适应性演化的分子遗传机制:以高海拔适应为例
郝艳,雷富民,2,3

郝艳,2014—2020年就读于中国科学院动物研究所,在鸟类学研究组攻读博士学位,导师是雷富民研究员。目前在中国科学院动物研究所接受博士后训练,主要研究方向为鸟类对极端环境适应的分子机制。博士期间,在不同的鸟类演化体系中,采用比较基因组学、比较转录组学等研究方法,结合形态学等表型数据及功能实验验证,从位点、基因、基因家族、生物功能通路、基因表达等角度检测了鸟类对青藏高原极端环境适应的趋同和趋异信号,并探讨了鸟类适应演化的可能机制。已获得中国博士后科学基金一等面上资助项目、中国科学院特别研究助理资助项目、国家自然科学基金委青年科学基金项目及第七届中国科协青年人才托举工程项目。博士论文《三对近缘高低海拔雀形目鸟类的比较转录组学研究》获得2021年中国科学院优秀博士生论文。
适应性演化的分子遗传机制:以高海拔适应为例
郝艳1,雷富民1,2,3
1. 中国科学院动物研究所动物进化与系统学重点实验室,北京 100101 2. 中国科学院大学生命科学学院,北京 100049 3. 中国科学院动物进化与遗传前沿交叉卓越创新中心,昆明 650223
自达尔文时代起,解析适应性演化的机制一直是进化生物学和生态学领域研究最重要的科学问题之一。适应性演化通常指在自然选择驱动下,物种为提高适合度而演化出特定的表型。表型的适应表现在形态、生理生化、组织学、行为学等多个层级。随着分子生物学和测序技术的发展,越来越多的研究揭示了适应性复杂性状的遗传基础。研究适应性演化的分子遗传机制有助于理解塑造生物多样性的进化驱动力以及阐明基因型、表型和环境之间的关联关系。目前已有主效基因、超基因、多基因遗传、非编码区调控、重复序列调控、基因渐渗等多种假说可以解释适应性演化的遗传机制。高海拔极端环境的强选择压力极大地促进了物种表型和遗传适应的发生,对多组学数据的剖析为物种适应性演化提供了新的见解。本文对适应性演化的遗传机制、高海拔极端环境适应研究进展以及目前面临的主要挑战进行了综述,并对未来的发展趋势进行了展望,以期为该领域的科研人员提供参考。
表型;非编码区;多组学;调控;高海拔
适应性演化是指物种在某种特定环境的选择压力下,演化出有利于提高其适合度的适应性表型[1]。不同物种不同方向的适应性演化塑造了丰富的生物多样性,因此长期以来备受科学界关注。早在1859年,达尔文就已经在其经典论著《物种起源》中提出在自然选择的作用下,物种会发生特定的改变,以适应环境选择压力[2]。孟德尔学派“遗传学之父”摩尔根提出“突变–选择”理论,认为演化是在不断的突变与选择中发生的[3]。新达尔文主义则认为突变仅是演化的原材料,自然选择在其中发挥了重要的作用[4]。而随着1968年日本学者木村资生“中性学说”的提出[5],将自然选择理论与中性理论的争论推上了高潮。早期分子数据中观察到的氨基酸的改变实际上多为中性或近中性的,只有少数重要的氨基酸替代才能引起蛋白质功能的变化,继而促进了表型适应演化[6]。基于这些理论框架,适应性演化领域的研究在不断地发展和完善。
早期针对适应性演化的研究多集中于形态、生理生化、组织学、行为学等方面,而随着分子生物学的发展,适应性演化研究逐步转向更深层次——对分子遗传机制的探索。尤其是第二代、第三代测序技术的进步,测序成本下降,以及大量基因组序列的公布[7~10],极大地推动了基因组学分析方法在野生类群适应性演化遗传机制研究中的广泛应用。同时群体基因组、转录组、蛋白质组、代谢组、微生物组等多组学研究的涌现,为解析适应性演化的机制带来新的契机和研究范式。确定与适应相关的关键基因和分子机制有助于明确“遗传变异—表型变化—环境选择”之间的关系,对于理解何种进化力塑造了生物多样性具有重要的意义。本文主要综述了目前适应性演化的分子遗传机制和假说,并以物种对高海拔极端环境适应为例,简要介绍了具体的适应机制和研究进展。
1 适应性演化的分子遗传机制
1.1 主效基因假说(major-effect gene hypothesis)
主效基因假说是指单个基因甚至是单个位点或少数基因的变异控制适应性表型的产生(图1A)[11]。早期的进化论支持者认为适应性演化的遗传基础是微小的突变,这一观点在1930年Ronald A. Fisher的经典数学分析中得到支持。但随着20世纪80年代数量性状和微生物实验进化学科的发展,科研人员发现基因组中存在少数对适应性演化起关键作用的基因[12]。当物种受到强烈的正选择作用时,可能会导致少数具有重要作用(large effects)的基因受到选择清除(selective sweep)的影响快速固定下来,从而达到表型适应[13]。通常这种主效基因的突变会使演化更容易且更迅速地发生[14]。
其中最经典的是写入教科书的实例——工业黑化的桦尺蛾(),它在极短时间内由野生型(浅色型)演化出适应环境污染的黑色型。Van’t Hof等[11]发现黑色型中存在一段核心序列有强烈的选择特征。有趣的是,这段区域与其他鳞翅目控制翅型的主要基因座重叠,暗示了该区域具有重要的颜色调控遗传基础[11,15]。袖蝶属蝴蝶()基因组中存在多样化热点的小部分基因座,相比基因组的其他区域更有可能参与和促进蝴蝶复杂翅型的趋同和趋异演化[15]。Cooke等[16]整合全基因组关联分析(genome-wide association studies,GWAS)和基因表达分析,发现虎皮鹦鹉()中编码聚酮合酶的基因中发生了一个核苷酸多态性(single-nucleotide polymorphisms, SNPs)位点的非同义替代,造成了氨基酸变异(R644W),并进一步利用酵母体系异源表达和质谱分析,证明了基因这一氨基酸的替代影响了聚酮合酶的活性,导致了野生型中的黄色色素沉积失败,从而产生了蓝色的羽毛。
除了着色的适应,鸟类喙型的特化和生态适应也被证明会受到主效基因的影响。达尔文雀(Darwin’s finches,)是通过自然选择作用发生适应性演化的另一典型案例。Grant夫妇针对分布于加拉帕戈斯群岛和科科斯岛的达尔文雀开展了长达几十年的研究,揭示了物种如何以及为什么发生了快速多样化[17]。其中鸟类喙型通常被认为是代表着对特殊生态位适应的一种重要表型[17,18]。Abzhanov等[19]使用包含6个达尔文雀的单系系统(monophyletic system)通过不同胚胎发育阶段的样品的原位杂交实验,证实了在上喙间质中的表达与喙型深度和宽度的形状变异密切相关。Lamichhaney等[20]通过对14种达尔文雀和2种近缘外群共120个个体进行群体基因组学比较,发现了一个长度为240 kb编码影响颅面发育的转录因子基因的单倍型,证明了该单倍型有助于达尔文雀喙型的多样化,从而扩大了该类群对食物资源的利用。最近,Cheng等[21]对青藏高原(Qinghai–Tibet Plateau, QTP)特有物种——地山雀()及其近缘的13种山雀科(Paridae)鸟类的群体基因组学进行比较分析,发现具有独特取食方式(地面取食)的地山雀相比其他物种在基因中发生了选择清除,上出现的两个非同义替代(R1493Q和P1501L)可能解释了地山雀喙长的变异,因而使其可以适应青藏高原开阔的生境。
1.2 超基因假说(supergene hypothesis)
超基因假说是指适应性表型受一个包含许多紧密排列的基因座的大段区域控制(图1B)。通常由于倒位作用,在平衡选择作用下,该区域重组水平被显著降低而促使基因共进化,并连锁发挥效应,以调控复杂多态性状[22~25]。性染色体被认为是超基因的一个特殊实例[24],由于重组抑制,除可以重组的假常染色体区(pseudoautosomal regions, PARs)外,不同物种Y或W染色体的非重组区逐步形成复杂的“进化层”(evolutionary strata)模式[26,27]。最近的一系列研究表明超基因在适应性演化中扮演了比之前科学界所认识的更为重要的角色。
