in gases and liquids but suffers from low selectivity when it is used alone [13, 14].Currently, plasma catalysis has been a hot topic of research on account of its potential for applications in a wide range of chemical reaction, environmental protection and energy-related processes.Moreover, the coupling of plasma and catalysis can steer the reactions in the desired direction, thus providing improved selectivity and reducing unwanted by-products.Overall,plasma catalysis can improve energy efficiency and product selectivity, and sometimes it shows a synergistic effect, which promotes low-temperature activity while providing a high NOxconversion in a wide operating window[15-18].During the removal of NOx,NTP generates plenty of reactive O species (ROS), such as·O and·OH, and NO can be oxidized to NO2by ROS.A fast SCR reaction (equation (2)) occurs when the amounts of NO and NO2are equimolar in an NTP system, and its reaction efficiency is faster than that of the standard SCR reaction(equation (1)) [19, 20].
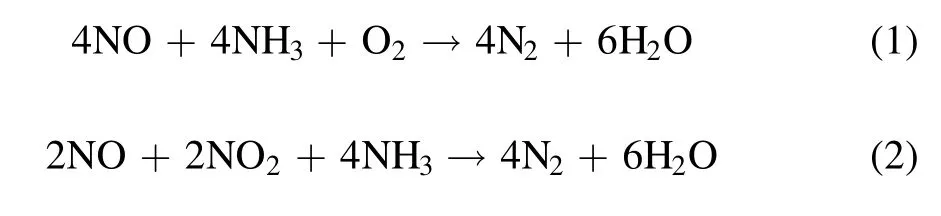
There are two combinations of NTP and NH3-SCR: inplasma catalysis (IPC) and post-plasma catalysis (PPC).IPC is usually called a single-stage plasma-catalytic system,in which the SCR catalyst is placed in the discharge zone,and its advantage is that active species can react on the SCR catalyst surface.In contrast, PPC is called a two-stage configuration, which involves placing the SCR catalyst downstream from the NTP.The oxidation of NO to NO2in NTP increases SCR catalytic performance in the two-stage configuration because the NO2removal is better than NO removal near the SCR catalyst [21-24].This work reports the deNOxperformance and catalytic stability of an NTPSCR system to reveal the effect of influencing parameters on NOxconversion via placing a CeMnZrOx@TiO2coreshell catalyst downstream from NTP.It is of great significance to investigate the NOxconversion of NH3-SCR assisted by NTP,which can provide technical guidance and theoretical support to remove NOxfrom marine diesel engine exhaust.
2.Experimental
2.1.Catalyst preparation
There are two steps in the preparation of the CeMnZrOx@TiO2catalyst.Firstly, for the CeMnZrOxcore a sol-gel method was adopted.The molar component loading of Ce:Mn:Zr was 7:2:1, and the corresponding Ce(NO3)2,Mn(NO3)2and Zr(NO3)2were dissolved in the deionized water.In addition, the C6H8O7was added at a 1:1 ratio, and the mixture was peptized by stirring for 6 h at room temperature to form a highly dispersed CeMnZrOxsol.After that,it was dried into gel at 100 °C for 12 h, followed by calcination in the muffle furnace at 500 °C for 4 h to obtain a CeMnZrOxcatalyst.Secondly, 300 mg CeMnZrOxcatalyst was dispersed in 400 ml ethanol.Subsequently, 36 ml tetrabutyl titanate and 3 ml hydrous ammonia were added separately and stirred continuously for 4 h at 60 °C.The sample was obtained by centrifugation, washed with ethanol and dried at 100 °C for 12 h, followed by calcination 500 °C for 4 h.Finally, all samples were pressed, crushed and sieved to 40-60 meshes for catalytic evaluation of NH3-SCR assisted by NTP.
