Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy
Lei Tang, Guo-Tong Xu, , Jing-Fa Zhang
Abstract Diabetic retinopathy, characterized as a microangiopathy and neurodegenerative disease, is the leading cause of visual impairment in diabetic patients.Many clinical features observed in diabetic retinopathy, such as capillary occlusion, acellular capillaries and retinal non-perfusion,aggregate retinal ischemia and represent relatively late events in diabetic retinopathy.In fact,retinal microvascular injury is an early event in diabetic retinopathy involving multiple biochemical alterations, and is manifested by changes to the retinal neurovascular unit and its cellular components.Currently, intravitreal anti-vascular endothelial growth factor therapy is the firstline treatment for diabetic macular edema, and benefits the patient by decreasing the edema and improving visual acuity.However, a significant proportion of patients respond poorly to anti-vascular endothelial growth factor treatments, indicating that factors other than vascular endothelial growth factor are involved in the pathogenesis of diabetic macular edema.Accumulating evidence confirms that low-grade inflammation plays a critical role in the pathogenesis and development of diabetic retinopathy as multiple inflammatory factors, such as interleukin-1β, monocyte chemotactic protein-1 and tumor necrosis factor -α, are increased in the vitreous and retina of diabetic retinopathy patients.These inflammatory factors, together with growth factors such as vascular endothelial growth factor, contribute to blood-retinal barrier breakdown, vascular damage and neuroinflammation, as well as pathological angiogenesis in diabetic retinopathy, complicated by diabetic macular edema and proliferative diabetic retinopathy.In addition, retinal cell types including microglia, Müller glia, astrocytes, retinal pigment epithelial cells, and others are activated, to secrete inflammatory mediators, aggravating cell apoptosis and subsequent vascular leakage.New therapies, targeting these inflammatory molecules or related signaling pathways, have the potential to inhibit retinal inflammation and prevent diabetic retinopathy progression.Here, we review the relevant literature to date, summarize the inflammatory mechanisms underlying the pathogenesis of diabetic retinopathy,and propose inflammation-based treatments for diabetic retinopathy and diabetic macular edema.
Key Words: anti-inflammation therapy; anti-vascular endothelial growth factor; diabetic retinopathy;hyperreflectivity foci; inflammation; inflammatory cells; inflammatory cytokines; leukostasis;microglia; Müller cells
Introduction
Diabetic retinopathy (DR) is a neurodegenerative disease featuring microvascular retinal lesions and causes acquired blindness in the workingage population worldwide (Altmann and Schmidt, 2018).The metabolic abnormalities of DR caused by chronic hyperglycemia include the activation of the polyol pathway, the hexosamine biosynthetic pathway, and the protein kinase C pathway, and advanced glycation end-products (AGEs) accumulation,resulting in increased reactive oxygen species in cells, and aggravation of retinal oxidative stress and inflammation (Whitehead et al., 2018).
Low-grade inflammation triggers a series of cellular abnormalities and tissue injury culminating in the retina (Altmann and Schmidt, 2018; Rübsam et al.,2018; Wang and Lo, 2018; Whitehead et al., 2018), as pro-inflammatory mediators, adhesion molecules, chemokines, and growth factors (Altmann and Schmidt, 2018; Lu et al., 2018; Rübsam et al., 2018; Wang and Lo,2018; Whitehead et al., 2018), are increased and extensively involved in the pathogenesis of DR.Inflammatory cells in the retina also respond to injury and stress.Noxious stimuli activate endothelial cells and pericytes to secrete pro-inflammatory factors, recruiting leukocyte which adhere to the vascular endothelium, causing leukostasis and subsequent capillary non-perfusion (Spencer et al., 2020).Glial cells, which provide structural support in the normal retina, are also activated in early DR and participate in microenvironmental homeostasis regulation (Sorrentino et al., 2016;Rübsam et al., 2018).In DR, microglia proliferate and migrate from the inner to outer retina, and secrete multiple inflammatory cytokines.These may increase vascular permeability and induce intraretinal fluid accumulation by disrupting tight junction proteins and triggering breakdown of the bloodretinal barrier (BRB) (Tang et al., 2022).Inflammation also aggravates retinal neurodegeneration in the early stage of DR (Wang and Lo, 2018).
Recently, anti-vascular endothelial growth factor (anti-VEGF) agents have been regarded as a promising treatment for patients with proliferative DR(PDR) or diabetic macular edema (DME), but there remains a significant proportion of patients who show poor or incomplete response to anti-VEGF treatment, indicating that DR is a multi-factorial disease.Indeed,the increased levels of inflammation-related chemokines and cytokines have been evidenced in serum, vitreous, aqueous humor and retina in DR patients, and antagonization of these inflammation-related molecules may delay or prevent the angiogenesis and neurodegeneration in DR (Semeraro et al., 2015), suggesting inflammation as a novel therapeutic target for DR treatment.Thus, the present review describes the pathogenetic role of inflammation in DR and proposes potential mechanism-based therapies targeting inflammation in DR.
Database Search Strategy
Literature review was performed using the PubMed database.The following combinations of key words were used to initially select articles for evaluation:diabetic retinopathy AND inflammation; microglia AND diabetic retinopathy;anti-inflammation therapy AND diabetic retinopathy; and hyperreflectivity foci AND diabetic retinopathy.Most of the selected studies (65% of all references)were published since 2016.An article published in 1993 was included due to its topic (macrophages) with relevance to the search.