鸟类独特的婚配系统和生态、行为适应为超基因假说提供了最好的例证。雄性流苏鹬()具有3种截然不同的形态,包括攻击性的“independents”、半合作的“satellites”和模仿雌性的“faeders”。研究发现“independents”为祖先状态,“satellites”和“faeders”型的出现是由于11号染色体中一段长约4.5 Mb包含有125个基因的区域发生了倒位,形成了多种不重组的单倍型。而倒位的一个断点影响了编码着丝粒蛋白基因的表达,导致了3种不同形态的雄性型在体型大小、羽色、行为、睾丸大小和类固醇代谢方面的差异[28,29]。而白喉带鹀()依据头部条纹颜色,分为白色条纹型和棕褐色条纹型。Tuttle和团队经过长达20多年的野外研究,发现白色条纹的雄性几乎只与棕褐色条纹的雌性交配(98.53%),而棕褐色条纹的雄性几乎只与白色条纹的雌性交配(98.46%),这种异型交配使得任何个体都只能有1/4的几率繁殖成功,继而造成了类似4种性别的婚配系统的出现[30,31]。在行为上,两种异型交配方式代表了不同繁殖策略的权衡,白色条纹的雄性更具攻击性,很少照顾子代,且往往一夫多妻;而棕褐色条纹的雄性会照顾子代,且一夫一妻;相同条纹颜色的雌性与雄性表现类似[32]。进一步的研究揭示了白色条纹型的ZAL2m染色体发生了大于100 Mb的倒位,形成包含1137 个基因的超基因,这些基因中许多都与不同型特异的行为和羽毛颜色相关[30]。类似地,具有3种生态型变异的朱顶雀()基因组中存在约55 Mb的染色体倒位,这段倒位使得超基因形成并维持平衡多态,最终控制了黑色素形成、类胡萝卜素着色和喙型发育等多种表型[33]。也有研究发现长115 Mb的超基因控制了西鹌鹑()表型和行为的变异,携带倒位的超基因的种群喉部颜色更深、体重更重、翅膀更圆润,同时长距离迁徙能力也发生丢失或变弱[34]。
此外,高度社会性昆虫蚂蚁分离的性比和社会性多态[35,36]、蝴蝶拟态的发生[37,38]、受过度开采影响的大西洋鳕鱼()对环境波动的抵抗和洄游的生活方式[39,40]、适应不同环境的向日葵()的生态分化[41]等也都被证明受到了超基因控制。
1.3 多基因遗传假说(polygenic inheritance hypothesis)
多基因遗传假说是指适应性表型受散布在基因组中的许多基因座控制,通常这些基因的作用是微效的[42],且可能在特定功能或通路中协同发挥作用(图1C)[43]。这样的多基因性状也被称为数量性状或复杂性状[44]。早期数量遗传学研究表明许多性状都是多基因控制的,有助于快速表型适应[42]。最近系统生物学的兴起为适应性演化研究带来新的活力。系统生物学研究强调了复杂性状是受到基因间及其调控因子间相互作用关系影响的,认为基因调控网络(gene regulatory networks, GRNs)在多基因适应演化中起到了重要的作用[44]。
人类的身高、体重和皮肤、头发和眼睛的颜色等都被认为是最典型的受多基因控制的性状。生态学领域也提出了一系列法则来解释这些适应性表型的变异,如生活在高纬度寒冷地区的种群体型变大的贝格曼定律、身体突出部分变短变圆的艾伦定律、生活在寒冷干旱地区的种群皮肤颜色变浅的葛洛格定律[42]。迄今通过GWAS分析已经确定了约800个与身高相关的常见高频和罕见低频变异[45]。有研究表明非洲和亚洲热带雨林中狩猎者—采集者趋同的“侏儒”表型是多基因适应的结果,且这些基因主要与“生长因子结合”和“心脏组织发育”功能相关[46]。通过对皮肤晒黑难易度的GWAS分析,研究人员新报道了14个基因,这些基因与色素沉积表型显著相关[47]。针对人类头发颜色的GWAS研究,确定了上百个与颜色相关的SNP位点,这些位点控制了多种头发颜色独立的产生[48,49]。研究人员还发现这些基因中许多不直接参与黑色素生成,而是与头发生长或头发质地相关,暗示了黑色素细胞可能与角质形成细胞相互作用一起影响发色色素沉着[48]。
1.4 非编码区调控假说(noncoding region regulation hypothesis)
非编码区调控假说是指基因组中非编码区的变异调控了单一或多种适应性表型的形成(图1D)[50]。King和Wilson在1975年开创性地提出非编码DNA在人类和非人灵长类动物表型差异中具有重要的意义[51]。非编码区变异,如顺式调控突变(-regulatory variation),通常只在特定的时间和空间发挥作用,具有更小的基因多效性影响(pleiotropic effects),更大的进化可塑性,同时可能调控基因表达[52]。在自然选择作用下,活跃增强子的微调作用为调控适应的发生提供了可能[53]。有研究表明基因的顺式调控突变导致了袖蝶属多种蝴蝶表现出红翅型[54],而基因的顺式调控突变导致了果蝇属()多种果蝇雄性产生趋同的特异性色素沉积[55]。类似地,基因的顺式调控增强子突变是导致和形态趋同的原因[56]。Merritt等[57]通过基因敲低实验、行为学实验,结合转录组和甲基化数据证实了白色条纹型的白喉带鹀基因(编码雌激素受体α)的顺式调控区的变异而不是编码区的非同义替代,调控了其基因表达水平的改变,继而产生了领域性的攻击行为。
随着大量基因组数据的公布,保守的非编码区(conserved noncoding elements, CNEs)也逐渐成为研究的热点。针对于人脑的研究发现,非编码区在人类脑演化中发挥了至关重要的作用,非编码区的差异是导致人类和非人灵长类动物认知功能差异的主要原因[58~60]。Sackton等[61]发现调控区(如保守的非外显子元件)的变异与古颚类物种飞行能力丢失这一性状的产生密切相关。Ferris等[62]揭示了不同冬眠动物的非编码区顺式调控元件发生了平行选择作用,这些元件潜在调控与人类肥胖相关的基因。对具有鸣唱能力的鸣禽、鹦鹉和蜂鸟的比较研究发现,在与语言能力或大脑表达特化相关的基因、与神经发育功能相关的基因、与语言交流障碍相关的基因的附近都发现了非编码区加速进化的信号,暗示了这些区域在调控语言学习能力中有潜在作用[63]。但目前大量适应性演化的研究仍主要关注于蛋白编码基因的变异,忽视了非编码区在适应性演化中的重要作用,因此无偏地对全基因组序列分析将有助于全面揭示物种适应性演化的机理。
1.5 重复序列调控假说(repeated region regulation hypothesis)
重复序列调控假说是指基因组中重复序列,尤其转座子(transposable elements, TEs)的变异调控了适应性表型的形成(图1E)[64]。重复序列依据重复单元的排列方式分为串联重复序列(tandem repeats)和散在重复序列(interspersed repeats)。串联重复序列主要分为卫星DNA和微卫星DNA(简单重复);散在重复序列又称为转座子,可分为Ⅰ型转座子,即逆转录转座子(retrotransposons,RNA转座子)和Ⅱ型转座子,即DNA转座子[65]。不同物种基因组中重复序列的比例有所差异。哺乳动物中重复序列平均约占整个基因组的34%~52%,而鸟类中重复序列比例较少,平均约占整个基因组的4%~10%[10]。
重复序列的积累或DNA获得/丢失会影响基因组大小。Lamichhaney等[66]发现澳大利亚特有蛙有着比大多鸟类更小的基因组,相比其他具有大基因组的两栖动物,其基因组的减小主要是为了控制转座子扩增,这可能与其生活史方式,如快速灵活的蝌蚪发育和饮食等有关。重复序列也可能会调控基因表达,驱动新表型的出现。Van’t Hof 等[64]对桦尺蛾表型黑化的遗传基础展开了进一步的研究,揭示了由于一个大的串联重复转座子插入到基因的第一个内含子,提高了基因的表达,调节了细胞周期,从而引起了桦尺蛾工业黑化表型的出现。Goubert等[67]在全基因组范围内检测了转座子在不同细胞类型的顺式调控作用、对增强子和其他远端调控元件的调控作用,以及对转录后基因表达的影响等,证明了转座子对表型变异具有重要作用。
1.6 基因渐渗假说(introgression hypothesis)
基因渐渗假说是指一个物种的有益遗传变异通过杂交转移到另一个物种,从而实现快速表型适应(图1F)[68]。自然界中遗传混合(admixture)的频率很高,广泛存在于动、植物中[41,69]。现代人类的基因组中也存在古人类尼安德特人和丹尼索瓦人的遗传信息[70,71]。在白喉带鹀的研究中还发现棕褐色条纹型的ZAL2染色体与白色条纹型的ZAL2m染色体倒位的区域存在很大分化,推测ZAL2m染色体可能是来自于已灭绝物种与白喉带鹀历史上的杂交事件导致的基因渐渗[30]。针对火蚁属()的研究,发现一个有“多蚁后”社会形态的物种中可以抵抗重组作用的超基因区的变异,通过渐渗杂交被重复地转移到其他物种,以维持火蚁属独特的社会等级和生态特征[72]。动物毛色的季节性变化也受到基因渐渗的重要影响。北美野兔()通常在冬季换上白色长毛以进行伪装,但在积雪较少的地区,一些北美野兔会保持棕色的皮毛。研究表明其与黑尾兔()的种间基因流促使黑尾兔色素沉积基因的渗入,导致冬季多态性表型的出现[73]。类似地,有研究发现山野兔()冬季皮毛颜色的多态性也是由于与其他野兔的杂交而获得了与特定毛色相关的等位基因[74]。适应性基因渐渗还可以在一定程度上对物种进行进化拯救(evolutionary rescue)。受环境污染影响的大底鳉()通过杂交渐渗从加拿大底鳉()基因组中获得了一个包含有缺失的芳香烃受体基因,这阻碍了大底鳉芳香烃受体信号的转导,从而增强了其对环境污染的抵抗能力[75]。