2.2.Catalyst characterization
The morphology analysis of the SCR catalysts was characterized by scanning electron microscopy (SEM, JEOL,Japan).The specific surface area of the SCR catalysts was determined by the Brunauer-Emmett-Teller (BET) method,and the pore volume and average pore diameter of the SCR catalysts were obtained from the desorption curve using the Barrett-Joyner-Halenda (BJH) method.Prior to the measurements,all samples were outgassed for 12 h at 200°C in a vacuum.X-ray diffraction(XRD)measurements were carried out on an XRD-7000 S (Shimadzu Corporation, Japan),which was operated at 40 kV and 40 mA using Cu Κα radiation.The scanning angle (2θ) scans ranged from 10° to 80° with a step of 0.02°, and the scanning speed was 5° min-1.H temperature-programmed reduction (H2-TPR)measurements of the SCR catalysts were performed using a Micromeritics AutoChem II 2920 chemisorption analyzer with a thermal conductivity detector (TCD).A 100 mg quantity of the catalyst was preprocessed at 200 °C for 1 h under Ar atmosphere and then cooled to room temperature.After that, the temperature was raised to 800 °C at 10 °C min-1with a flow of H2(10%)/Ar (50 ml min-1).Temperature-programmed desorption by ammonia (NH3-TPD)experiments were carried out on the Micromeritics AutoChem II 2920.100 mg catalyst was pretreated at 500 °C for 1 h in N2(50 ml min-1) and cooled down to 120 °C, then 10%NH3/N2(50 ml min-1) was passed through the catalyst bed for 1 h to obtain saturation.After purging with N2for 1 h,the temperature was increased to 800°C at a rate of 10°C min-1.
2.3.Catalytic evaluation of NH3-SCR assisted by NTP
The schematic diagram of NH3-SCR assisted by NTP is shown in figure 1.The reaction gas mixture simulated the marine diesel engine exhaust, which consisted of 500 ppm NO,500 ppm NH3, 0-300 ppm C3H6(when used), 100 ppm SO2(when used), and air as the balance gas.The total flow rate of feed gas was kept constant at 500 ml min-1,corresponding to a gas hourly space velocity (GHSV) of 30 000 h-1.The concentration of NOx(NOx= NO + NO2),N2O and NH3in the inlet and outlet gas was analyzed by a Fourier-transform infrared spectrometer (FT-IR, Shimadzu IRTracer-100).The NTP adopted DBD plasma reactor was powered by an alternating current (AC), and the output waveform was observed on an UNI-T UPO3254CS oscilloscope.A 1.0 ml quantity of SCR catalyst was employed,which was placed downstream from the NTP,and the reaction temperature was maintained at 150°C in order to simulate the actual condition of marine diesel engine exhaust.The mathematical expressions for NOxconversion and N2selectivity are as follows:


Figure 1.Schematic diagram of NH3-SCR assisted by NTP.
whereNOx,inis the NOxconcentration at the inlet of the DBD reactor,NOx,outsignifies the NOxconcentration at outlet of the DBD reactor, andN2Ooutrepresents the N2O concentration at the outlet of the DBD reactor.
The catalytic evaluations of the CeMnZrOx@TiO2and CeMnZrOxcatalysts were tested three times, and if the experimental data fluctuated by more than 10%, it was discarded.Otherwise,it was averaged.The error bar was used as an index to evaluate the difference between the average and the measurement.
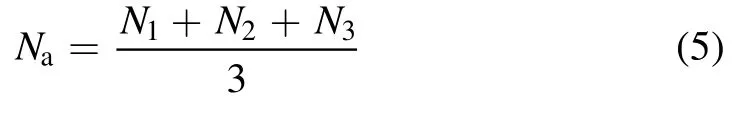

Table 1.Physical characteristics of the CeMnZrOx@TiO2 and CeMnZrOx catalysts.
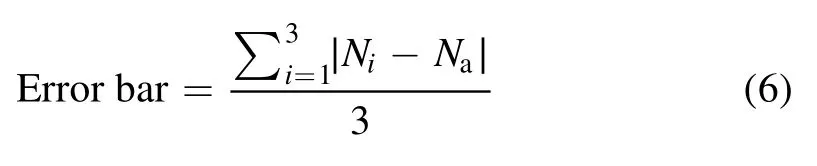
whereNasignifies the average of three experiment results.N,1N2andN3represent the results of the experiment.
