Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases
Syed Aoun Mehmood Sherazi Asim Abbasi Abdullah Jamil Mohammad Uzair Ayesha Ikram Shanzay QamarAdediji Ayomide Olamide Muhammad Arshad Peter J.Fried Milos Ljubisavljevic Ran Wang Shahid Bashir0
Abstract Aging is linked to the deterioration of many physical and cognitive abilities and is the leading risk factor for Alzheimer’s disease.The growing aging population is a significant healthcare problem globally that researchers must investigate to better understand the underlying aging processes.Advances in microarrays and sequencing techniques have resulted in deeper analyses of diverse essential genomes(e.g., mouse, human, and rat) and their corresponding cell types, their organ-specific transcriptomes,and the tissue involved in aging.Traditional gene controllers such as DNA- and RNA-binding proteins significantly influence such programs, causing the need to sort out long non-coding RNAs, a new class of powerful gene regulatory elements.However, their functional significance in the aging process and senescence has yet to be investigated and identified.Several recent researchers have associated the initiation and development of senescence and aging in mammals with several well-reported and novel long non-coding RNAs.In this review article, we identified and analyzed the evolving functions of long non-coding RNAs in cellular processes, including cellular senescence, aging, and age-related pathogenesis, which are the major hallmarks of long non-coding RNAs in aging.
Key Words: aging; Alzheimer’s disease; DNA sequence; epigenetics; immune; non-coding RNA;oligonucleotides; telomere-associated
Introduction
Aging is a complicated and interrelated biological process in which cell, tissue,and organ performance steadily decline, eventually leading to senescence.Decreased body functioning augments aging-associated illnesses (Gems and Kern, 2022; Shibu et al., 2022).Changes in its nature rely on various factors such as alteration in tissue composition, which conclusively contributes to dysregulation of homeostasis, energy metabolism, and neurodegeneration(Faniyi et al., 2022; Preston et al., 2022).Noncoding RNAs have a direct momentous impact on the gene expression process in aging (Liu et al., 2022a;Ni et al., 2022; Shi et al., 2022).
Noncoding RNAs (ncRNAs) are ubiquitously transcribed by more than 75%of the genome (Della Bella et al., 2022).Up to 2% of protein-coding RNAs are transcribed in mammalian genomes, including humans, whereas the rest are classified as noncoding RNAs (ncRNAs).These ncRNAs were formerly known as transcriptional “noise” and were divided into two classes (Fellah et al., 2022), housekeeping and regulatory ncRNAs (Quek et al., 2015).Among noncoding RNAs, microRNAs (miRNAs) are well studied.In contrast to miRNAs, long non-coding RNAs (lncRNAs) are less known in terms of their role in aging development control (Quek et al., 2015; Caponnetto et al.,2022).LncRNAs are a 200-nucleotide-long class of non-protein-coding RNAs that play critical roles in chromatin advancement, transcription, and posttranscriptional modifications, among other biological processes (Sharma et al., 2022).At the translational level, miRNAs influence gene expression.MiRNAs are thought to be negative regulators of gene expression because they bind to the 3-untranslated region of the target messenger RNA (mRNA).A single miRNA can control many target genes at the same time, either within a single route or across different pathways, because it can target multiple mRNAs.However, lncRNAs regulate target gene expressions by interacting with critical target proteins via their higher-order structures.Most lncRNAs are transcribed from promoters with a low proportion of CpG dinucleotides(Ratti et al., 2020).A wide range of expressed lncRNAs and their impacts on protein expression programs have only recently been discovered.LncRNAs influence gene expression trends at all stages of the process: posttranslation,posttranscription, and transcription (Luo et al., 2019; Iaccarino et al., 2022;Wu et al., 2022).They are arbitrarily categorized into context, antisense,telomere-associated, circular, promoter- and enhancer-associated, bidirectional, telomere-associated, pseudogene-associated, circular, and repeatrich lncRNAs, which differ in their chromosomal locations, orientations, and transcription methods (Morris et al., 2019; Rogers et al., 2021).
LncRNAs function as numerous regulators in various ways.(1) As chromatin modifiers, lncRNAs conscript various histone and DNA methyltransferases to inactivate the area of chromosome inactivation (e.g., lncRNA-p21, HOTAIR,and XIST) (Maulik et al., 2021; Okechukwu, 2021).(2) They act as “decoys”or “guides,” directing various transcriptional repressors/activators to attach to regulatory DNA elements.Examples include growth arrest-specific 5 and AIR (Wang et al., 2021b; Zhang et al., 2022).LncRNAs have strange functions,including inactivation of the X chromosome (Heskett et al., 2021).They also perform transcription in several ways, such as antisense, bidirectional, and overlapping transcriptions, to communicate with neighboring genes (Li et al., 2021b).Proliferation, senescence, differentiation, quiescence, cellular response to stress and immune agents, and several other cellular processes substantial to the biology of aging are all influenced by lncRNAs.
Database Search Strategy
PubMed, Web of Science, Google Scholar, and ProQuest were searched to retrieve papers.During searching, no filters were applied such as restricting the date, subjects, or publication type.All publications reviewed were in the English language.Literature retrieval was performed using all possible ways including the reference lists and authors’ files from the included studies.“RNA, long non-coding”, “aging”, “disease”, “Alzheimer’s disease”, “telomere”,“telomere stability”, and “neurodegenerative diseases” keywords were medical subject heading (MeSH) used in the search strategy.The last search was conducted on August 28, 2021.
Long Non-Coding RNAs and the Molecular Hallmarks of Aging
Aging is a process of physiological degeneration and, as a result, decreased function, which is related to senescence at the level of both the cells and organs.Specific changes in the reservoirs of expressed proteins control the phenotypic changes that characterize the aging process.DNA damage,chromosomal destabilization, telomere attrition, oncogene activation,mitochondrial dysfunction, as well as cell cycle-related stressors are all believed to trigger senescence in untransformed cells (Pignolo et al., 2021).As changes in gene expression drive senescence leading to aging, lncRNAs play an essential part in the aging process by controlling DNA expression at all levels (Zaniani et al., 2021).All non-coding RNAs are more specific for specific tissue types than protein-coding RNAs, highlighting their role in regulatingtissue identity and function (de Goede et al., 2021).This section describes the known functions of lncRNAs that affect the aging process through mediating various processes (Figure 1).The communication of cells, which these lncRNAs modulate, can be either intracellular communication or intercellular communication.
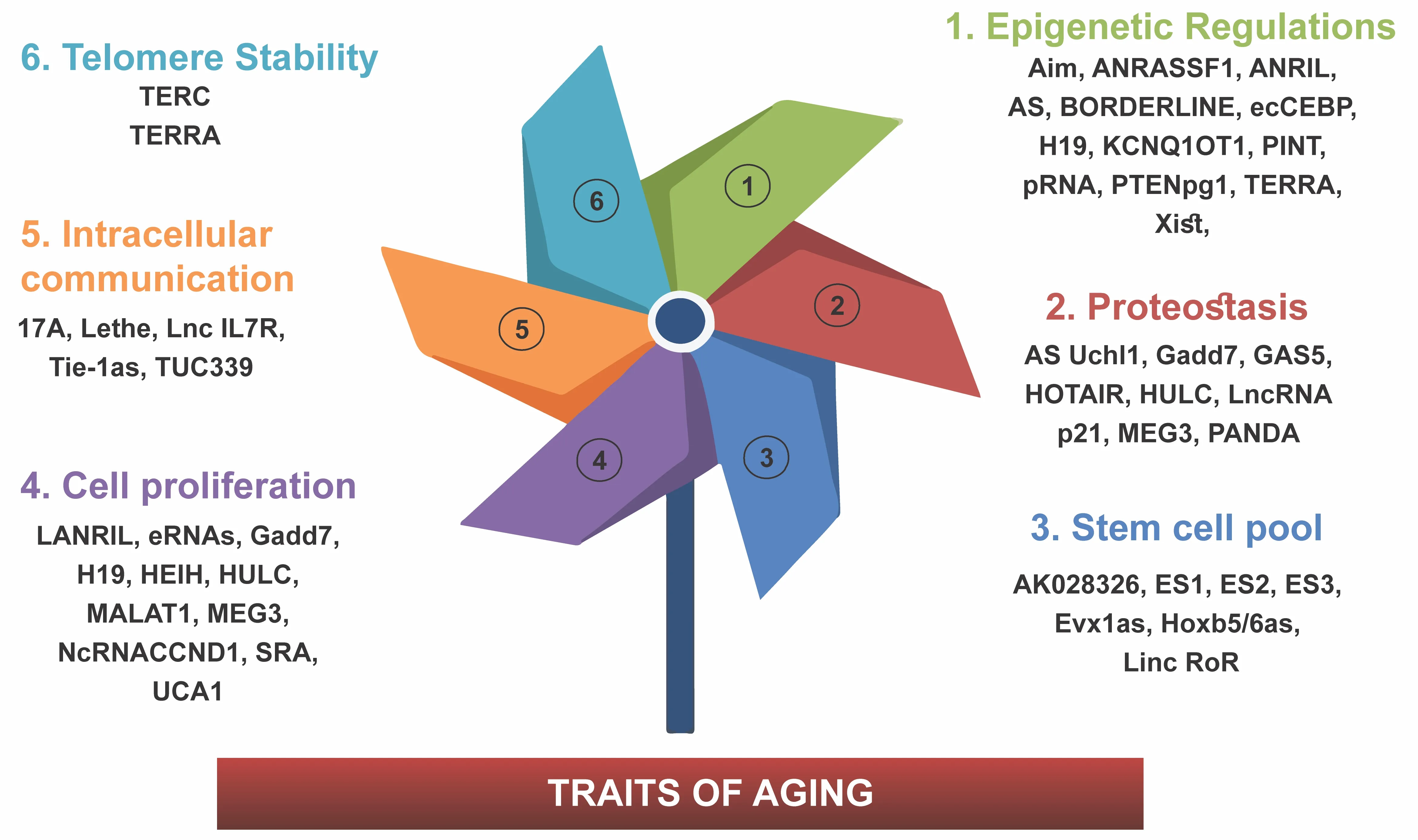
Figure 1|The schematic figure summarizes various lncRNAs in the aging process.