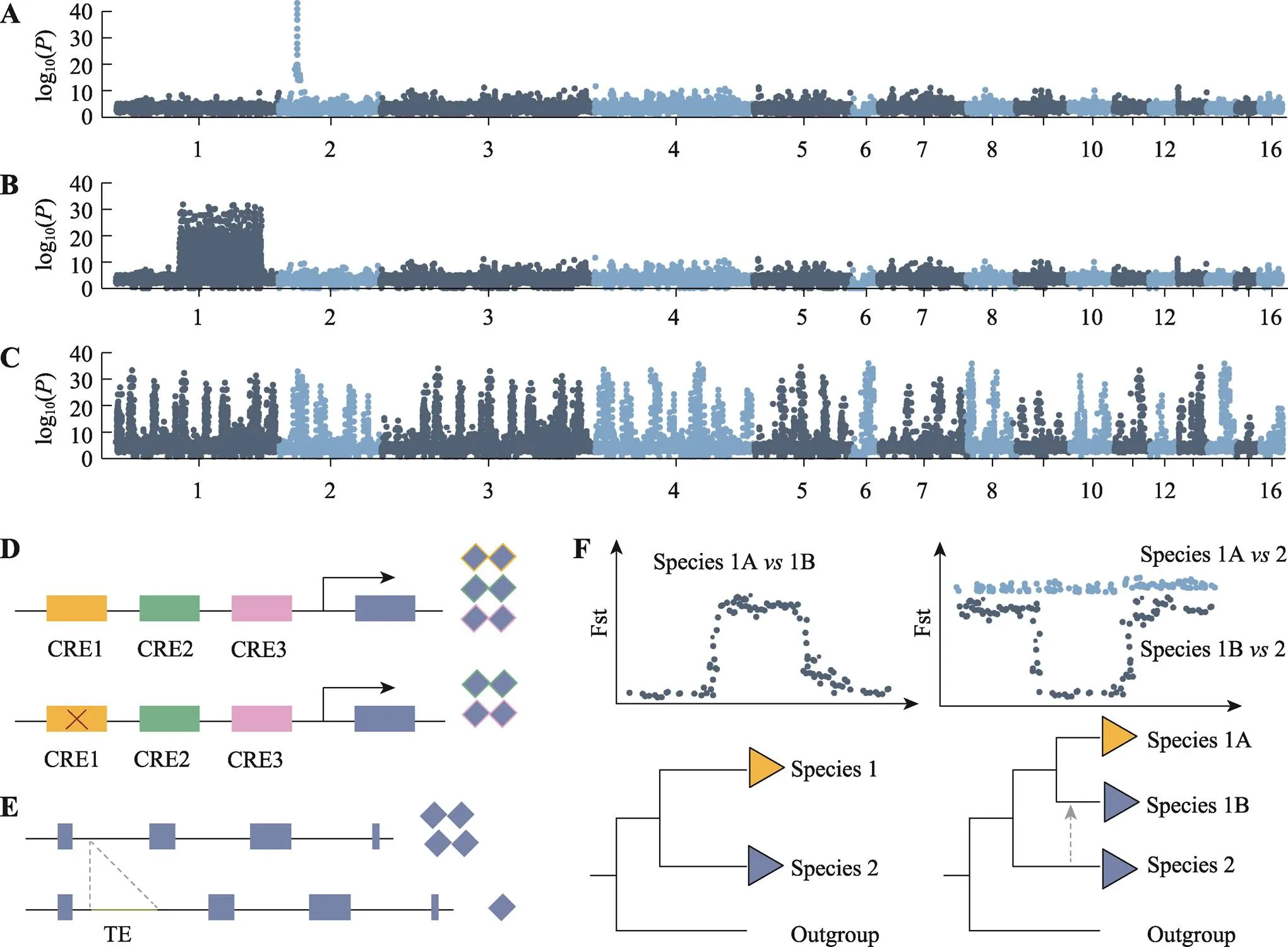
图1 适应性演化遗传机制
A:主效基因假说;B:超基因假说;C:多基因遗传假说;D:非编码区调控假说;E:重复序列调控假说;F:基因渐渗假说。CRE:-regulatory element;TE:transposable element。
2 物种对高海拔极端环境适应
高海拔地区具有低氧、低温、强紫外线辐射等典型的环境特征,因而成为了研究物种极端环境适应性演化的天然实验室。生活在这种极端环境下的生物,为应对强的选择压力,在表型和遗传层面发生了相应的适应性改变。世界范围内最典型的高海拔环境包括安第斯高原、埃塞俄比亚高原以及青藏高原。其中,被誉为“世界屋脊”和“地球第三极”的青藏高原平均海拔在4500 m以上,是全球面积最大、平均海拔最高的高原[76],长期以来受到国内外科学家的广泛关注。随着组学技术的发展,青藏高原地区已有大量典型动物类群的基因组和适应性演化机制被解析,成为全球高海拔适应研究中最具有代表性的区域。因此,本节将主要以青藏高原极端环境适应为例,简要概述不同类群的表型适应特征、遗传适应机制及其主要支持的适应性演化分子遗传假说。
2.1 物种对高海拔环境的表型适应
早期物种高海拔适应的研究主要集中在形态、生理、生化、行为等表型水平。
人类是高海拔适应研究最多和最深入的类群之一。相比仅有短期高原居住史的居民(原低海拔人群),有着久远高海拔适应历史的藏人演化出了更高的静息通气和低氧通气能力以及更低的低氧肺血管收缩和慢性高原病敏感性等特异的表型特征[77]。同时,不同于通过提高红细胞数目及血红蛋白(hemoglobin, Hb)浓度来增强氧气利用率的较短高原居住史的居民,藏人主要通过提高Hb-O2亲和力来高效利用氧气,从而有效避免了由于红细胞增多导致的血液黏稠和血栓形成,降低了罹患心脏病等高原疾病的风险[78]。
为适应极端环境压力,其他高海拔动物物种/种群相比其低海拔近缘物种或低海拔种群,也演化出一系列适应性表型。高原哺乳动物相比低海拔物种/种群,有着显著更高的心脏与体重比、更低的肺动脉压及更强的低氧适应能力[79,80]。对不同海拔的高原鼢鼠()种群的比较研究发现,相比于低海拔种群(<3000 m),高海拔的高原鼢鼠种群(4300 m)的红细胞数目、血红蛋白浓度以及红细胞比容显著增高,而右心室未发生肥厚、心肌细胞未发生肥大,暗示着心脏功能的增强[80]。对高低海拔不同种群的拉布拉多白足鼠()肌肉表型的研究发现,高海拔种群的腓肠肌有更高比例的氧化型纤维和毛细血管密度[81]。
普遍具飞行能力的鸟类也表现出许多高海拔适应性特征。例如,分布于青藏高原的地山雀、褐冠山雀()、黑冠山雀()及黑眉长尾山雀()相比各自近缘的低海拔物种,都显著提高了Hb-O2亲和力[82]。在青藏高原辐射成种的雀形目鸟类——雪雀类群(snowfinches)和近缘的低海拔树麻雀()的比较研究中发现,相比低海拔树麻雀,高海拔雪雀整体上有着更大的体重、心脏与体重比和飞行肌与体重比[83~85],以及更高的线粒体体积密度、胰岛素敏感性和需氧代谢能力[83]。但同时由于生态位竞争,这些同域分布的雪雀在形态、生理和行为等表型上也出现了一定程度的适应性分化[84,86]。此外,Shao等[87]结合几何形态学和物种的系统发育关系,发现青藏高原特有种地山雀为适应高原开阔的生境,演化出区别于其他山雀类群的长而下弯的喙和相对较长的跗跖,以便于其地面活动和觅食。
高海拔栖息的较为低等变温动物的鱼类、两栖类和爬行类,也发生了许多适应性表型变化,包括降低总代谢速率,即抑制代谢;增强对代谢副产物,如质子等的耐受;增强细胞损伤修复的能力来抵御组织氧气供应的不足等[88]。
整体来看,适应于高海拔环境的物种/类群有着趋同的表型适应特征,如更强的低氧、低温等耐受能力及更强的心血管功能,也有着趋异的物种/类群独特的表型适应特征。
2.2 物种对高海拔环境的遗传适应
遗传的适应可以驱动适应性表型的形成。自分子生物学兴起以来,科研人员们对物种高海拔适应的遗传机制进行了长期的探索。由于早期测序技术的限制,分子机制的研究主要集中于血红蛋白、细胞色素C氧化酶(cytochrome c oxidase, COX)等少数与高海拔适应功能相关的基因[82,89~91]。高海拔类群的这些蛋白编码基因某些位点的非同义氨基酸突变,可能会造成蛋白的结构变异,继而影响到O2的运输能力和氧化磷酸化过程中O2的还原。特别地,在不同的高海拔物种间发现Hb-O2亲和力趋同增加,可能是由于血红蛋白基因中某些相同位点的氨基酸趋同的改变[82,91],也可能是由于不同位点氨基酸的改变[82,90],且不同氨基酸替代所引起的变构调节机制也可能不同[82]。随着高通量测序和组学技术的发展和成熟,研究人员可以针对多种类群从全基因组水平挖掘与适应相关的遗传信号。表1汇总了不同类群高海拔适应研究所使用的测序技术及主要支持的假说。
利用群体基因组学研究方法以及先验知识(潜在候选基因集),Simonson等[92]在全基因组水平鉴定了与藏人高海拔适应相关的正选择区域,其中和单倍型的变异与其血红蛋白浓度降低显著相关。同期,Beall等[93]发现()中有多个SNPs发生了连锁不平衡,与藏人血红蛋白浓度降低也显著相关。Peng等[94]发现藏人的变异不仅和血红蛋白浓度降低有关,还和肺血管收缩响应变弱有关。Lorenzo等[95]还发现中两个突变(12C>G和380G>C)在阻止低氧诱导和HIF介导的红细胞增多中也发挥了作用。而Yang等[96]对藏人和东亚非藏人的上万个体进行基因组扫描,鉴定到了9个高海拔适应基因,其中和与藏人血液相关表型,如血红蛋白、同型半胱氨酸和叶酸的浓度强烈相关。基因rs1801133位与叶酸增加相关的等位基因频率在藏人中发生了比随机预期更显著的提高,可能与藏人高紫外辐射适应功能相关。等主效基因的变异很好地揭示了藏人高海拔低氧适应的遗传基础。最近,Xu等[79]通过结合比较基因组学和功能实验,证实了高海拔哺乳动物基因中氨基酸的改变(Q247R)驱动了其更大的心脏和更粗的肺动脉的形成,从而增强了高原低氧适应能力。这些高海拔适应机制主要支持了主效基因假说。
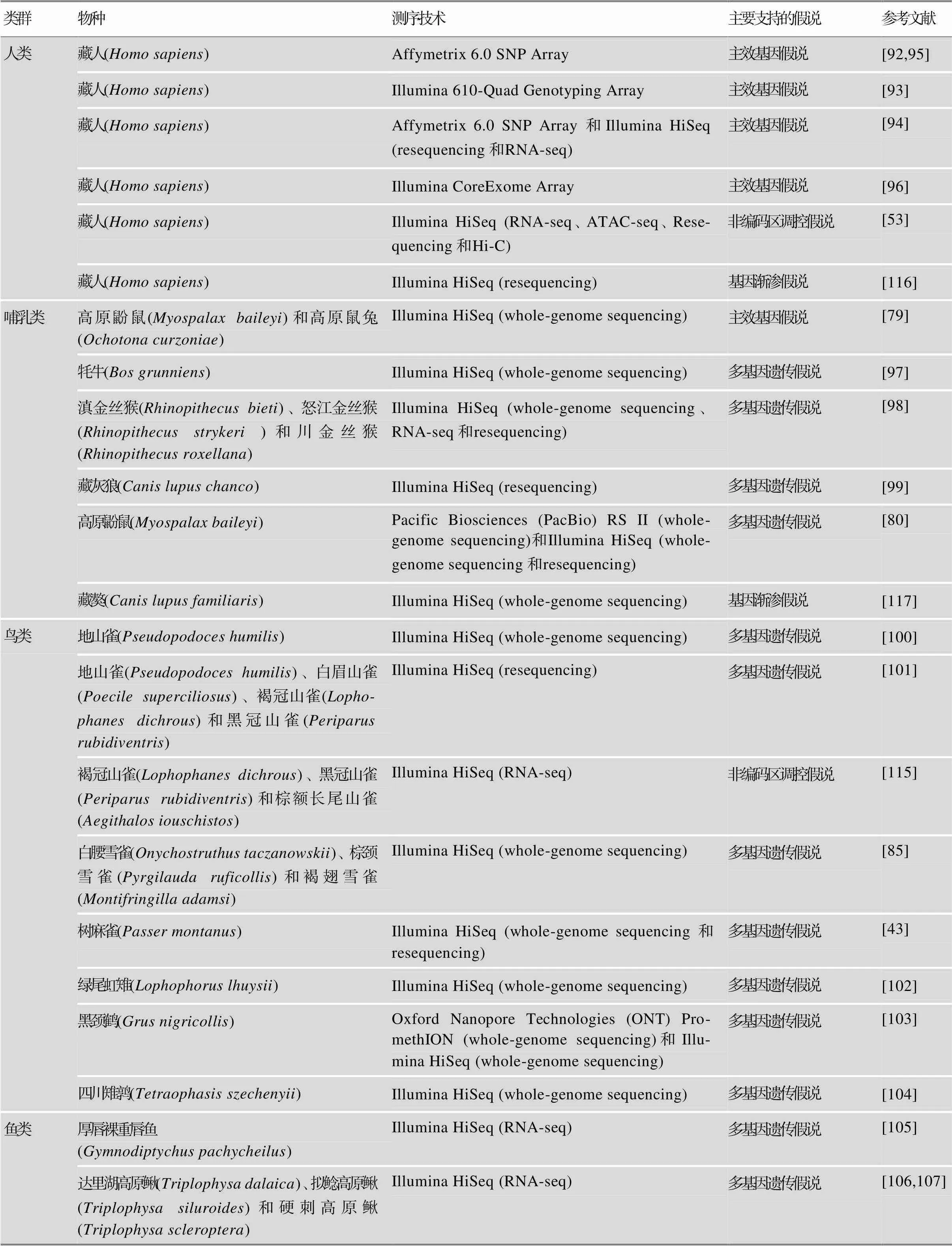
表1 高海拔适应研究的典型实例汇总
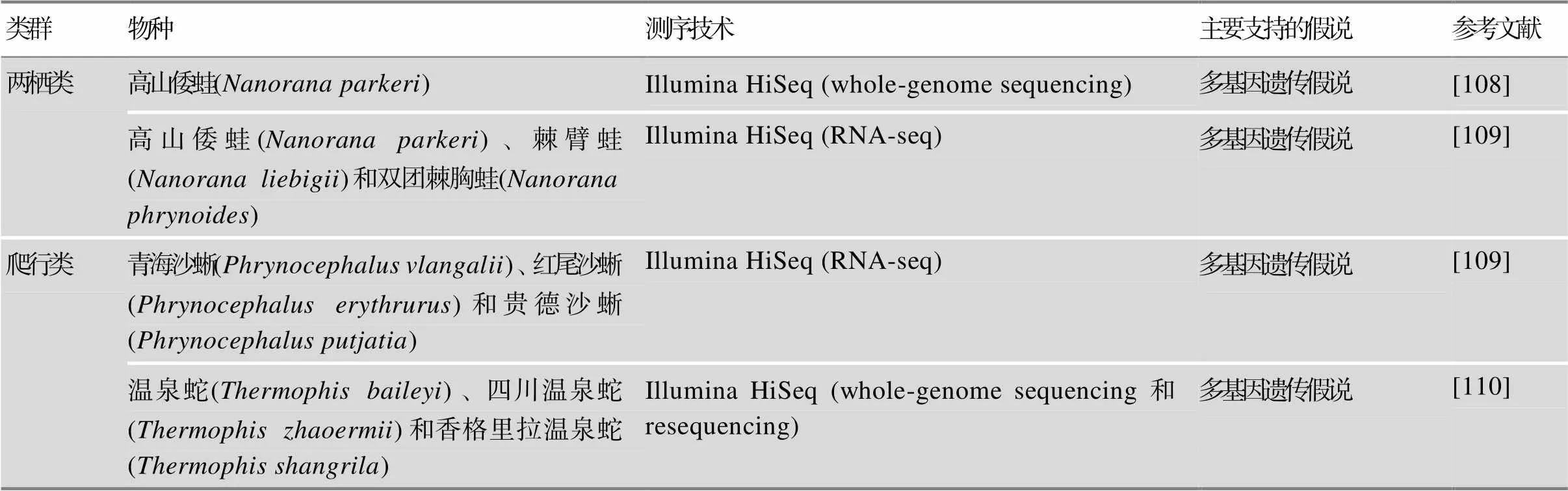
续表
然而,针对更多高海拔特有的动物类群的研究发现,高海拔适应是一个极端复杂的性状,主效基因可能不能完全解释高海拔适应机理,而更可能是遵循多基因或功能/通路/调控网络水平适应的规律,支持多基因遗传假说。哺乳类,如牦牛()[97]、金丝猴()[98]、藏灰狼()[99]和高原鼢鼠[80];鸟类,如地山雀[100,101]、山雀[101]、雪雀[85]、树麻雀[43]、绿尾虹雉()[102]、黑颈鹤()[103]和四川雉鹑()[104];鱼类,如厚唇裸重唇鱼()[105]和高原鳅()[106,107];两栖类,如高山倭蛙()[108,109];爬行类,如沙蜥()[109]和温泉蛇()[110]等物种都检测到多个与低氧响应、能量代谢和DNA修复等功能相关的正选择或加速进化基因,以应对低氧、低温以及强紫外辐射等极端环境条件。
物种高海拔适应的研究多集中在DNA序列水平,而蛋白编码序列的变异可能不直接影响基因表达的变化,而基因表达的改变在物种适应性演化中有着重要的作用[111~114]。Yu等[98]发现高海拔金丝猴在对极端环境和饮食适应过程中,能量消耗的组织(心脏、骨骼肌)和负责消化的组织(小肠、大肠、胃)的高表达基因集发生了特异的表达谱转变。针对3对近缘的高低海拔山雀、长尾山雀的比较转录组研究发现,高海拔类群在多个组织中都发生了适应性的趋同改变,且这种基因表达的改变与其表型适应,如高海拔鸟类具有更大的体型、更长的跗跖显著相关[115]。进一步研究发现高海拔适应相关的正选择基因和差异表达基因只有很少部分的交集,暗示了非编码区在调控基因表达中发挥了重要作用[115]。Xin等[53]结合RNA-seq、ATAC-seq等多组学数据,开发了vPECA(variant interpretation methodology)方法证明了非编码区调控元件的变异影响了相关转录因子的结合,继而下调了的表达,从而改变了的低氧响应和血管再生调控网络。这些研究则主要支持了非编码区调控假说。