3.Results and discussion
3.1.Characterizations of SCR catalysts
Figure 2 displays the SEM images of the CeMnZrOx@TiO2and CeMnZrOxcatalysts.As depicted in figure 2(a), the CeMnZrOx@TiO2core-shell structure catalyst presents a spherical particle and relatively smooth surface,and the outer surface is coated by a TiO2shell.By contrast,the CeMnZrOxcatalyst possesses a loose structure and rich porosity,which is beneficial for the high dispersion of metal oxides on the CeMnZrOxsurface [25].The BET surface area, pore volume and average pore diameter results of the SCR catalysts are summarized in table 1.Visibly, the BET surface area of the CeMnZrOx@TiO2catalyst is higher than that of CeMnZrOx,and the pore volume and average pore diameter of CeMnZrOx@TiO2are about 0.124 cm3g-1and 1.98 nm,respectively.
The XRD patterns of the CeMnZrOx@TiO2and CeMnZrOxcatalysts are presented in figure 3.As illustrated in figure 3, the CeMnZrOx@TiO2catalyst only exhibits CeO2crystalline phase, and the signals of MnOx, ZrO2and anatase TiO2phases are not observed over CeMnZrOx@TiO2, which means that the MnOx, ZrO2and TiO2species are homogeneously dispersed on the surface of CeMnZrOx@TiO2as amorphous oxides or aggregated in minicrystals that are too small to be detected by XRD.Moreover, it is worth mentioning that the intensity of the diffraction peak attributed to CeO2crystalline phase over CeMnZrOx@TiO2is reduced markedly compared to CeMnZrOx, which may be attributed to the coating of the TiO2shell.

Figure 2.SEM images of SCR catalysts.(a) CeMnZrOx@TiO2 and (b) CeMnZrOx.

Figure 3.XRD patterns of CeMnZrOx@TiO2 and CeMnZrOx catalysts.
Figure 4(a) illustrates the NH3-TPD curves of the CeMnZrOx@TiO2and CeMnZrOxcatalysts in the temperature range of 100 °C-800 °C.As plotted in figure 4(a), it is obvious that the CeMnZrOx@TiO2catalyst demonstrates two NH3desorption peaks at 302°C and 520°C,respectively.In addition, the NH3desorption signal of CeMnZrOxcatalyst occurs at 293 °C, which exhibits a board peak ranging from 100°C to 800°C.The H2-TPR profiles of CeMnZrOx@TiO2and CeMnZrOxcatalysts are presented in figure 4(b), and it can be seen that there are two reduction peaks at 210 °C and 435°C,respectively,which can be attributed to the reduction of CeO2and MnOx.Furthermore, it can be observed that the reduction peak of the CeMnZrOx@TiO2catalyst moves toward lower temperature, and the peak area of the CeMnZrOx@TiO2catalyst is larger than that of CeMnZrOxas elucidated in table 2, which indicates that there are more reductive sites in the surface of CeMnZrOx@TiO2.