LncRNA expression patterns and conservation functions during aging
The characteristics of lncRNAs in various organisms include poor conservation of DNA sequences, low expression patterns, and high temporal and spatial expression precision (Cornelis et al., 2016), which have been demonstrated in the brains of humans and macaques (Leypold and Speicher, 2021).It is interesting that compiled and novel lncRNAs, along with their low or nondetectable expression levels in the brain, are substantially more preserved than annotated or novel lncRNAs with high or nondetectable expression levels in the brain (Carrasco-León, 2021; Rufino-Ramos et al., 2022).
In lncRNAs, highly complex spatial and temporal speech patterns can be found.As a result, lncRNAs may regulate various cell types and behaviors,including gene expression differentiation and development control.Previous papers, for instance, included an analysis of 409 lncRNA expression patterns and categorized them into eight key coding sequences (D’haene and Vergult,2021).The expected abundantly presented and proliferation-associated lncRNAs, such as Gomafu and metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), were enhanced with the aging-associated decline in neurogenesis, which suggests that these lncRNAs are insensitive to mitogenic or environmental stimuli, or the stimuli themselves are decreased or not conveyed accurately during aging (Butler II, 2019).
Intracellular communication regulation
Cells interact in various ways and exchange different types of messages and information is called intercellular communication.These methods of communication entail a variety of mechanisms involved separately or concurrently that vary depending on the physiological or pathological state of cells (Liu et al., 2022b).During aging, the intercellular communication between cells is altered, leading to differences in communication between organs and body systems, including the immune system.Endocrine, neuroendocrine,synaptic, and inflammatory signaling are all affected at the intercellular stage during aging.This can be observed in neurohormonal alterations,including renin-angiotensin system deregulation or the insulin–insulin growth factor responses.With age, inflammatory reactions increase, while immunosurveillance against pathogens and premalignant cells decreases,resulting in opportunistic infections and malignant cell development.Immunosenescence, or changes in the immune system, and increased cytokine production by adipose tissue cells are the two main reasons for chronic inflammation in older people.Some age-dependent diseases, such as cardiovascular diseases, arthritis, and metabolic diseases such as diabetes,are only a few examples caused by inflammation and immunosenescence.These disease conditions are one of the significant reasons for body weakness, morbidity, and mortality among the elderly (Barbé-Tuana et al.,2020).LncRNAs are one of the components of intracellular communication(Li et al., 2010, 2014, 2018; Cui et al., 2014; Zgheib et al., 2017; Wei et al.,2018).Intracellular communication, occurring within a cell, is accomplished by molecular language and involves an anterograde and retrograde communication network between the nucleus and the organelles, allowing for this interactive information exchange necessary for cellular hemostasis (Barral et al., 2022).The subsections describe various points within the cells where lncRNAs exert control and regulate the aging process.
Telomerase stability
The linear physical ends of chromosomes are unavoidably eroded with each cell division (Jacome Burbano and Gilson, 2021), which occurs because of differences in replication between the leading and lagging strands of the same DNA template.DNA polymerase II is the main enzyme involved in making copies of a DNA template.As polymerase II completes copying the leading strand during replication, it fails to finish the lagging DNA strand of the same template.All cells with linear chromosomes, such as mammalian cells, experience this inevitable erosion (Aibara et al., 2021).After each round of replication, 50–200 base pairs are lost, creating an uncovered singlestranded fragment of DNA at the end of the molecule (Xue et al., 2022).This depletion leads to the aging process by limiting the number of cell divisions available.The number of divisions in each cell can have Hayflick’s limit (Chan et al., 2022).To avoid premature shortening of chromosomes,telomeres protect the linear ends of chromosomes.Telomeres are defensive nucleoprotein structures that contain double-stranded 5′-TTAGGG-3′ repeats that terminate in the 3′ protrusion of single-stranded G-rich overhangs at the end of chromosomes (Mamaev and Zvyagilskaya, 2021).As a result, telomeres maintain the stability of the genome at chromosome ends (Bonnell et al.,2021).
Telomeres transcribe lncRNAs from subtelomeric sequences called telomeric repeat-containing RNA (TERRA).These lncRNA molecules are characterized by 5′-(UUAGGG)-3 repeats at the 3′ and are involved in maintaining and regulating telomere homeostasis (Gala and Khattar, 2021) by controlling the overexpansion of the telomeric length.The TERRA suboptimal expression has been reported to cause constitutive heterochromatin loss (Sengupta and Sengupta, 2022), shortening of G-rich overhangs, formation of multiple telomeric ends, and even complete loss of telomeres (Abraham Punnoose et al., 2018; Markova et al., 2020).On the other hand, the telomerase RNA component, another lncRNA transcribed by telomeres, elongates telomeres,as telomeres shortening has been associated with instability of chromosomes and initiation of premature aging and decreased life span in mice (Table 1;Rossi and Gorospe, 2020).
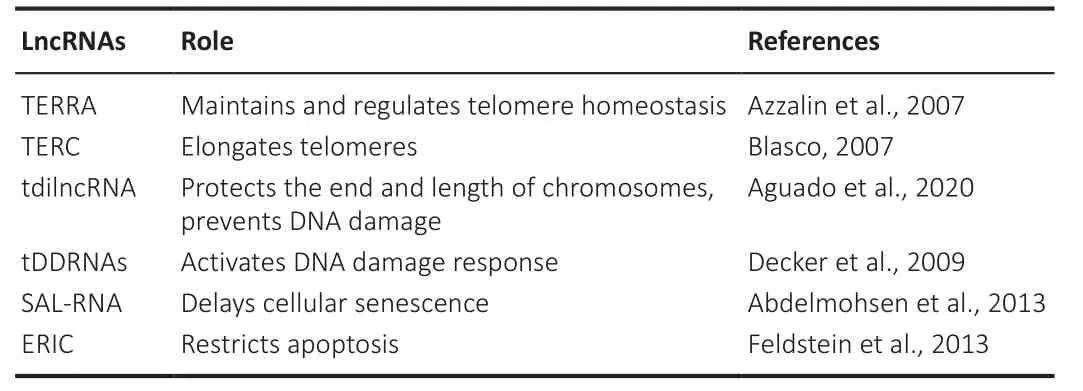
Table 1 | Roles of different types of lncRNAs in telomerase and genomic stability
Genomic instability
Genomic instability causes destructive changes in the integrity of neuronal and cellular functions, contributing to the aging process (Madabhushi et al., 2014).Increases in DNA lesions or double-stranded breaks (DSBs) and decreases in DNA repair cause a subsequent increase in genomic instability and chromosomal rearrangements.Therefore, DSBs also contribute to the aging process.The binding of RNA with DNA break points causes chromosomal rearrangement (Wang et al., 2020c).Recent evidence suggests that specific lncRNAs transcribed at telomeres, such as TERRA and telomeric damage-induced long ncRNAs (tdilncRNA), play important roles in age-related pathways by orchestrating the factors that govern telomere chromosome end protection, length, and DNA damage signaling (Aguado et al., 2020).However,lncRNA-assisted genomic instability in the aging process has been reported elusively (Table 1).LncRNAs accumulate at DSBs and form DNA-RNA hybrids,creating an R-loop structure.R-loops negatively affect genomic stability by interfering with DNA replication, transcription, and translation (Petermann et al., 2022).Moreover, age-related DSBs have been reported near telomeric genes.R-loops tend to initiate homologous rearrangement, increasing telomeric RNA-DNA hybrids and delaying cellular senescence.This might be due to the recombination-mediated telomere elongation events promoted by telomeric RNA-DNA hybrids (Brenner and Nandakumar, 2022).However,in the absence of telomerase and homologous recombination, telomeric RNA-DNA hybrids accumulate, resulting in cellular senescence.TERRA forms telomeric RNA-DNA hybrids, which regulate telomere length dynamics(Petti et al., 2019).The loss of telomeric heterochromatin upregulates TERRA and activates the DNA damage response, which could lead to cellular senescence (Aguado et al., 2020).TERRA is also reported to be upregulated in a premature aging syndrome, including immunodeficiency, centromeric instability, facial anomalies syndrome type I, and Hitchinson-Gilford progeria syndrome (Aguado et al., 2019).In Hitchinson-Gilford progeria syndrome, acondition marked by accelerated telomere shortening and premature cellular senescence, other lncRNAs, including telomeric damage-induced lncRNAs(tdilncRNAs) and telomeric DNA damage response RNAs, was reported to be upregulated (Decker et al., 2009).TdilncRNAs and telomeric DNA damage response RNAs are transcribed from deprotected telomeres or telomeres carrying damaged DNA (Aguado et al., 2020).As lncRNAs play a critical role in senescence, senescence-associated lncRNAs (SAL-RNAs) delay cellular senescence (Abdelmohsen et al., 2013).SAL-RNA regulates phosphatase and tensin homolog (PTEN)-induced putative protein kinase 1 (PINK1)-mediated senescence by regulating sirtuin 1 (SIRT1) (Jiang et al., 2021).Moreover,SAL-RNA increases and activates SIRT1, consequently deregulating alveolar epithelial cell type II senescence induced by cigarette smoke-medium suspension (Devadoss et al., 2019).The E2F family of transcription factors regulates gene expression during the cell cycle.E2Fs also regulate DNA repair,cellular differentiation, development, apoptosis, and autophagy.E2F1, a family member of the E2F gene family, upregulates the lncRNA expression of the E2F1-regulated inhibitor of cell death (ERIC) induced by DNA damage.Inhibition of ERIC expression induces an increase in apoptotic rate (Emanuele et al., 2020).