基因渐渗假说在高海拔适应性演化中也得到证实。有研究表明藏人的高海拔适应一定程度上是源自于丹尼索瓦人样的DNA渐渗[116]。类似地,藏獒快速高海拔低氧适应能力的获得也可能由于藏灰狼的基因渐渗[117]。而超基因假说和重复序列调控假说是否可以解释高海拔适应性演化还有待进一步的研究。
此外,有着物种“第二基因组”美誉的肠道微生物在高海拔适应中也发挥了潜在的作用。Zhang等[118]比较了高海拔牦牛、藏绵羊和近缘低海拔物种的瘤胃微生物,发现高海拔类群之间有着类似的微生物群落结构和组成,且显著提高了挥发性脂肪酸的产生和显著降低了甲烷的产生,同时相似的通路
也被鉴定出来,暗示了微生物与宿主共进化以适应高海拔极端环境。Bo等[119]对高海拔鸟类肠道微生物的研究发现,不同高原鸟类的肠道菌群均主要由厚壁菌门、蓝藻菌门和变形菌门组成,其中厚壁菌门与挥发性脂肪酸的合成有关,这可能参与了高海拔类群能量代谢通路的调控。
3 适应性演化领域现存的主要挑战
3.1 方法论的更新
适应性变异的检测方法和相关方法论已经相对成熟,但目前分析方法多针对于编码区。一方面是由于蛋白编码基因在功能上通常有重要作用;另一方面它相对保守、易于检测,可以应用分子进化领域的框架和理论。而越来越多的研究表明基因组中占比更大的非编码区和重复序列等在适应性演化中也发挥着重要的调控作用。目前针对于非编码区的研究方法多聚焦在保守的非编码元件[59,120],而非保守的非编码元件也可能具有重要作用。Wang等[121]开发了功夫熊猫方法(Kung-Fu Panda, KFP),基于泊松概率(Poisson probability)检验将加速进化的检测扩展到了非保守的区域。未来应有更多针对于非编码区和重复序列加速进化的检测方法被开发和应用。
3.2 基因型和表型的关联
在种内水平,尽管数量遗传学和群体遗传学利用数量性状基因座定位(quantitative trait locus mapping, QTL)和GWAS等方法建立起了基因型和表型数据的关联,但这些分析大都需要一个物种多个个体、群体或生态型的样品,并要求大量样本和1对1的基因型和表型对应的数据,这对于非模式物种很难实现。在种上水平,表型和基因型的连接变得更加困难。通常的物种水平研究都假定单个具有特殊表型或具有类似表型(趋同演化)的物种因受到潜在的选择作用,相比于其他对比物种在序列水平发生了特定的改变,继而分析这些改变在适应性表型中可能发挥的功能,以建立起基因型和表型的潜在联系。有研究将蛋白编码基因的非同义替代速率/同义替代速率(ratio of nonsynonymous substitutions per nonsynonymous site to synonymous substitutions per synonymous site, dN/dS)与表型数据或潜在影响表型的基因表达数据,进行相关分析[122~124];最近也有整合模型尝试建立表型的改变和位点特异性序列进化之间的联系[125]。但这样的间接推测或关联分析很难真正揭示基因的变异在表型改变中起到的作用。因此,相关的实验方案、分析及统计方法和研究思路需要不断探索、发展和优化。
3.3 基因表达可塑性的作用
可塑性近些年成为进化遗传学和适应性演化领域中重要的研究主题之一。其中有两个最关键的科学问题:一个是检测可塑性和遗传变异在适应演化中的相对作用;另一个是评估可塑性在适应、驯化(acclimation)和不良适应(maladaptation)中的作用[126~129]。基因表达具有时间(包括不同发育阶段)、空间特异性,因此对基因表达的调控有助于物种可塑性地快速响应环境变化或拓殖到新生境[130]。但由于实验条件和技术的限制,目前野生类群很少开展实验,区分基因表达的变化是响应局域环境的可塑性改变还是进化的可塑性改变(具有遗传学基础)[115]。最近有研究结合细胞的转录表达和群体的遗传分化探究了短期应激和长期适应的关系,发现这两个过程有着相似的响应途径,暗示了短期应激可能促进了适应的发生[131]。未来野生类群同质园实验(common garden experiment)或相互移植实验(reciprocal transplant experiment)的技术突破将有助于更好地理解基因表达可塑性在适应性演化中的作用[43,80,86,132]。
3.4 非模式体系的实验验证
组学技术作为强有力的工具可以帮助科研人员快速挖掘与适应性表型相关的遗传信号,但现有的统计方法或模型都具有一定的局限性,这就导致了分析结果中不可避免地存在假阳性。因此,进行相关的实验验证尤为重要。目前针对筛选到的信号的功能实验验证主要依赖于模式体系,包括利用已有的成熟细胞系和小鼠、果蝇、斑马鱼、酵母、鸡等模式动物的模型。而非模式物种如果直接利用现有的模式体系进行实验验证,可能会受到所使用的模式物种的遗传背景的干扰,从而无法获得最直接的功能证据,甚至可能得到一些不可靠的结论。所以,亟需构建非模式物种本身或近缘物种的实验体系。最近,Qu等[85]构建了野生鸟类大山雀()的胚胎成纤维细胞系,证实了高海拔雪雀类群基因的非同义突变明显增强了其DNA损伤修复能力。但总体来看,构建非模式物种的实验体系仍面临着诸多的挑战,而CRISPR/Cas9基因编辑技术的发展和应用使非模式体系实验验证成为了可能。
4 结语与展望
物种的适应性演化是进化生物学和生态学领域研究的核心问题。研究物种的适应性演化分子遗传机制有助于深入理解物种多样性形成机制,对于生物多样性保护和种质资源保存具有重要意义。目前,已针对不同类群独特的形态、生理生化、组织结构和行为等表型的遗传机制进行了广泛探索。依据目前的研究进展,关于适应性演化的分子遗传机制的假说大体包括主效基因、超基因、多基因遗传、非编码区调控、重复序列调控、基因渐渗等。高海拔极端环境为物种适应性演化提供了天然实验室,塑造了高原生物独特的表型特征。当物种不能适应这种极端环境时,就可能出现肺水肿、脑水肿、心脏病等高原疾病。因此,研究极端环境中的土著物种如何适应和演化,对于研究相关疾病或癌症的发病机制具有潜在的医学意义。
如前所述,目前适应性演化领域仍存在诸多亟待解决的重要科学问题。分析方法的改进、组学技术的发展和实验手段的完善将有助于解决这些困难。适应的发生往往不局限在单一层面,而是反映在细胞、生理生化、酶学、组织学、形态、行为等多个层面、多个维度。相应地,分子遗传机制也往往支持多种不同的假说。因此,联合表型组、基因组(包括群体)、转录组(包括单细胞)、蛋白组、代谢组、微生物组、表观组(如ChIP-seq和ATAC-seq)等组学数据,结合CRISPR/Cas9基因编辑等功能实验,多维度解析适应性演化的机制必将成为未来的发展趋势。
[1] Svensson EI, Berger D. The role of mutation bias in adaptive evolution., 2019, 34(5): 422–434.
[2] Darwin CR. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life.London: John Murray, 1859.
[3] Morgan T. The Scientific Basis of Evolution. London: Faber and Faber, Ltd., 1932.
[4] Nei M. Molecular Evolutionary Genetics. New York: Columbia University Press, 1987.
[5] Kimura M. Evolutionary rate at the molecular level., 1968, 217(5129): 624–626.
[6] Nei M. Selectionism and neutralism in molecular evolution., 2005, 22(12): 2318–2342.