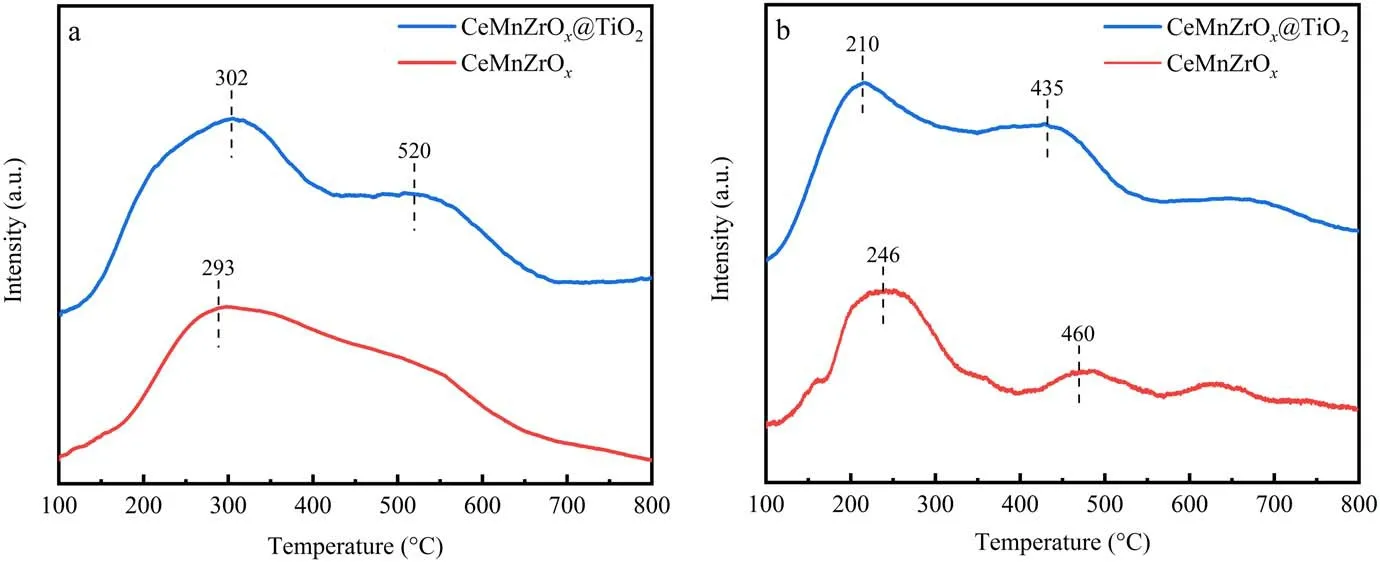
Figure 4.Chemical adsorption profiles of CeMnZrOx@TiO2 and CeMnZrOx catalysts.(a) NH3-TPD and (b) H2-TPR.
3.2.Effect of specific energy input on NOx conversion
The discharge power of the DBD reactor was determined by checking the charge-voltage (Q-U) Lissajous, and the specific energy input of the NTP system was calculated in the following equations (7) and (8).
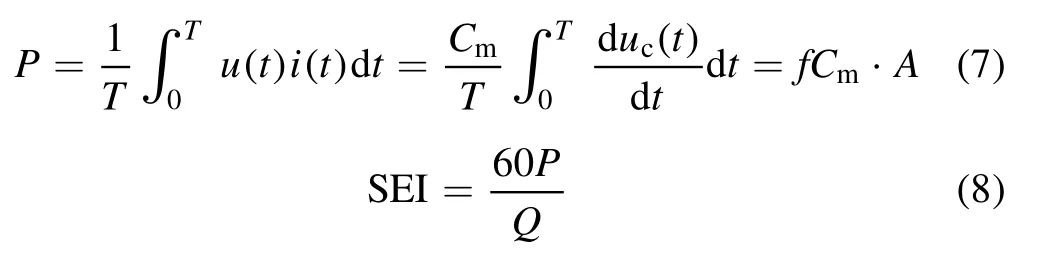
P represents the discharge power (W).T is the period of the discharge cycle (s).u(t) is the high voltage applied between electrodes (kV).i(t) is the current change with time(A).f is the discharge frequency of the AC power signal(Hz).uc(t) is the voltage at both ends of Cm(kV).Cmis the measuring capacitance,which is equal to 0.36 μF.A is the area of Q-U Lissajous graphics.SEI represents the specific energy input of the reaction system (J l-1).Q signifies the total gas flow rate (l min-1).

Table 2.NH3 and H2 consumption of CeMnZrOx@TiO2 and CeMnZrOx catalysts.