Epigenetic regulation
As the study of organisms that have undergone alterations in gene expression rather than alterations in genetic coding, epigenetics uses molecular markers to influence gene expression (Tsaballa et al., 2021).Histone modification,DNA methylation, and noncoding ribonucleic acid regulation constitute well-known epigenetic factors.Taken together, these factors establish the chromatin architecture, genetic loci accessibility to transcriptional machinery,and gene expression levels (Cavalcante et al., 2020).Human interaction with the environment and lifestyle affects the epigenome of some genes and influences their expression, affecting susceptibility to various diseases and aging (Reiner and Karzbrun, 2020).Several studies have shown a crucial role of lncRNAs in the epigenetics of aging (Table 2; Du et al., 2004; Barsyte-Lovejoy et al., 2006; Chaumeil et al., 2006; Schmitz et al., 2010; Kotake et al.,2011; Di Ruscio et al., 2013; Johnsson et al., 2013; Keller et al., 2013; Marín-Béjar et al., 2013; Travers et al., 2013; Wan et al., 2013; Xu et al., 2014).
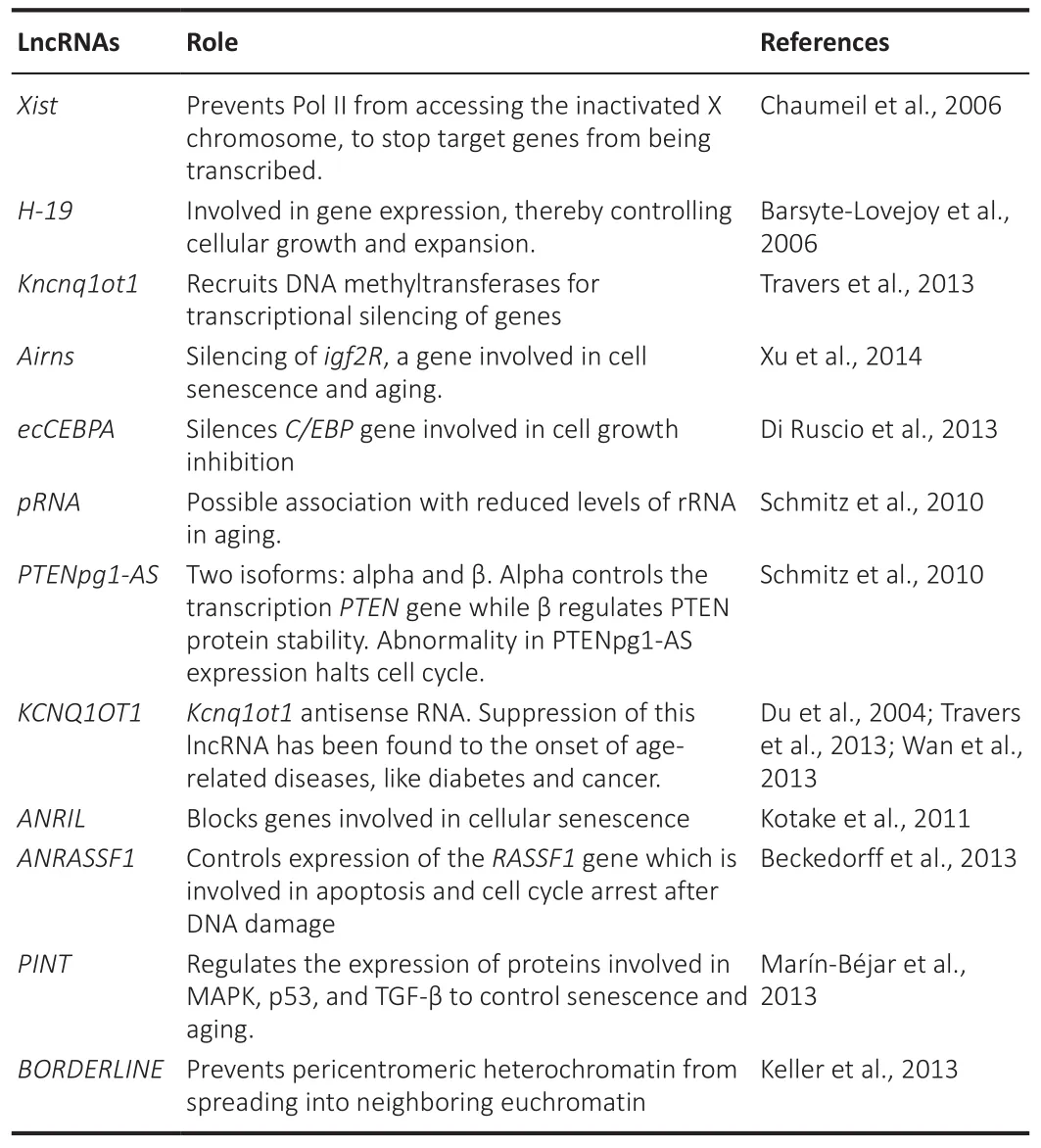
Table 2 |Roles of different types of lncRNAs in epigenetic control
Proteostasis
The term proteostasis is a combination of protein and homeostasis.The complex control of a healthy and functioning proteome is known as proteostasis.The proteostasis network, which is composed of competing and interconnected biological pathways inside cells, regulates protein biosynthesis, folding, transport, and depletion both inside and outside a cell (Rahman, 2021).Despite the extraordinary robustness and flexibility of proteostasis networks, if stress factors are persistent, the proteostasis equilibrium becomes difficult to sustain, and proteotoxicity occurs.Several lines of evidence indicate a strong connection between proteostasis and healthy aging (Dick et al., 2021).With aging, most organisms experience a slow loss of proteostasis.The stability of the proteasome affects the longevity of species, as the longest-living organisms maintain robust proteomes(Witkowski et al., 2021).By controlling the expression of proteins involved in aging, lncRNAs play a significant role in proteostasis and aging (Table 3; Zhang et al., 2003; Ropolo et al., 2009; Hung et al., 2011; Ummanni et al., 2011; Liu et al., 2012, 2015; Zhou et al., 2017).