[7] Feng SH, Stiller J, Deng Y, Armstrong J, Fang Q, Reeve AH, Xie D, Chen GJ, Guo CX, Faircloth BC, Petersen B, Wang ZJ, Zhou Q, Diekhans M, Chen WJ, Andreu-Sánchez S, Margaryan A, Howard JT, Parent C, Pacheco G, Sinding MHS, Puetz L, Cavill E, Ribeiro ÂM, Eckhart L, Fjeldså J, Hosner PA, Brumfield RT, Christidis L, Bertelsen MF, Sicheritz-Ponten T, Tietze DT, Robertson BC, Song G, Borgia G, Claramunt S, Lovette IJ, Cowen SJ, Njoroge P, Dumbacher JP, Ryder OA, Fuchs J, Bunce M, Burt DW, Cracraft J, Meng GL, Hackett SJ, Ryan PG, Jønsson KA, Jamieson IG, da Fonseca RR, Braun EL, Houde P, Mirarab S, Suh A, Hansson B, Ponnikas S, Sigeman H, Stervander M, Frandsen PB, van der Zwan H, van der Sluis R, Visser C, Balakrishnan CN, Clark AG, Fitzpatrick JW, Bowman R, Chen N, Cloutier A, Sackton TB, Edwards SV, Foote DJ, Shakya SB, Sheldon FH, Vignal A, Soares AER, Shapiro B, González-Solís J, Ferrer-Obiol J, Rozas J, Riutort M, Tigano A, Friesen V, Dalén L, Urrutia AO, Székely T, Liu Y, Campana MG, Corvelo A, Fleischer RC, Rutherford KM, Gemmell NJ, Dussex N, Mouritsen H, Thiele N, Delmore K, Liedvogel M, Franke A, Hoeppner MP, Krone O, Fudickar AM, Milá B, Ketterson ED, Fidler AE, Friis G, Parody-Merino ÁM, Battley PF, Cox MP, Lima NCB, Prosdocimi F, Parchman TL, Schlinger BA, Loiselle BA, Blake JG, Lim HC, Day LB, Fuxjager MJ, Baldwin MW, Braun MJ, Wirthlin M, Dikow RB, Ryder TB, Camenisch G, Keller LF, DaCosta JM, Hauber ME, Louder MIM, Witt CC, McGuire JA, Mudge J, Megna LC, Carling MD, Wang B, Taylor SA, Del-Rio G, Aleixo A, Vasconcelos ATR, Mello CV, Weir JT, Haussler D, Li QY, Yang HM, Wang J, Lei FM, Rahbek C, Gilbert MTP, Graves GR, Jarvis ED, Paten B, Zhang GJ. Dense sampling of bird diversity increases power of comparative genomics., 2020, 587(7833): 252–257.
[8] Consortium Z. A comparative genomics multitool for scientific discovery and conservation., 2020, 587(7833): 240–245.
[9] Chen L, Qiu Q, Jiang Y, Wang K, Lin ZS, Li ZP, Bibi F, Yang YZ, Wang JH, Nie WH, Su WT, Liu GC, Li QY, Fu WW, Pan XY, Liu C, Yang J, Zhang CZ, Yin Y, Wang Y, Zhao Y, Zhang C, Wang ZK, Qin YL, Liu W, Wang B, Ren YD, Zhang R, Zeng Y, da Fonseca RR, Wei B, Li R, Wan WT, Zhao RP, Zhu WB, Wang YT, Duan SC, Gao Y, Zhang YE, Chen CY, Hvilsom C, Epps CW, Chemnick LG, Dong Y, Mirarab S, Siegismund HR, Ryder OA, Gilbert MTP, Lewin HS, Zhang GJ, Heller R, Wang W. Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits., 2019, 364(6446): eaav6202.
[10] Zhang GJ, Li C, Li QY, Li B, Larkin DM, Lee C, Storz JF, Antunes A, Greenwold MJ, Meredith RW, Ödeen A, Cui J, Zhou Q, Xu LH, Pan HL, Wang ZJ, Jin LJ, Zhang P, Hu HF, Yang W, Hu J, Xiao J, Yang ZK, Liu Y, Xie QL, Yu H, Lian JM, Wen P, Zhang F, Li H, Zeng YL, Xiong ZJ, Liu SP, Zhou L, Huang ZY, An N, Wang J, Zheng QM, Xiong YQ, Wang GB, Wang B, Wang JJ, Fan Y, da Fonseca RR, Alfaro-Núñez A, Schubert M, Orlando L, Mourier T, Howard JT, Ganapathy G, Pfenning A, Whitney O, Rivas MV, Hara E, Smith J, Farré M, Narayan J, Slavov G, Romanov MN, Borges R, Machado JP, Khan I, Springer MS, Gatesy J, Hoffmann FG, Opazo JC, Håstad O, Sawyer RH, Kim H, Kim KW, Kim HJ, Cho S, Li N, Huang YH, Bruford MW, Zhan XJ, Dixon A, Bertelsen MF, Derryberry E, Warren W, Wilson RK, Li SB, Ray DA, Green RE, O'Brien SJ, Griffin D, Johnson WE, Haussler D, Ryder OA, Willerslev E, Graves GR, Alström P, Fjeldså J, Mindell DP, Edwards SV, Braun EL, Rahbek C, Burt DW, Houde P, Zhang Y, Yang HM, Wang J, Avian Genome Consortium, Jarvis ED, Gilbert MTP, Wang J. Comparative genomics reveals insights into avian genome evolution and adaptation., 2014, 346(6215): 1311–1320.
[11] Van't Hof AE, Edmonds N, Dalíková M, Marec F, Saccheri IJ. Industrial melanism in British peppered moths has a singular and recent mutational origin., 2011, 332(6032): 958–960.
[12] Orr HA. The genetic theory of adaptation: a brief history., 2005, 6(2): 119–127.
[13] Jain K, Stephan W. Modes of rapid polygenic adaptation., 2017, 34(12): 3169–3175.
[14] Messer PW, Petrov DA. Population genomics of rapid adaptation by soft selective sweeps., 2013, 28(11): 659–669.
[15] Papa R, Martin A, Reed RD. Genomic hotspots of adaptation in butterfly wing pattern evolution., 2008, 18(6): 559–564.
[16] Cooke TF, Fischer CR, Wu P, Jiang TX, Xie KT, Kuo J, Doctorov E, Zehnder A, Khosla C, Chuong CM, Bustamante CD. Genetic mapping and biochemical basis of yellow feather pigmentation in budgerigars., 2017, 171(2): 427–439.e21.
[17] Grant PR, Grant BR. How and why species multiply. The radiation of Darwin's finches. Princeton University Press, 2008.
[18] Bright JA, Marugán-Lobón J, Cobb SN, Rayfield EJ. The shapes of bird beaks are highly controlled by nondietary factors., 2016, 113(19): 5352–5357.
[19] Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ.and morphological variation of beaks in Darwin's finches., 2004, 305(5689): 1462–1465.
[20] Lamichhaney S, Berglund J, Almén MS, Maqbool K, Grabherr M, Martinez-Barrio A, Promerová M, Rubin CJ, Wang C, Zamani N, Grant BR, Grant PR, Webster MT, Andersson L. Evolution of Darwin’s finches and their beaks revealed by genome sequencing., 2015, 518(7539): 371–375.
[21] Cheng YL, Miller MJ, Zhang DZ, Song G, Jia CX, Qu YH, Lei FM. Comparative genomics reveals evolution of a beak morphology locus in a high-altitude songbird., 2020, 37(10): 2983–2988.
[22] Hunt BG. Supergene evolution: recombination finds a way., 2020, 30(2): R73–R76.
[23] Gutiérrez-Valencia J, Hughes PW, Berdan EL, Slotte T. The genomic architecture and evolutionary fates of supergenes., 2021, 13(5): evab057.
[24] Thompson MJ, Jiggins CD. Supergenes and their role in evolution., 2014, 113(1): 1–8.
[25] Schwander T, Libbrecht R, Keller L. Supergenes and complex phenotypes., 2014, 24(7): R288– R294.
[26] Xu LH, Auer G, Peona V, Suh A, Deng Y, Feng SH, Zhang GJ, Blom MPK, Christidis L, Prost S, Irestedt M, Zhou Q. Dynamic evolutionary history and gene content of sex chromosomes across diverse songbirds., 2019, 3(5): 834–844.
[27] Zhou Q, Zhang JL, Bachtrog D, An N, Huang QF, Jarvis ED, Gilbert MTP, Zhang GJ. Complex evolutionary trajectories of sex chromosomes across bird taxa., 2014, 346(6215): 1246338.
[28] Küpper C, Stocks M, Risse JE, Dos Remedios N, Farrell LL, McRae SB, Morgan TC, Karlionova N, Pinchuk P, Verkuil YI, Kitaysky AS, Wingfield JC, Piersma T, Zeng K, Slate J, Blaxter M, Lank DB, Burke T. A supergene determines highly divergent male reproductive morphs in the ruff., 2016, 48(1): 79–83.
[29] Lamichhaney S, Fan GY, Widemo F, Gunnarsson U, Thalmann DS, Hoeppner MP, Kerje S, Gustafson U, Shi CC, Zhang H, Chen WB, Liang XM, Huang LH, Wang JH, Liang EJ, Wu Q, Lee SMY, Xu X, Höglund J, Liu X, Andersson L. Structural genomic changes underlie alternative reproductive strategies in the ruff ()., 2016, 48(1): 84–88.
[30] Tuttle EM, Bergland AO, Korody ML, Brewer MS, Newhouse DJ, Minx P, Stager M, Betuel A, Cheviron ZA, Warren WC, Gonser RA, Balakrishnan CN. Divergence and functional degradation of a sex chromosome-like supergene., 2016, 26(3): 344–350.
[31] Campagna L. Supergenes: the genomic architecture of a bird with four sexes., 2016, 26(3): R105– R107.
[32] Tuttle EM. Alternative reproductive strategies in the white-throated sparrow: behavioral and genetic evidence., 2003, 14(3): 425–432.
[33] Funk ER, Mason NA, Pálsson S, Albrecht T, Johnson JA, Taylor SA. A supergene underlies linked variation in color and morphology in a Holarctic songbird., 2021, 12(1): 6833.
[34] Sanchez-Donoso I, Ravagni S, Rodríguez-Teijeiro JD, Christmas MJ, Huang Y, Maldonado-Linares A, Puigcerver M, Jiménez-Blasco I, Andrade P, Gonçalves D, Friis G, Roig I, Webster MT, Leonard JA, Vilà C. Massive genome inversion drives coexistence of divergent morphs in common quails., 2022, 32(2): 462–469.e6.
[35] Lagunas-Robles G, Purcell J, Brelsford A. Linked supergenes underlie split sex ratio and social organization in an ant., 2021, 118(46): e2101427118.
[36] Yan Z, Martin SH, Gotzek D, Arsenault SV, Duchen P, Helleu Q, Riba-Grognuz O, Hunt BG, Salamin N, Shoemaker D, Ross KG, Keller L. Evolution of a supergene that regulates a trans-species social polymorphism., 2020, 4(2): 240–249.
[37] Charlesworth D, Charlesworth B. Mimicry: the hunting of the supergene., 2011, 21(20): R846–R848.
[38] Zhang W, Westerman E, Nitzany E, Palmer S, Kronforst MR. Tracing the origin and evolution of supergene mimicry in butterflies., 2017, 8(1): 1269.
[39] Sodeland M, Jentoft S, Jorde PE, Mattingsdal M, Albretsen J, Kleiven AR, Synnes AEW, Espeland SH, Olsen EM, Andrè C, Stenseth NC, Knutsen H. Stabilizing selection on Atlantic cod supergenes through a millennium of extensive exploitation., 2022, 119(8): e2114904119.
[40] Matschiner M, Barth JMI, Tørresen OK, Star B, Baalsrud HT, Brieuc MSO, Pampoulie C, Bradbury I, Jakobsen KS, Jentoft S. Supergene origin and maintenance in Atlantic cod., 2022, 6(4): 469–481.
[41] Todesco M, Owens GL, Bercovich N, Légaré JS, Soudi S, Burge DO, Huang K, Ostevik KL, Drummond EBM, Imerovski I, Lande K, Pascual-Robles MA, Nanavati M, Jahani M, Cheung W, Staton SE, Muños S, Nielsen R, Donovan LA, Burke JM, Yeaman S, Rieseberg LH. Massive haplotypes underlie ecotypic differentiation in sunflowers., 2020, 584(7822): 602–607.