The effect of SEI on NOxconversion and N2selectivity in the NTP-SCR system is shown in figure 5(a).Obviously,the NOxconversion is very low if using NTP alone because NO is mostly converted into NO2in the NTP gas-phase reaction, and NO2does not react with NH3adequately to generate N2and H2O without an SCR catalyst.The conclusion of NO being oxidized to NO2can be drawn from figure 5(b).In addition, the N2selectivity is also poor due to the reactions of(9)and(10).Furthermore,it can be seen that the NOxconversions of CeMnZrOx@TiO2and CeMnZrOxare just 43.9%and 36.2%without NTP,respectively.In sharp contrast to this result,the NOxconversion improves gradually with the increase of SEI when NTP is operated, and the NOxconversion of CeMnZrOx@TiO2reaches the maximum value of 73.96% at an SEI of 28 J l-1.However, the NOxconversion increases slowly when the SEI exceeds 28 J l-1, which might be ascribed to the enhanced reverse reaction(equation (24)) due to the presence of a higher concentration of atomic O species.
In the NTP-SCR system, NO can be oxidized by atomic O species generated by the NTP gas-phase reaction,which is beneficial to the SCR reaction to a certain degree.Zhao et al revealed that the rate constant of O2dissociation by electron collision reactions was almost two orders of magnitude higher than that of N2dissociation, and the concentration of atomic O species increases with increasing SEI.In this case, the rate constants of NOxconversion decreased with increasing atomic O species concentration because the atomic O species were electronegative and thus reduced electron concentration[26].The above experimental phenomenon is consistent with the research of Jiang et al [27], who also found that the heat effect caused by NTP had a negligible impact on enhanced NOxconversion, while the intermediates generated in the NTP system might significantly contribute to the improved SCR catalytic performance of NOxon the catalyst surface in the NH3-SCR process.Moreover, the N2selectivity is maintained at 100%,which is almost independent of SEI.The byproducts of N2O and N2O5are not detected in the outlet gas,which indicates that the NH3-SCR assisted by NTP does not cause secondary pollution.The following chemical reactions occur in the NTP-SCR system according to the relevant reports [28-30].

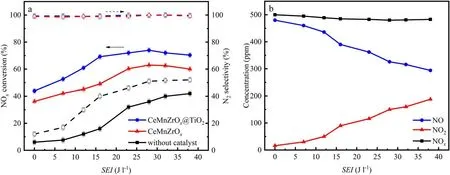
Figure 5.Effect of SEI on catalytic performance.(a)NOx conversion and N2 selectivity;(b)concentrations of NO,NO2 and NOx at the outlet of the DBD reactor.
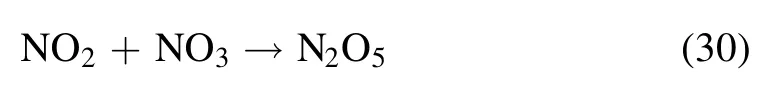
where O2* is an excited O2molecule, andN2* is an excited N2molecule.