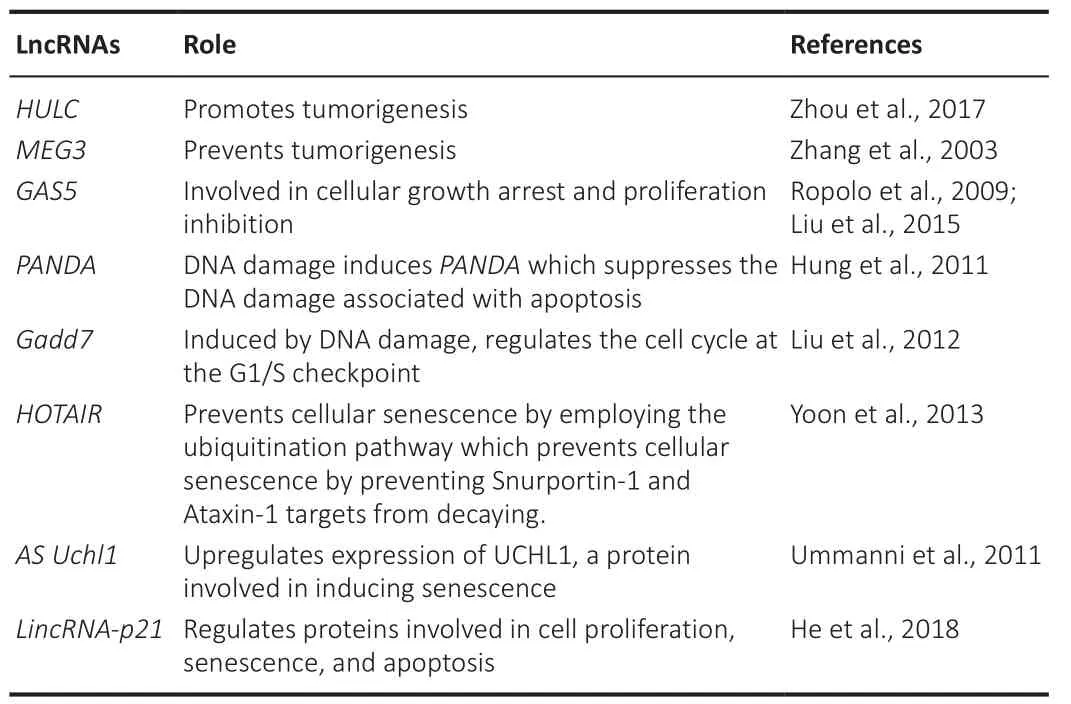
Table 3 | Role of lncRNAs in proteostasis
Stem cell differentiation
Over time, the process of organismal aging is characterized by functional deterioration due to histological and biochemical alterations in tissues and organ systems.Deteriorating functionality reduces the ability to react to damage or stress.Stem cells play a role in tissue homeostasis, regeneration,and reconstruction (Mannino et al., 2022).Adult stem cells are specialized cells that are used to replace tissue throughout an organism’s lifetime.In mosttissues, stem cells are often in a dormant state, but in response to external signals, they can be coaxed back into the cell cycle.The differentiation of stem cells into progenitors and effector cells repairs and replaces old body cells and tissues.Progenitors are comparatively undifferentiated cell forms from asymmetric stem cell differentiation that cannot self-renew once stem cells divide.Through subsequent rounds of proliferation, progenitors divide them into differentiated effector cells.According to several studies, lncRNAs control stem cell self-renewal and multilineage differentiation (Dinger et al., 2008;Ng et al., 2012; Cao et al., 2016; Feng et al., 2018).Table 4 describes lncRNAs and their roles in stem cell activities that affect the aging process.

Table 4 |Role of lncRNAs in stem cell activity
Cell proliferation
Cell proliferation is the mechanism of a cell growing and dividing into two daughter cells.Controlling cell proliferation is critical for tissue formation during growth and regeneration (Gupte et al., 2018).Cell proliferation slows as people get older.The precise cause of this occurrence is unknown.However, processes beneficial to the young, such as increasing reproductive capacity, can lead to later disease development.Pathways that stimulate cell growth and proliferation, for example, can play a role in cancer.Therefore, it is likely that cell proliferation decreases with increasing age, either because the processes in the young cause damage or because the activities that are advantageous to the young are detrimental to the old (Courchesne et al.,2019).LncRNAs play a significant role in regulating cell proliferation in various stages, thereby controlling the fate of growing cells.Table 5 summarizes lncRNAs involved in cellular proliferation (Wang et al., 2008; Xu et al., 2010;Yap et al., 2010; Yang et al., 2011; Du et al., 2012; Melo et al., 2013; Tripathi et al., 2013; Deng et al., 2016; Dan et al., 2018; Zhao et al., 2018; Ghafouri-Fard and Taheri, 2019).
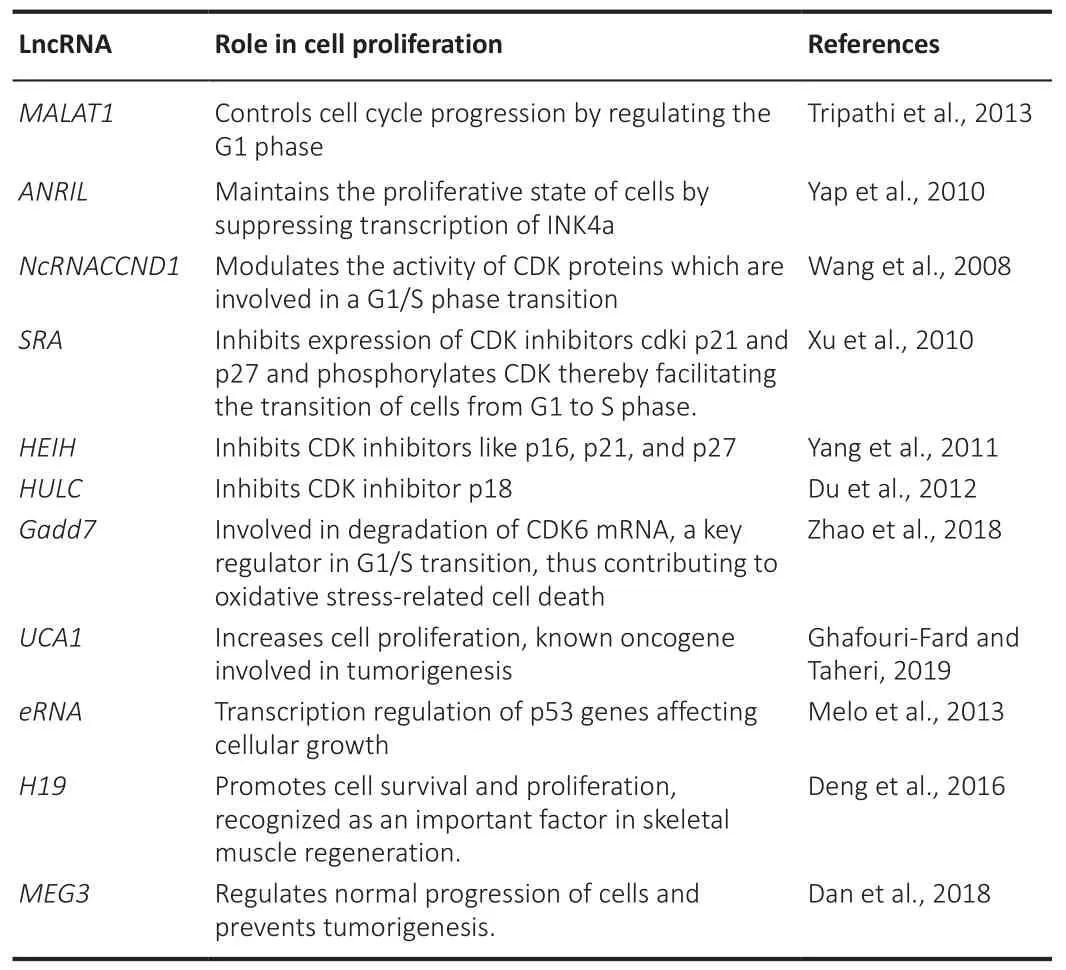
Table 5 |Role of lncRNAs in cellular proliferation
Effect of Long Non-Coding RNAs in Aging-Associated Diseases
LncRNAs play a significant role in several molecular processes associated with aging.As aging leads to several diseases, including metabolic,neurodegenerative, muscular, and immunity bases, lncRNA plays a significant role in these diseases by disturbing or altering several pathways (Table 6;Mishra and Kumar, 2021).