[42] Pritchard JK, Pickrell JK, Coop G. The genetics of human adaptation: hard sweeps, soft sweeps, and polygenic adaptation., 2010, 20(4): R208– R215.
[43] Qu YH, Chen CH, Xiong Y, She HS, Zhang YE, Cheng YL, DuBay S, Li DM, Ericson PGP, Hao Y, Wang HY, Zhao HF, Song G, Zhang HL, Yang T, Zhang C, Liang LP, Wu TY, Zhao JY, Gao Q, Zhai WW, Lei FM. Rapid phenotypic evolution with shallow genomic differentiation during early stages of high elevation adaptation in Eurasian Tree Sparrows., 2020, 7(1): 113–127.
[44] Fagny M, Austerlitz F. Polygenic adaptation: integrating population genetics and gene regulatory networks., 2021, 37(7): 631–638.
[45] Marouli E, Graff M, Medina-Gomez C, Lo KS, Wood AR, Kjaer TR, Fine RS, Lu YC, Schurmann C, Highland HM, Rüeger S, Thorleifsson G, Justice AE, Lamparter D, Stirrups KE, Turcot V, Young KL, Winkler TW, Esko T, Karaderi T, Locke AE, Masca NGD, Ng MCY, Mudgal P, Rivas MA, Vedantam S, Mahajan A, Guo XQ, Abecasis G, Aben KK, Adair LS, Alam DS, Albrecht E, Allin KH, Allison M, Amouyel P, Appel EV, Arveiler D, Asselbergs FW, Auer PL, Balkau B, Banas B, Bang LE, Benn M, Bergmann S, Bielak LF, Blüher M, Boeing H, Boerwinkle E, Böger CA, Bonnycastle LL, Bork-Jensen J, Bots ML, Bottinger EP, Bowden DW, Brandslund I, Breen G, Brilliant MH, Broer L, Burt AA, Butterworth AS, Carey DJ, Caulfield MJ, Chambers JC, Chasman DI, Chen YDI, Chowdhury R, Christensen C, Chu AY, Cocca M, Collins FS, Cook JP, Corley J, Galbany JC, Cox AJ, Cuellar-Partida G, Danesh J, Davies G, de Bakker PIW, de Borst GJ, de Denus S, de Groot MCH, de Mutsert R, Deary IJ, Dedoussis G, Demerath EW, den Hollander AI, Dennis JG, Di Angelantonio E, Drenos F, Du MM, Dunning AM, Easton DF, Ebeling T, Edwards TL, Ellinor PT, Elliott P, Evangelou E, Farmaki AE, Faul JD, Feitosa MF, Feng S, Ferrannini E, Ferrario MM, Ferrieres J, Florez JC, Ford I, Fornage M, Franks PW, Frikke-Schmidt R, Galesloot TE, Gan W, Gandin I, Gasparini P, Giedraitis V, Giri A, Girotto G, Gordon SD, Gordon-Larsen P, Gorski M, Grarup N, Grove ML, Gudnason V, Gustafsson S, Hansen T, Harris KM, Harris TB, Hattersley AT, Hayward C, He L, Heid IM, Heikkilä K, Helgeland Ø, Hernesniemi J, Hewitt AW, Hocking LJ, Hollensted M, Holmen OL, Hovingh GK, Howson JMM, Hoyng CB, Huang PL, Hveem K, Ikram MA, Ingelsson E, Jackson AU, Jansson JH, Jarvik GP, Jensen GB, Jhun MA, Jia YC, Jiang XJ, Johansson S, Jørgensen ME, Jørgensen T, Jousilahti P, Jukema JW, Kahali B, Kahn RS, Kähönen M, Kamstrup PR, Kanoni S, Kaprio J, Karaleftheri M, Kardia SLR, Karpe F, Kee F, Keeman R, Kiemeney LA, Kitajima H, Kluivers KB, Kocher T, Komulainen P, Kontto J, Kooner JS, Kooperberg C, Kovacs P, Kriebel J, Kuivaniemi H, Küry S, Kuusisto J, La Bianca M, Laakso M, Lakka TA, Lange EM, Lange LA, Langefeld CD, Langenberg C, Larson EB, Lee IT, Lehtimäki T, Lewis CE, Li HX, Li J, Li-Gao RF, Lin HH, Lin LA, Lin X, Lind L, Lindström J, Linneberg A, Liu YH, Liu YM, Lophatananon A, Luan JA, Lubitz SA, Lyytikäinen LP, Mackey DA, Madden PAF, Manning AK, Männistö S, Marenne G, Marten J, Martin NG, Mazul AL, Meidtner K, Metspalu A, Mitchell P, Mohlke KL, Mook-Kanamori DO, Morgan A, Morris AD, Morris AP, Müller-Nurasyid M, Munroe PB, Nalls MA, Nauck M, Nelson CP, Neville M, Nielsen SF, Nikus K, Njølstad PR, Nordestgaard BG, Ntalla I, O'Connel JR, Oksa H, Loohuis LMO, Ophoff RA, Owen KR, Packard CJ, Padmanabhan S, Palmer CNA, Pasterkamp G, Patel AP, Pattie A, Pedersen O, Peissig PL, Peloso GM, Pennell CE, Perola M, Perry JA, Perry JRB, Person TN, Pirie A, Polasek O, Posthuma D, Raitakari OT, Rasheed A, Rauramaa R, Reilly DF, Reiner AP, Renström F, Ridker PM, Rioux JD, Robertson N, Robino A, Rolandsson O, Rudan I, Ruth KS, Saleheen D, Salomaa V, Samani NJ, Sandow K, Sapkota Y, Sattar N, Schmidt MK, Schreiner PJ, Schulze MB, Scott RA, Segura-Lepe MP, Shah S, Sim XL, Sivapalaratnam S, Small KS, Smith AV, Smith JA, Southam L, Spector TD, Speliotes EK, Starr JM, Steinthorsdottir V, Stringham HM, Stumvoll M, Surendran P, Hart LM, Tansey KE, Tardif JC, Taylor KD, Teumer A, Thompson DJ, Thorsteinsdottir U, Thuesen BH, Tönjes A, Tromp G, Trompet S, Tsafantakis E, Tuomilehto J, Tybjaerg-Hansen A, Tyrer JP, Uher R, Uitterlinden AG, Ulivi S, van der Laan SW, Van Der Leij AR, van Duijn CM, van Schoor NM, van Setten J, Varbo A, Varga TV, Varma R, Edwards DRV, Vermeulen SH, Vestergaard H, Vitart V, Vogt TF, Vozzi D, Walker M, Wang FJ, Wang CA, Wang S, Wang YQ, Wareham NJ, Warren HR, Wessel J, Willems SM, Wilson JG, Witte DR, Woods MO, Wu Y, Yaghootkar H, Yao J, Yao P, Yerges-Armstrong LM, Young R, Zeggini E, Zhan XW, Zhang WH, Zhao JH, Zhao W, Zhao W, Zheng H, Zhou W, EPIC-InterAct Consortium, CHD Exome+ Consortium, ExomeBP Consortium, T2D-Genes Consortium, GoT2D Genes Consortium, Global Lipids Genetics Consortium, ReproGen Consortium, MAGIC Investigators, Rotter JI, Boehnke M, Kathiresan S, McCarthy MI, Willer CJ, Stefansson K, Borecki IB, Liu DJ, North KE, Heard-Costa NL, Pers TH, Lindgren CM, Oxvig C, Kutalik Z, Rivadeneira F, Loos RJF, Frayling TM, Hirschhorn JN, Deloukas P, Lettre G. Rare and low-frequency coding variants alter human adult height., 2017, 542(7640): 186–190.
[46] Bergey CM, Lopez M, Harrison GF, Patin E, Cohen JA, Quintana-Murci L, Barreiro LB, Perry GH. Polygenic adaptation and convergent evolution on growth and cardiac genetic pathways in African and Asian rainforest hunter-gatherers., 2018, 115(48): E11256–E11263.
[47] Visconti A, Duffy DL, Liu F, Zhu G, Wu WT, Chen Y, Hysi PG, Zeng CQ, Sanna M, Iles MM, Kanetsky PA, Demenais F, Hamer MA, Uitterlinden AG, Ikram MA, Nijsten T, Martin NG, Kayser M, Spector TD, Han JL, Bataille V, Falchi M. Genome-wide association study in 176,678 Europeans reveals genetic loci for tanning response to sun exposure., 2018, 9(1): 1684.
[48] Morgan MD, Pairo-Castineira E, Rawlik K, Canela-Xandri O, Rees J, Sims D, Tenesa A, Jackson IJ. Genome-wide study of hair colour in UK Biobank explains most of the SNP heritability., 2018, 9(1): 5271.
[49] Hysi PG, Valdes AM, Liu F, Furlotte NA, Evans DM, Bataille V, Visconti A, Hemani G, McMahon G, Ring SM, Smith GD, Duffy DL, Zhu G, Gordon SD, Medland SE, Lin BD, Willemsen G, Jan Hottenga J, Vuckovic D, Girotto G, Gandin I, Sala C, Concas MP, Brumat M, Gasparini P, Toniolo D, Cocca M, Robino A, Yazar S, Hewitt AW, Chen Y, Zeng CQ, Uitterlinden AG, Ikram MA, Hamer MA, van Duijn CM, Nijsten T, Mackey DA, Falchi M, Boomsma DI, Martin NG, Hinds DA, Kayser M, Spector TD. Genome-wide association meta-analysis of individuals of European ancestry identifies new loci explaining a substantial fraction of hair color variation and heritability., 2018, 50(5): 652–656.
[50] Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution., 2008, 134(1): 25–36.
[51] King MC, Wilson AC. Evolution at two levels in humans and chimpanzees., 1975, 188(4184): 107–116.
[52] Hao Y, Qu YH, Song G, Lei FM. Genomic insights into the adaptive convergent evolution., 2019, 20(2): 81–89.
[53] Xin JX, Zhang H, He YX, Duren Z, Bai CJ, Chen L, Luo X, Yan DS, Zhang CY, Zhu X, Yuan QY, Feng ZY, Cui CY, Qi XB, Ouzhuluobu, Wong WH, Wang Y, Su B. Chromatin accessibility landscape and regulatory network of high-altitude hypoxia adaptation., 2020, 11(1): 4928.
[54] Reed RD, Papa R, Martin A, Hines HM, Counterman BA, Pardo-Diaz C, Jiggins CD, Chamberlain NL, Kronforst MR, Chen R, Halder G, Nijhout HF, McMillan WO.drives the repeated convergent evolution of butterfly wing pattern mimicry., 2011, 333(6046): 1137–1141.
[55] Signor SA, Liu Y, Rebeiz M, Kopp A. Genetic convergence in the evolution of male-specific color patterns in., 2016, 26(18): 2423–2433.
[56] Frankel N, Wang S, Stern DL. Conserved regulatory architecture underlies parallel genetic changes and convergent phenotypic evolution., 2012, 109(51): 20975–20979.
[57] Merritt JR, Grogan KE, Zinzow-Kramer WM, Sun D, Ortlund Eric A, Yi SV, Maney DL. A supergene-linked estrogen receptor drives alternative phenotypes in a polymorphic songbird., 2020, 117(35): 21673–21680.
[58] Babbitt CC, Fedrigo O, Pfefferle AD, Boyle AP, Horvath JE, Furey TS, Wray GA. Both noncoding and protein-coding RNAs contribute to gene expression evolution in the primate brain., 2010, 2: 67–79.