3.3.Effect of C3H6 on NOx conversion
Previous studies have demonstrated that hydrocarbons can be attributed an important role in the removal of NOxusing NTP technology, and the reaction paths for NOxremoval can change significantly from those without hydrocarbon additives.C3H6is a representative hydrocarbon on account of its superior efficiency of NO oxidation, which can significantly enhance NO to NO2conversion performance as an essential step for the subsequent NH3-SCR reaction paralleled with economy in the input energy cost.Therefore, the effect of C3H6on NOxconversion over CeMnZrOx@TiO2and CeMnZrOxcatalysts was investigated at 28 J l-1,and figure 6 indicates the detailed results.Clearly, it can be seen that the NOxconversion improves greatly when introducing the C3H6additive to the NTP-SCR system due to the reactions between the hydrocarbons and NOx.Meanwhile, the concentration of NO2increases significantly in the NTP gas-phase reaction as depicted in figure 3, which is beneficial for the fast SCR reaction in the subsequent device.The experimental result is similar to the research of Jiang et al [27], who reported that the NOxconversion improved gradually with increasing C3H6concentration, and that the addition of C3H6not only promoted the conversion of NO into NO2in the NTP system but also enhanced the catalytic reduction of NOxon the catalyst surface.Further research found that the oxidation of C3H6with O atoms could generate oxygenates in first-stage plasma,and there might be two effects that could result in the enhanced reduction of NOx.On one hand,the O atoms might be consumed by C3H6, which limits the reverse reaction equation (24).On the other hand, NO might react with oxygenates to produce N2,as shown in equation(38).Guan et al also found that the NOxremoval efficiency was improved significantly at lower temperatures in the NH3-SCR system because C3H6could be decomposed into useful intermediates such as methyl, methoxy radicals, and partial oxidation products of C3H6conversion, including peroxyl radicals and hydro-peroxy radicals [31].In the presence of C3H6, specific pathways between NO and hydrocarbons could occur in the NTP-SCR system, such as the following chemical reactions[32-34]:

Figure 6.Effect of C3H6 on catalytic performance at an SEI of 28 J l-1.(a)NOx conversion and N2 selectivity;(b)concentrations of NO,NO2 and NOx at the outlet of the DBD reactor.

3.4.Effect of H2O on NOx conversion
It is noted that in practical applications, H2O is an inevitable combustion product in marine diesel engine exhaust and could generate a variety of strong oxidizing free radicals though NTP gas-phase reaction, which usually contributes to the oxidative removal of NOx[35].Numerous investigations have indicated that the conversion of NO to NO2is an important intermediate step in the reduction of NOxto N2,and the most efficient approach to achieve it is using NTP combined with an SCR catalyst.First of all, NO is oxidized to NO2in the presence of HC and H2O using NTP technology.Then, the catalyst reduces NO2to N2by selective reduction using NH3as a reductant.However, the adsorbed H2O molecules can consume high-energy electrons due to their electronegativity, and the specific energy input of the NTP system declines significantly when the H2O content exceeds the limit [36-38].Beyond that, H2O can also poison and deactivate the catalyst by inhibition of the active sites.In light of the characteristics of marine diesel engine exhaust, the effect of H2O on NOxconversion at a specific energy input of 28 J l-1was investigated under different H2O contents of 0,3 vol.%,5 vol.%,7 vol.%and 10 vol.%.As can be seen from figure 7, the NOxconversion over CeMnZrOx@TiO2increases slightly from nearly 73.96% to about 82.67% when H2O is added to the NTP-SCR system.However, the NOxconversion over CeMnZrOxincreases first and then decreases when the H2O concentration ranges from 0 to 10 vol.%.That is because H2O can inhibit the removal of NOxdue to the competitive adsorption of NO, NH3and H2O on the active sites, and it gives the conclusion that the TiO2shell of CeMnZrOx@TiO2can prevent the adsorption of H2O.Similarly,the concentration of NO2increases slightly when adding an appropriate amount of H2O to the NTP-SCR system.Jiang et al reported that another reaction pathway for NOxremoval exists on the SCR catalyst surface in the presence of H2O[27].The related chemical reactions are involved when there is H2O in the NTP system according to the relevant reports,which are as follows [39-42]:
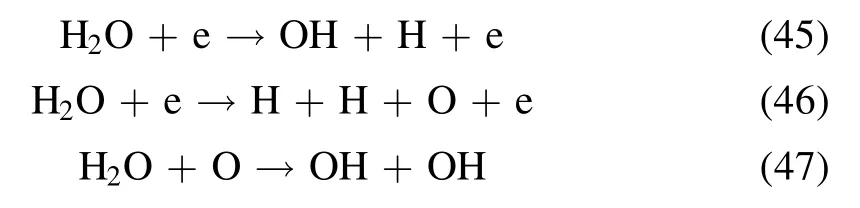
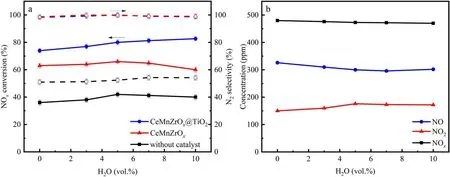
Figure 7.Effect of H2O on catalytic performance at an SEI of 28 J l-1.(a)NOx conversion and N2 selectivity;(b)concentrations of NO,NO2 and NOx at the outlet of the DBD reactor.