Table 6| LncRNAs and their role in intercellular communication
LncRNAs affecting aging-associated diabetes
LncRNAs play significant functions in almost the entire process, from pancreas development to β-cell functioning and insulin production (Table 7).Insulin exocytosis has been reported to correlate directly with HI-LNC901 (Guček et al., 2019).The development of the pancreas and the functioning of β-cells have been linked to the involvement of PLUTO (PDX1-associated LncRNA),as it regulates the transcriptional activity of PDX1 (pancreatic and duodenal homeobox 1) (Akerman et al., 2017).Modulation of β-cell formation and function have been linked to the β-cell long intergenic non-coding RNA, βlinc1(β-cell long intergenic noncoding RNA 1) (Arnes et al., 2016).Moreover,elevated levels of Kcnq1ot1, HI-LNC78, and HI-LNC80 and low levels of HILNC45 observed in pancreatic islets of diabetic individuals or the presence of high glucose levels suggests their blood glucose level-sensing potential (Morán et al., 2012).Furthermore, lncRNA ANRIL (antisense noncoding RNA at the INK4 locus) associates with polycomb repressive complexes 1 and 2 to prevent the transcription of the tumor suppressors and inducers of senescence p15INK4 and p16INK4A (Latres et al., 2000; Pasmant et al., 2007; Kotake et al., 2011; Hannou et al., 2015).Genome-wide association studies have also recognized ANRIL as a primary source of mutations in type 2 diabetes (Pasmant et al., 2011; Hannou et al., 2015).ANRIL may also contribute to maintaining the capacity of proliferation of β-cells with age.
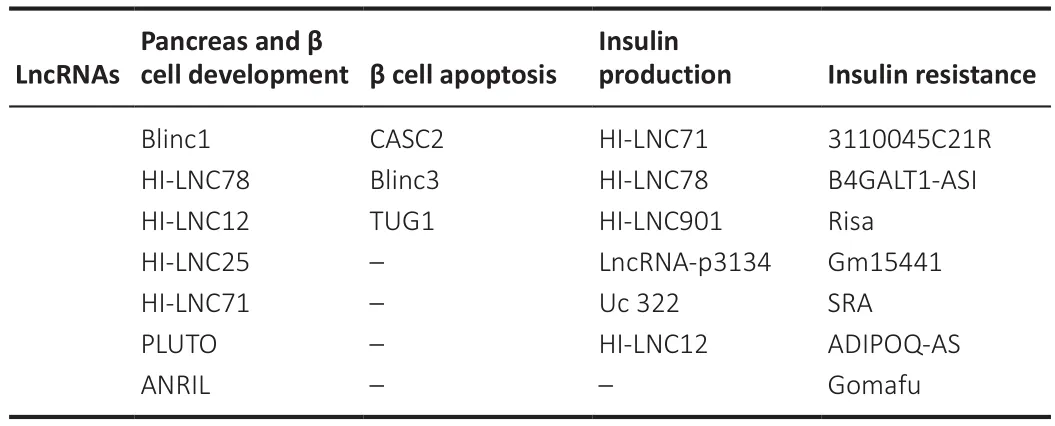
Table 7 |Long non-coding RNA (lncRNA) involved in diabetes complications, most especially in T2D, and their area of function (Li et al., 2010)
LncRNAs affecting aging-associated neurodegenerative diseases
Aging is linked to physical deterioration, resulting in an increased risk of disease and death (Rose, 2009; Ismail et al., 2021).Neurodegenerative diseases are a specific class that is strongly associated with aging.Alzheimer’s disease (AD) and Parkinson’s disease, the two highly prevalent neurodegenerative disorders, often occur in the elderly, and the incidence rates of such diseases increase with age.Molecular research has also shown that the brain tissue of the elderly showed irregular deposits of accumulated proteins such as hyperphosphorylated tau (p-tau), amyloid (A), and synuclein(Elobeid et al., 2016).
Long noncoding RNAs play a significant role in age-related neurodegenerative diseases.BACE1-antisense transcript (BACE1-AS) has a high level of reciprocity with BACE1 mRNA; it improves the stability of BACE1 mRNA by shielding this mRNA from miR-485-5p-mediated degradation (Faghihi et al., 2008,2010).The expression levels of the BACE1 protein activity increase with the aging of the brain and AD.Elevated BACE1 levels in AD can be correlated with enhanced BACE1-AS abundance, insinuating which BACE1-AS could account for an essential function in the development or progression of AD(Fukumoto et al., 2002; Faghihi et al., 2008; Modarresi et al., 2011).Knocking down BACE1 and BACE1-AS in AD mouse models decreases BACE1 protein levels and insoluble amyloid-beta (Aβ) peptides (Modarresi et al., 2011).LncRNA-17A is another biologically important RNA in AD that is linked to a malfunction in GABABR2 (aminobutyric acid B receptor 2) alternative splicing.When lncRNA-17A is overexpressed, GABABR2 variant A is usually repressed,while improving the expression of variant B and favoring the aggregation of peptides Aβ42 and Aβ40, which are obtained from the breakdown of the amyloid precursor protein (APP) and highly implicated in AD pathogenesis.These observations suggest that lncRNA-17A may play a vital role in GABA signaling and Aβ production (Massone et al., 2011).BCYRN1 levels were found to be reduced by > 60% in areas of the cortex of patients with AD between the ages of 49 and 86 years.However, compared with those in age-matched normal brains, BCYRN1 levels were enhanced in brains with AD (Mus et al.,2007)
Moreover, age-dependent changes in linc00507 expression patterns have also been reported in the cortex.LINC00507 is specifically expressed in the primate cortex and has age-dependent expression patterns.Linc00507 also showed high expression levels in patients with AD, negatively regulating miR-181c-5p by binding between linc00507 and miR-181c-5p.In turn, linc00507 downregulates the targets of miR-181c-5p, namely MAPK and TTBK1, by directly sponging miR-181c-5p as competing endogenous RNA (ceRNA).Linc00507 also induces tau phosphorylation by activating GSK3β in AD (Yan et al., 2020; Ni et al., 2022).
Brain cytoplasmic (BC) RNA causes the aging of the brain and AD.LncRNA transcripts such as mouse BC1 RNA and human BC200 RNA are carried to dendritic processes as ribonucleoprotein particles and bind to poly (A)-binding protein (PABP1), a translation initiation regulator, influencing gene expressions at the translational level (Muddashetty et al., 2002).BC200 can bind to RNA-binding proteins associated with aberrant mRNA transport,causing abnormal protein localization.BC200 overexpression can cause synaptic and dendritic degeneration in AD in the aging brain.BC1 interacts with a delicate X syndrome protein to trigger APP mRNA translation, according to a study (FMRP).In AD mice, the inhibition of the expression of BC1 or BC1-FMRP reduces its accumulation in the brain and increases spatial learning and memory (Zhang et al., 2018).
The lncRNA expression of GOMAFU was suppressed when mice cortical neurons and neurons produced from human-induced pluripotent stem cells were depolarized with KCl.The interactions of GOMAFU with Quaking gene(QKI) and serine- and arginine-rich splicing factor 1 (SRSF1) attenuate the alternative splicing of Erb-B2 receptor tyrosine kinase 4 (ERBB4) pre-mRNAsand disrupted-in-schizophrenia 1 (DISC1), implying that potassium-activated ion channels develop splicing factors accessible for alternative splicing by downregulating GOMAFU expression (Barry et al., 2014).Modification of the alternative splicing of genes linked to aging-associated neurological illnesses,including DISC1, of which some splice variants have been linked to age-related recurrent severe depression, could explain these findings (Thomson et al.,2013; Kimbro et al., 2014).
Differential expression of numerous lncRNAs, including BC089918, was caused by sciatic nerve damage.BC089918 is frequently downregulated after an injury, and its depletion increases dorsal root ganglion neuron proliferation.Its role in the repair of neurons after sciatic nerve injury could have implications for nerve injury and recovery in the elderly (Yu et al., 2013; Ni et al., 2022).
LncRNA rhabdomyosarcoma 2-associated transcript (RMST) is brain-specific and required for neurogenesis.It is transcriptionally suppressed by the neuronal transcription factor repressor element-1 silencing transcription factor.RMST established a complex with the transcription factor SRYBox transcription factor 2 (SOX2) in neural cells, which allowed SOX2 to be recruited to specific DNA locations to carry out a neurogenic program.RMST is also linked to hnRNP A2/B1, a protein whose dysfunction is linked to neurodegeneration (Kim et al., 2013; Ng et al., 2013).These roles are summarized in Tables 8 and 9 (Wang et al., 2018).