[59] Pollard KS, Salama SR, King B, Kern AD, Dreszer T, Katzman S, Siepel A, Pedersen JS, Bejerano G, Baertsch R, Rosenbloom KR, Kent J, Haussler D. Forces shaping the fastest evolving regions in the human genome., 2006, 2(10): e168.
[60] Prabhakar S, Noonan JP, Pääbo S, Rubin EM. Accelerated evolution of conserved noncoding sequences in humans., 2006, 314(5800): 786.
[61] Sackton TB, Grayson P, Cloutier A, Hu ZR, Liu JS, Wheeler NE, Gardner PP, Clarke JA, Baker AJ, Clamp M, Edwards SV. Convergent regulatory evolution and loss of flight in paleognathous birds., 2019, 364(6435): 74–78.
[62] Ferris E, Gregg C. Parallel accelerated evolution in distant hibernators reveals candidateelements and genetic circuits regulating mammalian obesity., 2019, 29(9): 2608–2620.e4.
[63] Cahill JA, Armstrong J, Deran A, Khoury CJ, Paten B, Haussler D, Jarvis ED. Positive selection in noncoding genomic regions of vocal learning birds is associated with genes implicated in vocal learning and speech functions in humans., 2021, 31(11): 2035–2049.
[64] Van’t Hof AE, Campagne P, Rigden DJ, Yung CJ, Lingley J, Quail MA, Hall N, Darby AC, Saccheri IJ. The industrial melanism mutation in British peppered moths is a transposable element., 2016, 534(7605): 102–105.
[65] Wells JN, Feschotte C. A field guide to eukaryotic transposable elements., 2020, 54: 539–561.
[66] Lamichhaney S, Catullo R, Keogh JS, Clulow S, Edwards SV, Ezaz T. A bird-like genome from a frog: mechanisms of genome size reduction in the ornate burrowing frog,., 2021, 118(11): e2011649118.
[67] Goubert C, Zevallos NA, Feschotte C. Contribution of unfixed transposable element insertions to human regulatory variation., 2020, 375(1795): 20190331.
[68] Harrison RG, Larson EL. Hybridization, introgression, and the nature of species boundaries., 2014, 105 Suppl 1: 795–809.
[69] Zhang W, Dasmahapatra KK, Mallet J, Moreira GRP, Kronforst MR. Genome-wide introgression among distantly relatedbutterfly species., 2016, 17: 25.
[70] Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai WW, Fritz MHY, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prüfer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Höber B, Höffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, Novod N, Affourtit J, Egholm M, Verna C, Rudan P, Brajkovic D, Kucan Ž, Gušic I, Doronichev VB, Golovanova LV, Lalueza-Fox C, de la Rasilla M, Fortea J, Rosas A, Schmitz RW, Johnson PLF, Eichler EE, Falush D, Birney E, Mullikin JC, Slatkin M, Nielsen R, Kelso J, Lachmann M, Reich D, Pääbo S. A draft sequence of the Neandertal genome., 2010, 328(5979): 710–722.
[71] Reich D, Green RE, Kircher M, Krause J, Patterson N, Durand EY, Viola B, Briggs AW, Stenzel U, Johnson PLF, Maricic T, Good JM, Marques-Bonet T, Alkan C, Fu QM, Mallick S, Li H, Meyer M, Eichler EE, Stoneking M, Richards M, Talamo S, Shunkov MV, Derevianko AP, Hublin JJ, Kelso J, Slatkin M, Pääbo S. Genetic history of an archaic hominin group from Denisova cave in Siberia., 2010, 468(7327): 1053–1060.
[72] Stolle E, Pracana R, López-Osorio F, Priebe MK, Hernández GL, Castillo-Carrillo C, Arias MC, Paris CI, Bollazzi M, Priyam A, Wurm Y. Recurring adaptive introgression of a supergene variant that determines social organization., 2022, 13(1): 1180.
[73] Jones MR, Mills LS, Alves PC, Callahan CM, Alves JM, Lafferty DJR, Jiggins FM, Jensen JD, Melo-Ferreira J, Good JM. Adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares., 2018, 360(6395): 1355–1358.
[74] Giska I, Farelo L, Pimenta J, Seixas FA, Ferreira MS, Marques JP, Miranda I, Letty J, Jenny H, Hackländer K, Magnussen E, Melo-Ferreira J. Introgression drives repeated evolution of winter coat color polymorphism in hares., 2019, 116(48): 24150–24156.
[75] Oziolor EM, Reid NM, Yair S, Lee KM, Guberman VerPloeg S, Bruns PC, Shaw JR, Whitehead A, Matson CW. Adaptive introgression enables evolutionary rescue from extreme environmental pollution., 2019, 364(6439): 455–457.
[76] Ruddiman WF, Kutzbach JE. Plateau uplift and climatic change., 1991, 264(3): 66–75.
[77] Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives., 1998, Suppl 27: 25–64.
[78] Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives., 2007, 104 Suppl 1(Suppl 1): 8655–8660.
[79] Xu DM, Yang CP, Shen QS, Pan SK, Liu Z, Zhang TZ, Zhou X, Lei ML, Chen P, Yang H, Zhang T, Guo YT, Zhan XJ, Chen YB, Shi P. A single mutation underlying phenotypic convergence for hypoxia adaptation on the Qinghai-Tibetan Plateau., 2021, 31(9): 1032– 1035.
[80] Zhang T, Chen J, Zhang J, Guo YT, Zhou X, Li MW, Zheng ZZ, Zhang TZ, Murphy RW, Nevo E, Shi P. Phenotypic and genomic adaptations to the extremely high elevation in plateau zokor ()., 2021, 30(22): 5765–5779.
[81] Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation., 2015, 32(8): 1962–1976.
[82] Zhu XJ, Guan YY, Signore AV, Natarajan C, DuBay SG, Cheng YL, Han NJ, Song G, Qu YH, Moriyama H, Hoffmann FG, Fago A, Lei FM, Storz JF. Divergent and parallel routes of biochemical adaptation in high-altitude passerine birds from the Qinghai-Tibet Plateau., 2018, 115(8): 1865–1870.
[83] Xiong Y, Fan LQ, Hao Y, Cheng YL, Chang YB, Wang J, Lin HY, Song G, Qu YH, Lei FM. Physiological and genetic convergence supports hypoxia resistance in high-altitude songbirds., 2020, 16(12): e1009270.
[84] She HS, Jiang ZY, Song G, Ericson PGP, Luo X, Shao SM, Lei FM, Qu YH. Quantifying adaptive divergence of the snowfinches in a common landscape., 2021, 0: 1–14.
[85] Qu YH, Chen CH, Chen XM, Hao Y, She HS, Wang MX, Ericson PGP, Lin HY, Cai TL, Song G, Jia CX, Chen CY, Zhang HL, Li J, Liang LP, Wu TY, Zhao JY, Gao Q, Zhang GJ, Zhai WW, Zhang C, Zhang YE, Lei FM. The evolution of ancestral and species-specific adaptations in snowfinches at the Qinghai-Tibet Plateau., 2021, 118(13): e2012398118.
[86] Li DM, Davis JE, Sun YF, Wang G, Nabi G, Wingfield JC, Lei FM. Coping with extremes: convergences of habitat use, territoriality, and diet in summer but divergences in winter between two sympatric snow finches on the Qinghai-Tibet Plateau., 2020, 15(6): 533–543.
[87] Shao SM, Quan Q, Cai TL, Song G, Qu YH, Lei FM. Evolution of body morphology and beak shape revealed by a morphometric analysis of 14 Paridae species., 2016, 13: 30.
[88] Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability., 2007, 69: 145–170.
[89] Projecto-Garcia J, Natarajan C, Moriyama H, Weber RE, Fago A, Cheviron ZA, Dudley R, McGuire JA, Witt CC, Storz JF. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds., 2013, 110(51): 20669–20674.
[90] Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Muñoz-Fuentes V, Green AJ, Kopuchian C, Tubaro PL, Alza L, Bulgarella M, Smith MM, Wilson RE, Fago A, McCracken KG, Storz JF. Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level., 2015, 11(12): e1005681.
[91] Scott GR, Schulte PM, Egginton S, Scott ALM, Richards JG, Milsom WK. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose., 2011, 28(1): 351–363.
[92] Simonson TS, Yang YZ, Huff CD, Yun HX, Qin G, Witherspoon DJ, Bai ZZ, Lorenzo FR, Xing JC, Jorde LB, Prchal JT, Ge RL. Genetic evidence for high-altitude adaptation in Tibet., 2010, 329(5987): 72–75.
[93] Beall CM, Cavalleri GL, Deng LB, Elston RC, Gao Y, Knight J, Li CH, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu YM, Xu Z, Yang L, Zaman MJ, Zeng CQ, Zhang L, Zhang XL, Zhaxi PC, Zheng YT. Natural selection on() associated with low hemoglobin concentration in Tibetan highlanders., 2010, 107(25): 11459–11464.
[94] Peng Y, Cui CY, He YX, Ouzhuluobu, Zhang H, Yang DY, Zhang Q, Bianbazhuoma, Yang LX, He YB, Xiang K, Zhang XM, Bhandari S, Shi P, Yangla, Dejiquzong, Baimakangzhuo, Duojizhuoma, Pan YY, Cirenyangji, Baimayangji, Gonggalanzi, Bai CJ, Bianba, Basang, Ciwangsangbu, Xu SH, Chen H, Liu SM, Wu TY, Qi XB, Su B. Down-regulation oftranscription and genetic adaptation of Tibetans to high-altitude hypoxia., 2017, 34(4): 818–830.
[95] Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing JC, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation., 2014, 46(9): 951–956.
[96] Yang J, Jin ZB, Chen J, Huang XF, Li XM, Liang YB, Mao JY, Chen X, Zheng ZL, Bakshi A, Zheng DD, Zheng MQ, Wray NR, Visscher PM, Lu F, Qu J. Genetic signatures of high-altitude adaptation in Tibetans., 2017, 114(16): 4189–4194.
[97] Qiu Q, Zhang GJ, Ma T, Qian WB, Wang JY, Ye ZQ, Cao CC, Hu QJ, Kim J, Larkin DM, Auvil L, Capitanu B, Ma J, Lewin HA, Qian XJ, Lang YS, Zhou R, Wang LZ, Wang K, Xia JQ, Liao SG, Pan SK, Lu X, Hou HL, Wang Y, Zang XT, Yin Y, Ma H, Zhang J, Wang ZF, Zhang YM, Zhang DW, Yonezawa T, Hasegawa M, Zhong Y, Liu WB, Zhang Y, Huang ZY, Zhang SX, Long RJ, Yang HM, Wang J, Lenstra JA, Cooper DN, Wu Y, Wang J, Shi P, Wang J, Liu JQ. The yak genome and adaptation to life at high altitude., 2012, 44(8): 946–949.
[98] Yu L, Wang GD, Ruan J, Chen YB, Yang CP, Cao X, Wu H, Liu YH, Du ZL, Wang XP, Yang J, Cheng SC, Zhong L, Wang L, Wang X, Hu JY, Fang L, Bai B, Wang KL, Yuan N, Wu SF, Li BG, Zhang JG, Yang YQ, Zhang CL, Long YC, Li HS, Yang JY, Irwin DM, Ryder OA, Li Y, Wu CI, Zhang YP. Genomic analysis of snub-nosed monkeys () identifies genes and processes related to high-altitude adaptation., 2016, 48(8): 947–952.