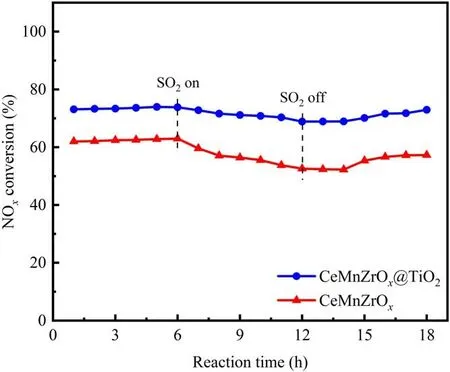
Figure 8.Catalytic performance of SO2 tolerance assisted by NTP in the presence of 100 ppm SO2 at an SEI of 28 J l-1.
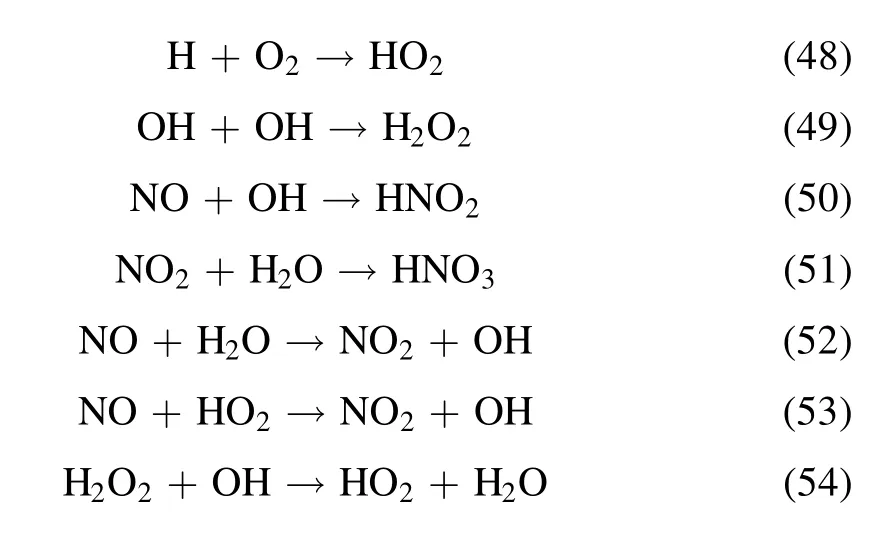
3.5.Catalytic performance of SO2 tolerance assisted by NTP
It is generally acknowledged that SO2easily forms unstable sulfite ions and further reacts with adsorbed O to generate metal sulfates,which seriously inhibit the catalytic activity of the SCR catalyst and eventually lead to SCR catalyst deactivation[43-46].The SO2tolerance of the CeMnZrOx@TiO2and CeMnZrOxcatalysts at a specific energy input of 28 J l-1was explored in the presence of 100 ppm SO2, and the detailed results are presented in figure 8.As depicted in figure 8,the catalytic activity of CeMnZrOx@TiO2decreased slightly after introducing the SO2, and then remained almost constant.After that, the NOxconversion recovered to the original level and remained stable for 6 h when the SO2was removed, which demonstrated that the addition of SO2had little effect on the SCR activity of CeMnZrOx@TiO2.By contrast, the NOxconversion of CeMnZrOxwas severely suppressed when SO2was added to the NTP-SCR system,and the NOxconversion of that was restored to a certain extent but was lower than the initial value when the SO2was cut off.Combined with the above observations, the CeMnZrOx@TiO2core-shell catalyst possesses a stronger tolerance to SO2than CeMnZrOx, and it can be deduced that the formation of (NH4)2SO4, NH4HSO4and metal sulfates may be restrained due to the presence of the TiO2shell,which can react with SO2and serve as a protective layer.