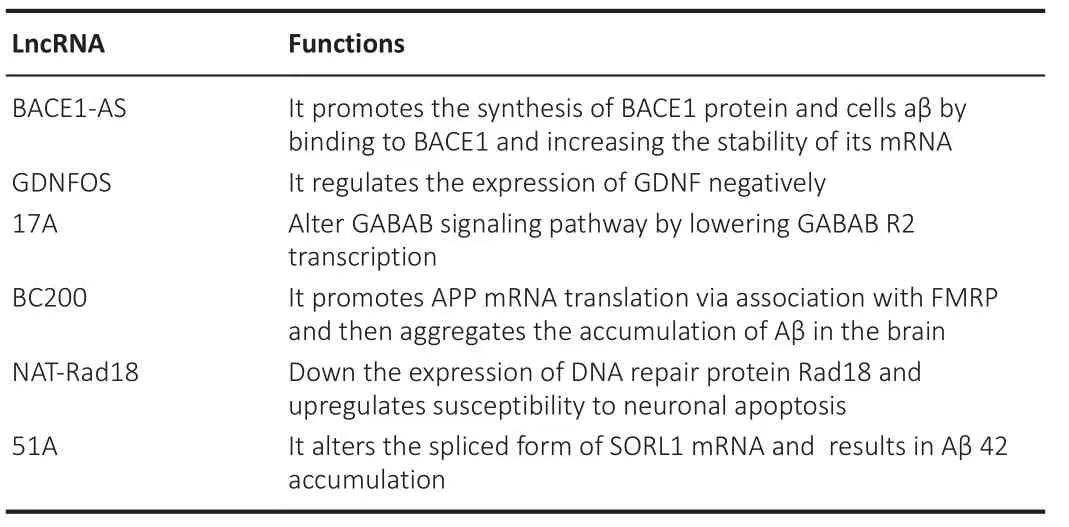
Table 8 | Long non-coding RNAs involved in Alzheimer’s disease and their functions
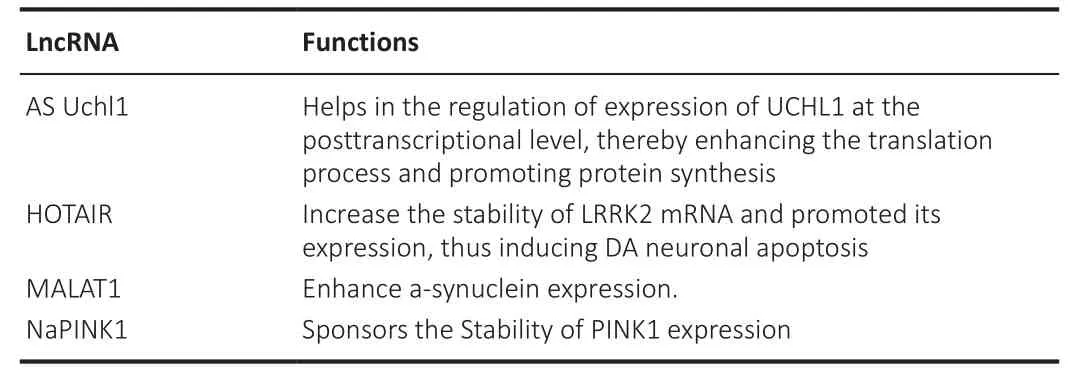
Table 9 | Long non-coding RNAs involved in Parkinson’s disease and their functions
Numerous lncRNAs have been shown to be expressed differently in neurodegenerative diseases, including AD, and most of them are closely associated with pathophysiological metabolic pathways; consequently,lncRNAs could be used as prominent therapeutic targets for improving the prognoses of neurodegenerative disorders.
LncRNAs affecting age-associated muscle pathology
Mature individuals usually develop muscular dysfunctions, which ultimately cause age-linked weakness and sarcopenia (Saini, 2019).Many lncRNAs related to age-linked muscle dysfunctions are evolving.The lncRNA H19 is involved in cell differentiation (Yang et al., 2018), expressed from the maternal allele, and is extremely rich in embryonic tissues (Andergassen et al., 2017).In contrast to many tissues that involve robust suppression of H19 levels after birth and in aged people, skeletal muscles uphold high H19 levels,signifying the crucial role of H19 in skeletal muscle function (Kader, 2015).H19 and its encoded miRNAs, miR-675-3p and miR-675-5p, are overexpressed throughout thein vitro
differentiation of myoblasts andin vivo
regeneration of muscles in humans and mice and primary muscle cells.Cultured myoblasts of the mouse cellosaurus cell line (C2C12) are induced to differentiate by H19.Furthermore, miR-675-3p and miR-675-5p support the role of H19 in muscle differentiation and regeneration by maintaining the bone morphogenetic protein pathway, which includes the transcription factors SMAD1 and SMAD5,and the DNA replication initiation component CDC6 (cell division cycle 6)(Dey et al., 2014a).Likewise, H19 can work as a molecular sponge; it has the potential to bind to let-7 and limit its accessibility.The diminished H19 levels reported in the muscle cells of patients with diabetes in response to elevated insulin levels increase let-7 expression levels (Tian et al., 2018).Therefore, H19 can have a crucial function in skeletal muscle regeneration,a procedure that is compromised in elderly individuals (Scimè et al., 2010).The muscle-definite linc-MD1 exhibits a snare action for miR-133, restraining its suppressive influence on Elavl1 (ELAV-like RNA binding protein 1) mRNA expression levels in mouse myoblasts.A feedstuff-frontward regulating mechanism was proposed; HuR improved the linc-MD1 sponge function by allowing it to use miR-133 and miR-135, thereby controlling differentiation.HuR is expressed in differentiated muscles at decreased levels and plays a crucial role in muscle degeneration and sarcopenia.As a result, linc-MD1 works in muscle regeneration throughout the weakening process via HuR.SIRT1 AS (SIRT1 antisense RNA) is a natural antisense lncRNA that has recently been linked to myogenesis (Zhu et al., 2017).Sirt1 mRNA and Sirt1 AS lncRNA levels decreased steadily during C2C12 myogenic development.Sirt1 AS overexpression increased NAD-dependent deacetylase SIRT1 (sirtuin 1) levels.Sirt1 AS contradicted the negative regulation of SIRT1 by miR-34a in C2C12 cells.SIRT1 assists in the prevention of aging by maintaining a strong myogenic program, and Sirt1 AS is linked to muscle aging.MALAT1 levels increase during muscle cell development in mice and humans and silencing MALAT1 decreases myoblast and endothelial cell proliferation.Malat1 levels decreased critically after the treatment of mouse gastrocnemius muscle with recombinant myostatin (Watts et al., 2013).These roles are summarized in Table 10 (Pardo and Boriek, 2011; Lu et al., 2013; Schirwis et al., 2013; Watts et al., 2013; Dey et al., 2014b; Wang et al., 2014b).
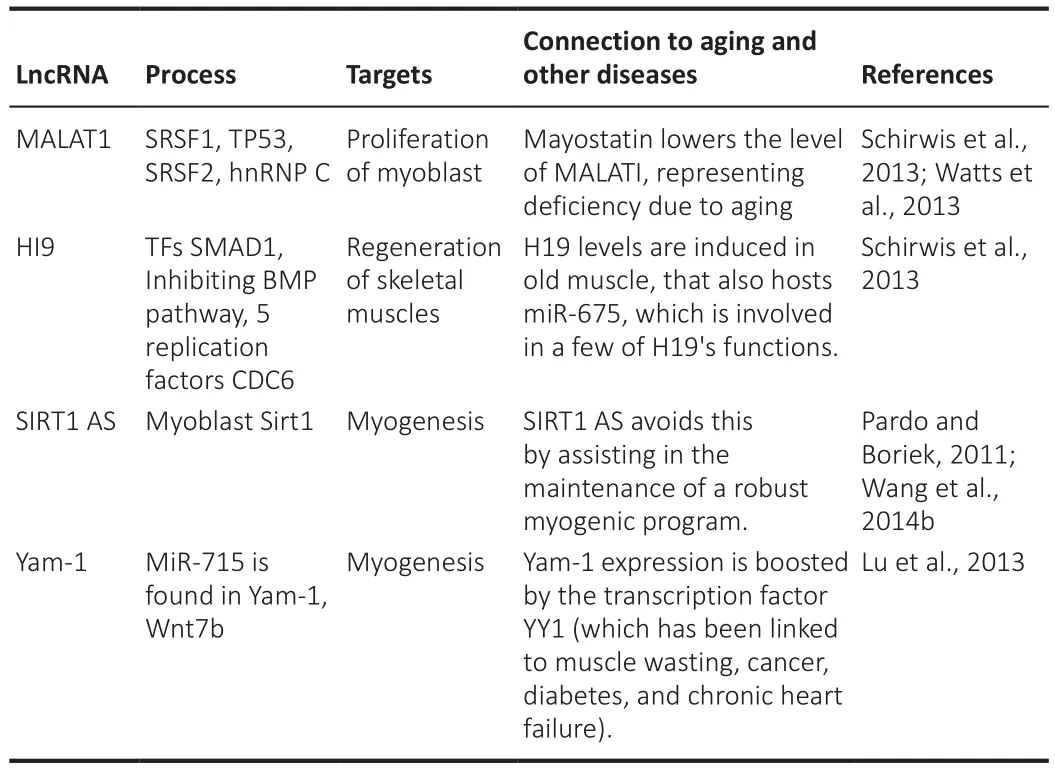
Table 10 |LncRNA in aging-associated muscle pathology
LncRNAs affecting aging-associated immune dysfunction
Abnormal immunological responses are a predictor of aging and age-related diseases and are connected to the continuous release of proinflammatory cytokines through senescent cells.LncRNAs influence cytokine production and regulate the subcellular localization of the transcription factors involved in cellular defense pathways in response to pathogenic conditions and viral infections (Table 11; Wang et al., 2020e).The influence of lncRNAs on the immune reaction seems to be evolutionarily conserved through species (Chen et al., 2017).Long intergenic noncoding RNA cyclooxygenase 2 (lincRNA-Cox2)and the Toll-like receptor (TLR2/TLR1) agonist Pam3CSK4 were overexpressed in bone marrow-derived macrophages after treatment with lipopolysaccharide and required the transcription factor NFκB (nuclear factor-kappa B) and the TLR adaptor myeloid differentiation primary response 88 (MYD88).LincRNACox2 particularly inhibits immune gene transcription by interacting with the RNA-binding proteins heterogeneous nuclear ribonucleoproteins (hnRNP A/B and hnRNP A2/B1), despite improving interleukin 6 (IL6) expression via TLRs(Atianand and Fitzgerald, 2014).These findings suggest that lincRNA-COX2 both activates and suppresses immune response genes in macrophages and promotes the actions of NFB, a key player in aging and age-related diseases.The lncRNA PACER (p50-associated COX-2 extragenic RNA) increases COX2 expression, connects to the suppressive subunit of NFκB, p53, and restricts its link with the COX2 gene promoter, allowing transcriptional upregulation of COX2 expression by NFκB (Khyzha et al., 2017).Tumor necrosis factor(TNF), a