[99] Zhang WP, Fan ZX, Han E, Hou R, Zhang L, Galaverni M, Huang J, Liu H, Silva P, Li P, Pollinger JP, Du LM, Zhang XY, Yue BS, Wayne RK, Zhang ZH. Hypoxia adaptations in the grey wolf () from Qinghai-Tibet Plateau., 2014, 10(7): e1004466.
[100] Qu YH, Zhao HW, Han NJ, Zhou GY, Song G, Gao B, Tian SL, Zhang JB, Zhang RY, Meng XH, Zhang Y, Zhang Y, Zhu XJ, Wang WJ, Lambert D, Ericson PGP, Subramanian S, Yeung C, Zhu HM, Jiang Z, Li RQ, Lei FM. Ground tit genome reveals avian adaptation to living at high altitudes in the Tibetan Plateau., 2013, 4: 2071.
[101] Cheng YL, Miller MJ, Zhang DZ, Xiong Y, Hao Y, Jia CX, Cai TL, Li SH, Johansson US, Liu Y, Chang YB, Song G, Qu YH, Lei FM. Parallel genomic responses to historical climate change and high elevation in East Asian songbirds., 2021, 118(50): e2023918118.
[102] Cui K, Li WJ, James JG, Peng CJ, Jin JZ, Yan CC, Fan ZX, Du LM, Price M, Wu YJ, Yue BS. The first draft genome of: a step forward for Phasianidae genomic diversity and conservation., 2019, 111(6): 1209–1215.
[103] Zhou C, Yu HR, Geng Y, Liu W, Zheng S, Yang N, Meng Y, Dou L, Price M, Ran JH, Yue BS, Wu YJ. A high-quality draft genome assembly of the black-necked crane () based on Nanopore sequencing., 2019, 11(12): 3332–3340.
[104] Zhou C, James JG, Xu Y, Tu HM, He XC, Wen QC, Price M, Yang N, Wu YJ, Ran J, Meng Y, Yue BS. Genome-wide analysis sheds light on the high-altitude adaptation of the buff-throated partridge ()., 2020, 295(1): 31–46.
[105] Yang LD, Wang Y, Zhang ZL, He SP. Comprehensive transcriptome analysis reveals accelerated genic evolution in a Tibet fish,., 2014, 7(1): 251–261.
[106] Wang Y, Yang LD, Wu B, Song ZB, He SP. Transcriptome analysis of the plateau fish (): implications for adaptation to hypoxia in fishes., 2015, 565(2): 211–220.
[107] Wang Y, Yang LD, Zhou K, Zhang YP, Song ZB, He SP. Evidence for adaptation to the Tibetan Plateau inferred from Tibetan loach transcriptomes., 2015, 7(11): 2970–2982.
[108] Sun YB, Xiong ZJ, Xiang XY, Liu SP, Zhou WW, Tu XL, Zhong L, Wang L, Wu DD, Zhang BL, Zhu CL, Yang MM, Chen HM, Li F, Zhou L, Feng SH, Huang C, Zhang GJ, Irwin D, Hillis DM, Murphy RW, Yang HM, Che J, Wang J, Zhang YP. Whole-genome sequence of the Tibetan frogand the comparative evolution of tetrapod genomes., 2015, 112(11): E1257–E1262.
[109] Sun YB, Fu TT, Jin JQ, Murphy RW, Hillis DM, Zhang YP, Che J. Species groups distributed across elevational gradients reveal convergent and continuous genetic adaptation to high elevations., 2018, 115(45): E10634–E10641.
[110] Li JT, Gao YD, Xie L, Deng C, Shi P, Guan ML, Huang S, Ren JL, Wu DD, Ding L, Huang ZY, Nie H, Humphreys DP, Hillis DM, Wang WZ, Zhang YP. Comparative genomic investigation of high-elevation adaptation in ectothermic snakes., 2018, 115(33): 8406–8411.
[111] Feigin CY, Newton AH, Doronina L, Schmitz J, Hipsley CA, Mitchell KJ, Gower G, Llamas B, Soubrier J, Heider TN, Menzies BR, Cooper A, O’Neill RJ, Pask AJ. Genome of the Tasmanian tiger provides insights into the evolution and demography of an extinct marsupial carnivore., 2018, 2(1): 182–192.
[112] Carroll SB, Prud’homme B, Gompel N. Regulating evolution., 2008, 298(5): 60–67.
[113] Gallant JR, Traeger LL, Volkening JD, Moffett H, Chen PH, Novina CD, Phillips GN, Anand R, Wells GB, Pinch M, Güth R, Unguez GA, Albert JS, Zakon HH, Samanta MP, Sussman MR. Genomic basis for the convergent evolution of electric organs., 2014, 344(6191): 1522–1525.
[114] Verta JP, Jones FC. Predominance of-regulatory changes in parallel expression divergence of sticklebacks., 2019, 8: e43785.
[115] Hao Y, Xiong Y, Cheng YL, Song G, Jia CX, Qu YH, Lei FM. Comparative transcriptomics of 3 high-altitude passerine birds and their low-altitude relatives., 2019, 116(24): 11851–11856.
[116] Huerta-Sánchez E, Jin X, Asan, Bianba ZM, Peter BM, Vinckenbosch N, Liang Y, Yi X, He MZ, Somel M, Ni PX, Wang B, Ou XH, Huasang, Luosang JB, Cuo ZXP, Li K, Gao GY, Yin Y, Wang W, Zhang XQ, Xu X, Yang HM, Li YR, Wang J, Wang J, Nielsen R. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA., 2014, 512(7513): 194–197.
[117] Miao BP, Wang Z, Li YX. Genomic analysis reveals hypoxia adaptation in the Tibetan mastiff by introgression of the gray wolf from the Tibetan Plateau., 2017, 34(3): 734–743.
[118] Zhang ZG, Xu DM, Wang L, Hao JJ, Wang JF, Zhou X, Wang WW, Qiu Q, Huang XD, Zhou JW, Long RJ, Zhao FQ, Shi P. Convergent evolution of rumen microbiomes in high-altitude mammals., 2016, 26(14): 1873–1879.
[119] Bo TB, Song G, Tang SY, Zhang MR, Ma ZW, Lv HR, Wu Y, Zhang DZ, Yang L, Wang DH, Lei FM. Incomplete concordance between host phylogeny and gut microbial community in Tibetan wetland birds., 2022, 13: 848906.
[120] Prabhakar S, Noonan JP, Pääbo S, Rubin EM. Accelerated evolution of conserved noncoding sequences in humans., 2006, 314(5800): 786.
[121] Wang YT, Dai GY, Gu ZL, Liu GP, Tang K, Pan YH, Chen YJ, Lin X, Wu N, Chen HS, Feng S, Qiu S, Sun HD, Li Q, Xu C, Mao YN, Zhang YE, Khaitovich P, Wang YL, Liu QX, Han JDJ, Shao Z, Wei G, Xu C, Jing NH, Li HP. Accelerated evolution of anenhancer shapes mammalian social hierarchies., 2020, 30(5): 408–420.
[122] Figuet E, Nabholz B, Bonneau M, Mas Carrio E, Nadachowska-Brzyska K, Ellegren H, Galtier N. Life history traits, protein evolution, and the nearly neutral theory in amniotes., 2016, 33(6): 1517–1527.
[123] Axelsson E, Hultin-Rosenberg L, Brandström M, Zwahlén M, Clayton DF, Ellegren H. Natural selection in avian protein-coding genes expressed in brain., 2008, 17(12): 3008–3017.
[124] Drummond DA, Bloom JD, Adami C, Wilke CO, Arnold FH. Why highly expressed proteins evolve slowly., 2005, 102(40): 14338–14343.
[125] Levy Karin E, Wicke S, Pupko T, Mayrose I. An integrated model of phenotypic trait changes and site-specific sequence evolution., 2017, 66(6): 917–933.
[126] Campbell-Staton SC, Velotta JP, Winchell KM. Selection on adaptive and maladaptive gene expression plasticity during thermal adaptation to urban heat islands., 2021, 12(1): 6195.
[127] Ho WC, Zhang JZ. Evolutionary adaptations to new environments generally reverse plastic phenotypic changes., 2018, 9(1): 350.
[128] Ho WC, Li DY, Zhu Q, Zhang JZ. Phenotypic plasticity as a long-term memory easing readaptations to ancestral environments., 2020, 6(21): eaba3388.
[129] Velotta JP, Robertson CE, Schweizer RM, McClelland GB, Cheviron ZA. Adaptive shifts in gene regulation underlie a developmental delay in thermogenesis in high-altitude deer mice., 2020, 37(8): 2309–2321.
[130] Gibbons TC, Metzger DCH, Healy TM, Schulte PM. Gene expression plasticity in response to salinity acclimation in threespine stickleback ecotypes from different salinity habitats., 2017, 26(10): 2711–2725.
[131] Chen XM, Ji YZ, Cheng YL, Hao Y, Lei XH, Song G, Qu YH, Lei FM. Comparison between short-term stress and long-term adaptive responses reveal common paths to molecular adaptation., 2022, 25(3): 103899.
[132] Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice., 2012, 109(22): 8635–8640.
Genetic mechanism of adaptive evolution: the example of adaptation to high altitudes
Yan Hao1, Fumin Lei1,2,3
Since Darwinʼs time, elucidating the mechanism of adaptive evolution has been one of the most important scientific issues in evolutionary biology and ecology. Adaptive evolution usually means that species evolve special phenotypic traits to increase fitness under selective pressures. Phenotypic adaptation can be observed at different hierarchical levels of morphology, physiology, biochemistry, histology, and behavior. With the breakthroughs of molecular biology and next-generation sequencing technologies, mounting evidence has uncovered the genetic architecture driving adaptive complex phenotypes. Studying the molecular genetic mechanisms of evolutionary adaption will enable us to understand the forces shaping biodiversity and set up genotype-phenotype-environment interactions. Genetic bases of adaptive evolution have been explained by multiple hypotheses, including major-effect genes, supergenes, polygenicity, noncoding regions, repeated regions, and introgression. The strong selection pressure exerted by high-altitude extreme environments greatly promotes the occurrence of phenotypic and genetic adaptation in species. Studies on multi-omics data provide new insights into adaptive evolution. In this review, we systematically summarize the genetic mechanism of adaptive evolution, research progress in adaptation to high-altitude environmental conditions, and existing challenges and discuss the future perspectives, thereby providing guidance for researchers in this field.
phenotype; noncoding region; multi-omics; regulation; high altitudes
2022-04-13;
2022-06-25;
2022-07-19
国家自然科学基金项目(编号:32100332,3213000355),第二次青藏高原综合科学考察研究项目(编号:2019QZKK0304),青年人才托举工程项目(编号:2021QNRC001)和中国博士后科学基金(编号:2021M700144)资助[Supported by the National Natural Science Foundation of China (Nos. 32100332, 3213000355), the Second Tibetan Plateau Scientific Expedition and Research (STEP) Program (No. 2019QZKK0304), the Young Elite Scientists Sponsorship Program by CAST (No. 2021QNRC001), and the China Postdoctoral Science Foundation (No. 2021M700144)]
郝艳,博士,研究方向:鸟类适应性进化。E-mail: haoyan@ioz.ac.cn
雷富民,研究员,博士生导师,研究方向:鸟类学。E-mail: leifm@ioz.ac.cn
10.16288/j.yczz.22-108
(责任编委: 于黎)