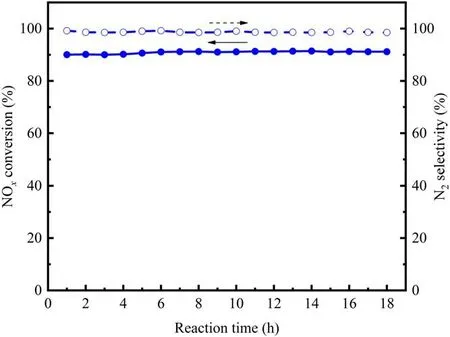
Figure 9.Catalytic stability of the CeMnZrOx@TiO2 catalyst in the presence of 300 ppm C3H6, 5 vol.% H2O and 100 ppm SO2 at an SEI of 28 J l-1.
3.6.Catalytic stability of CeMnZrOx@TiO2 catalyst
The long-time stability is of vital importance for the SCR catalyst in the NTP-SCR system and can provide a valuable reference for practical applications.The catalytic stability of CeMnZrOx@TiO2at a GHSV of 20 000 h-1was evaluated in this section.The stability test was carried out at an specific energy input of 28 J l-1,and the tested concentrations of NO,NH3, C3H6and SO2were 500 ppm, 500 ppm, 300 ppm and 100 ppm, respectively.The H2O concentration was kept at 10%, and the experimental result is depicted in figure 9.As can be seen from figure 9, the running time of the whole progress was 18 h without interruption, and the result of this continuous test illustrated that the activity of CeMnZrOx@TiO2depressed little within 18 h of reaction.Remarkably, the NOxconversion was maintained at 91.25%during the test period, which indicates that the CeMnZrOx@TiO2catalyst exhibits superior catalytic stability over the entire range of the time frame investigated.The good stability of the CeMnZrOx@TiO2catalyst in the NTP-SCR system provides the possibility for practical elimination of NOxfrom marine diesel engine exhaust.
4.Conclusions
This work has verified that the catalytic performance of NH3-SCR assisted by NTP for NOxremoval is an effective approach, and the NOxconversion of CeMnZrOx@TiO2is enhanced significantly when assisted by NTP compared to the catalytic activity of NH3-SCR without NTP, even in the presence of C3H6and H2O.The CeMnZrOx@TiO2core-shell structure catalyst possesses excellent low-temperature activity and superior catalytic stability in the NTP-SCR system,and it exhibits strong tolerance to SO2poisoning because the TiO2shell prevents the generation of (NH4)2SO4, NH4HSO4and metal sulfates from blocking the active sites.It is expected that this work can provide a new strategy for the removal of NOxfrom marine diesel engine exhaust and it has huge potential for industrial applications in the future.
Acknowledgments
This work was supported by National Key Research and Development Project of China (No.2019YFC1805503),National Engineering Laboratory for Mobile Source Emission Control Technology (No.NELMS2019A13), the Open Project Program of the State Key Laboratory of Petroleum Pollution Control (No.PPC2019013), and Major Science and Technology Projects of Shanxi Province(No.20181102017).
 Plasma Science and Technology2022年5期
Plasma Science and Technology2022年5期
- Plasma Science and Technology的其它文章
- Synthesis of Ag-decorated vertical graphene nanosheets and their electrocatalytic efficiencies
- Upgrade of an integrated Langmuir probe system on the closed divertor target plates in the HL-2A tokamak
- Numerical study of atmospheric-pressure argon plasma jet propagating into ambient nitrogen
- Effects of O2 addition on the plasma uniformity and reactivity of Ar DBD excited by ns pulsed and AC power supplies
- Design and first result of combined Langmuir-magnetic probe on J-TEXT tokamak
- A GPU-based general numerical framework for plasma simulations in terms of microscopic kinetic equations with full collision terms