proinflammatory cytokine linked to inflammation, aging, cellular senescence, and age-related diseases, promotes the regulation of various lncRNAs, including Lethe, a pseudogene-encoded lncRNA transcribed by NFκB.Lethe deficiency produces a negative regulation of NFκB inhibitor alpha(NFκBIA) and nuclear factor kappa B subunit 2 (NFκB2), indicating that Lethe likely operates as an NFB suppressor.As a result, the ectopic expression of Lethe suppressed the stimulation of NFκB objectives such as IL6, superoxidedismutase 2 (SOD2), interleukin 8 (IL8), and Nfkbia, supporting the theory that Lethe contributes to the negative response regulation of NFκB objective genes controlled by TNF to avoid an immune response.TNF- and HNRNPL-related immunoregulatory long noncoding RNA (THRIL) was weakened after TNF management (Simion et al., 2019).These findings support the involvement of lncRNAs in complexes regulated by NFκB and TNF, two key regulators of aging,age-related diseases, and immunological responses (Kulski, 2019).THRIL then stimulates TNF transcription via its promoter by engaging with its binding partner heterogeneous nuclear ribonucleoprotein L (hnRNP L) (Bureau et al.,1993; Clark and Peterson, 1994; Bihl et al., 1999; Baune et al., 2008; Tilstra et al., 2011; Carpenter et al., 2013; Kida et al., 2013; Rapicavoli et al., 2013; Gray et al., 2014; Wang et al., 2014a).
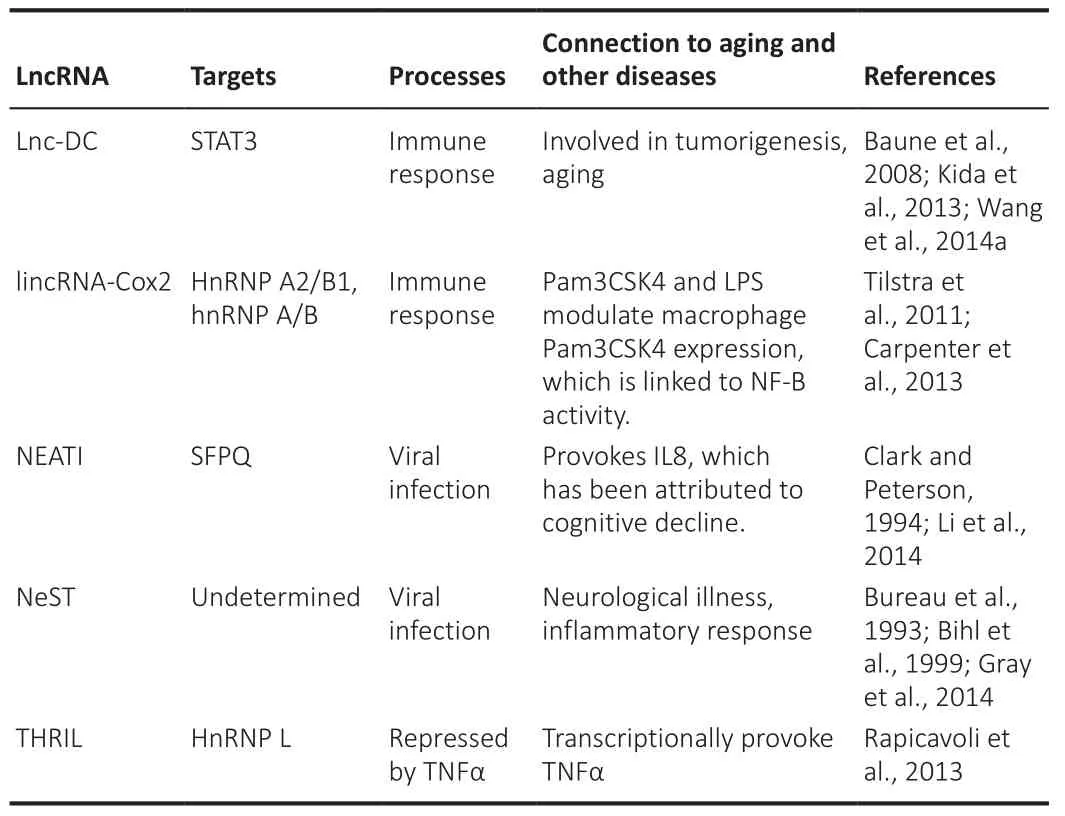
Table 11 |LncRNA in aging-associated immune decline
Future of Aging and Long Non-Coding RNAs in Other Diseases
As discussed, long noncoding RNAs exert a considerable role in regulating cellular processes.LncRNAs interfere with numerous detrimental pathways of the aging process (Zhang et al., 2021).Long non-coding transcripts have emerged as an important regulator of gene control as rapid developments continue in genome sequencing (Xu et al., 2021).Owing to high-throughput techniques and research advancements, lncRNAs are being widely recognized as potent antiaging targets.To devise effective lncRNA modulation strategies, their noncoding potential and peculiarities (e.g., conformational sophistication, cellular localization, or interactions) must be taken into consideration.Modified oligonucleotides are a well-known method for targeting lncRNAs.Antisense oligonucleotides have long been utilized to study thein vitro
andin vivo
actions of various lncRNAs (Kim et al., 2021; Li et al., 2022; Pierce et al., 2022).As different oligonucleotides act differently in different cells and tissues, there are still challenges with the distribution and targeting ofin vivo
antiaging treatments that involve base modifications such as locked nucleic acids.Furthermore, the delivery path is frequently unreliable, which may result in off-target results.However, the developed catalytic oligonucleotides with base modifications for stability and specificity against lncRNAs continue to be the best strategies for achieving adequate downregulation quantities of mature lncRNAs without requiring genetic modification, such as the approach used to treat Angelman syndrome in mice(Fantoni et al., 2021; Schmid et al., 2021).Angelman syndrome is caused by a mutation or deficiency of ubiquitin-protein ligase E3A (UBE3A) in maternal DNA, besides lncRNA; ubiquitin protein ligase E3A-ATS (UBE3AATS) silences the paternal clone of UBE3A (Khan et al., 2018; Elgersma and Sonzogni,2021).Aiming at UbE3AATS in a mouse with antisense oligonucleotide improved the cognitive deficiencies linked with the disorder (Milazzo et al.,2021).Whether the same technique can be applied to humans is still unclear.Survival-associated mitochondrial melanoma-specific oncogenic noncoding RNA (SAMMSON) is an example of a melanogenesis-related lncRNA.In a human xenograft model, targeting SAMMSON with intravenous antisense oligonucleotide greatly decreased tumor growth and cell proliferation(Huang et al., 2021b; Pang et al., 2022).Furthermore, modified antisense oligonucleotides have been utilized successfully to treat clinical disorders involving lncRNAs, such as hypercholesterolemia and inflammatory bowel disease (Feng et al., 2019; Nakamura et al., 2020; Di Mauro et al., 2021; Wang et al., 2022).Moreover, modified antisense oligonucleotides have recently progressed to clinical trials for neuromuscular and neurodegenerative disorders such as Duchene muscular dystrophy with a monogenic origin(Gebski et al., 2005; McClorey et al., 2005; Wilton and Fletcher, 2005a, b;Koo and Wood, 2013; Sousa-Franco et al., 2019).MALAT1 lncRNAs play a significant role in cancers such as hepatocellular carcinoma, bladder cancer,colorectal carcinoma, and lung cancer (Liu et al., 2019; Xie et al., 2021).Cell proliferation, relocation, and invasion were aided by MALAT1 lncRNA in these cancer types (Kim et al., 2018; Liao et al., 2019; Wang et al., 2021a).Highly upregulated in liver cancer (HULC) lincRNA also facilitates hepatitis B virus-induced cell proliferation and anchorage-independent growth in hepatocellular carcinoma (Kanki et al., 2020; Wu et al., 2020).H19 imprinted maternally expressed transcript (H19) has been shown to act biologically in cervical, stomach, bladder, breast, and esophageal cancers.In addition,H19 lncRNA tends to stimulate growth after hypoxia recovery, and cell cycle progression, and hinder apoptosis (Li et al., 2019; Cáceres-Durán et al., 2020;Ghafouri-Fard et al., 2020; Wang et al., 2020a, d; Huang et al., 2021a; Lim et al., 2021; Heydarnezhad Asl et al., 2022).Therefore, manipulating the functions of lncRNAs has anticancer potential (Li et al., 2021a).As a result,more efforts will likely be made to improve lncRNA-based cancer treatment.Nucleic acid-based techniques are commonly employed to target RNA, either by maintaining the quantity of lncRNAs in cancer cells or by changing their structures or sequences (Li et al., 2021a).Inhibiting lncRNAs in cancer cells using RNA interference techniques has been the most effective approach.RNA selectivity and knockdown efficiency are high in small interfering RNA (siRNAs)and short hairpin RNA or small hairpin RNA (shRNAs).The sophistication with which siRNAs and shRNAs are synthesized, besides their adaptability in terms of precise targeting, make them promising therapeutic agents.The stability of these nucleic acid drugs has been significantly enhanced owing to numerous chemical modifications (Swaminathan et al., 2021).Currently, research has identified a few lncRNAs that are commonly linked to various cancer types(Table 12), and it has been shown that targeting these lncRNAs has significant inhibitory effects on cancer cells (Zhong et al., 2019).As lncRNAs play such an important role in cell biology, scientists expect to see lncRNAs active in crucial stages of disease progression in the near future.These lncRNAs could be used as new therapeutic targets and precursors for developing novel disease therapies (Di Martino et al., 2021).However, the clinical application of lncRNAs has certain issues.Furthermore, the molecular or cellular roles of lncRNAs are unknown.Different insights into the processes and cellular pathways of lncRNAs will provide fresh perspectives on the management,prognosis, and intervention of neurodegenerative illnesses related to aging.Biological drug pharmaceutical technology has advanced significantly.Biological therapeutics targeting RNA molecules in cells are immensely effective, and they constitute the foundation for lncRNA-based antiaging and disease therapies (Wang et al., 2020b).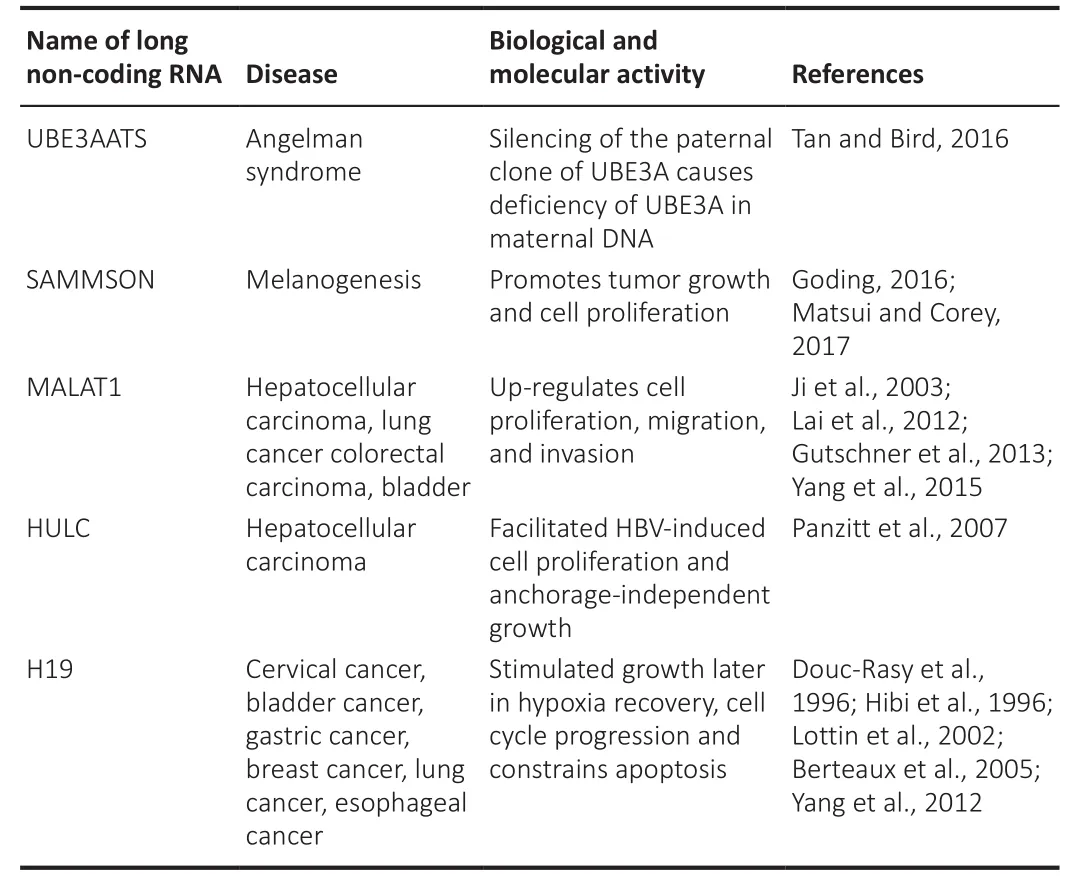
Table 12 |Biological and molecular activity in diseases
The great sensitivity of lncRNAs in aging-related diseases is a distinguishing feature, allowing them to be specific and precise biomarkers for defining the early stages of diseases and assessing the efficacy of therapies and diagnostic procedures.Although lncRNAs are typically resistant to traditional posttranscriptional regulation, their expression may be regulated by various mechanisms such as DNA methylation, chromatin changes, and so on.Furthermore, abnormally expressed lncRNAs can be retrieved noninvasively,which has the potential to be more cost-effective and less damaging.In comparison with protein-based antitumor medications, lncRNAs are more refined and less toxic, and because lncRNA expression is low, just a few inhibitors are required to make a difference.The overarching purpose of this study was to help elucidate the function of these unknown yet developing compounds in aging-related diseases.Reverse transcription-quantitative polymerase chain reaction assays can be used to quantify the expression levels of distinct lncRNAs to detect aberrant lncRNA expression levels inaging.At present, no lncRNA-based medications are available.Medications that target lncRNAs in aging-related illnesses will provide important clinical insights.
Conclusion
To recognize this prospective, comprehensive detection of essential diseaseassociated lncRNAs, thorough evaluation of the potential anti-disease effect of modulating lncRNAs, and ongoing efforts to improve RNA-targeting agents and approaches are efficiently required.In addition, lncRNA-targeted methods face challenges due to a lack of understanding of their structure,folding, interactions, and functional mechanisms.Some lncRNAs have a high attrition rate and a poor abundance of transcripts.They are only expressed for a short time and may be fundamentally unrecoverable.Extensive research is a prerequisite to determine how the secondary and tertiary structures of lncRNAs interact with proteins.Furthermore, the functions and effects of RNA editing and other post-transcriptional (i.e., A-to-I RNA editing) and chemical modifications (i.e., RNA mono-uridylation) of lncRNAs are other facets of lncRNAs that would necessitate further work to fully characterize the roles of lncRNA.LncRNAs may also be promising therapeutic targets for neurodegenerative disorders.LncRNA knockdown using antisense oligonucleotides could be a viable treatment option.However, in therapies for the treatment of neurodegenerative disorders, no antisense oligonucleotides targeting lncRNA have yet been developed.
Author contributions:
Conception, data collection, data analysis and interpretation: SAMS; data collection and writing: AA; editing & formatting:AJ; editing & critical review: MU; writing and data collection: AI, SQ; writing support: AAO; supervision and investigation: MA; revision and contribution to the final version of the manuscript: PJF, ML; critical feedback: RW; conceived of the presented idea, review the final draft, gives feedback and supervised the work: SB.All authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Patient-specific monocyte-derived microglia as a screening tool for neurodegenerative diseases
- Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy
- Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain
- Neurosteroids as stress modulators and neurotherapeutics: lessons from the retina
- Myelinosome organelles in pathological retinas:ubiquitous presence and dual role in ocular proteostasis maintenance
- Anti-IgLON5 disease: a novel topic beyond neuroimmunology