Role of Inflammatory Cytokines in Diabetic Retinopathy
Data increasingly implicate chronic inflammation in DR pathogenesis,especially in the development of pathological vascularization and macular edema (Rangasamy et al., 2012; Trotta et al., 2022).In hyperglycemic conditions, upregulated pro-inflammatory molecules promote the synthesis of chemokines, inflammatory cytokines and other factors (Semeraro et al.,2015), aggregating leukostasis, cell apoptosis, and capillary leakage in the retina.The upregulated cytokines which mediate inflammation in DR patients are listed in Table 1.

Table 1 |Cytokines mediated inflammation in patients with diabetic retinopathy
Inflammatory cytokines
Serum and vitreous levels of interleukin-1β (IL-1β), mainly produced by macrophages, are raised in DR patients (Demircan et al., 2006).As the potent up-regulators of adhesion molecules, both IL-1β and tumor necrosis factor-α(TNF-α) prompt nuclear factor κappa-B (NF-κB) transcription by binding its receptor, and result in increased expressions of IL-6 and IL-8, as well as activating caspase-1.Increased IL-1β is triggered by cleaved caspase-1 and promotes neovascularization in diabetes-induced mice (reviewed by Boss et al 2017).TNF-α, another inflammatory initiator mediating NF-κB activation,mainly induces leukocyte-capillary adhesion, and subsequent pericyte loss and capillary degeneration in diabetic rat retinas (Behl et al., 2009; Joussen et al., 2009).
Similarly, other inflammatory factors, such as IL-6, IL-8, and IL-12, are also found in high concentrations in the vitreous of patients with progressive PDR(Doganay et al., 2002; Zorena et al., 2008; Gverović Antunica et al., 2012).Upon pro-inflammatory cytokine stimulation, endothelial cells produce intercellular adhesion molecules, recruiting leukocyte adhesion to capillaries.Once attached, these leukocytes induce capillary blockage and disrupt tight junctions between endothelial cells, accompanied by acellular capillary formation, vascular leakage and diabetic macular edema (DME) (Lutty, 2013).Several studies have revealed significant positive correlations between vitreous IL-6 and the subtype of DME with serous retinal detachment(Coscas et al., 2013; Chung et al., 2019).Thein vitro
studies demonstrated that inflammatory cytokines, rather than hyperglycemic stimuli, are largely responsible for endothelial apoptosis (Doganay et al., 2002).Adhesion molecules and integrins
In addition, adhesion molecules such as intercellular adhesion molecule-1(ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are indirectly involved in the inflammatory response (Chibber et al., 2007).Hyperglycemia and oxidative stress upregulate the expressions of ICAM-1 and VCAM-1, which further induces leukocyte-capillary endothelium adhesion at an early stage of DR.Mice deficient in ICAM-1 are significantly prevented from vascular injury in experimental DR, typically in pericyte ghost and capillary degeneration(Joussen et al., 2004).
Integrins, such as cluster of differentiation 11 (CD11)/CD18, have been found to be responsible for mediating leukocyte adhesion in early DR.Leukocyte activation, via integrins binding to the adhesion molecule, such as ICAM-1, on the endothelial surface, amplifies the leukostasis and retinal non-perfusion.Blockade of integrins attenuates the diabetes-induced leukostasis and vascular leakage in the retina (Van Hove et al., 2021).In addition, administration of leukocyte antagonist has been confirmed to significantly reduce retinal vasculopathy and inflammatory factors such as VEGF and TNF-α in diabetic rats (Rao et al., 2010).
VEGF and placental growth factor
VEGF is considered the predominant factor in the progression of DR.Accumulation of VEGF in the retina can trigger glial activation, which produces VEGF and numerous other vascular permeable factors associated with retinal neovascularization and macular edema.Elevated VEGF, binding to its receptors, induces endothelial cell proliferation, migration and neovascularization with increasing leakage (Ishida et al., 2003).In addition,VEGF has been shown to exacerbate cytokine release and leukostasis by promoting ICAM-1 expression of endothelial cells, which further amplifies the inflammatory response.Moreover, as a pro-inflammatory molecule, VEGF itself also promotes the expressions of other pro-inflammatory cytokines such as macrophage inflammatory protein-1α, monocyte chemotactic protein-1(MCP-1) and IL-8.Specific blockade of VEGF decreased the levels of TNF-α,ICAM-1 and NF-κB in diabetic mice, alleviating retinal leukostasis and thus BRB breakdown (Ishida et al., 2003; Wang et al., 2010).
Placental growth factor (PlGF), a member of the VEGF family, is also detected at high levels in the vitreous of PDR patients (Mitamura et al., 2002).PlGF plays a physiological role in cell survival, and its dysregulation may correlate with DR development (Sensenbrenner, 1993).In contrast, PlGF deficiency in diabetic mice prevents BRB breakdown and retinal cell death (Huang et al., 2015).
Chemokines and other inflammatory mediators
Under inflammatory conditions, chemokines, such as CXC motif chemokine ligand 10 (CXCL10), C-C motif ligand-5 (CCL-5), and CCL-2 (also known as MCP-1), are upregulated in the vitreous and serum of DR patients (Demircan et al., 2006; Maier et al., 2008; Murugeswari et al., 2008).These chemokines facilitate leukocyte activation and recruitment, causing leakage of fluids and neutrophils from the vessels into retinal tissue.Inducible nitric oxide synthase(iNOS), together with cyclooxygenase-2 (Tang and Kern, 2011) participate in inflammation during DR progression.
Matrix metalloproteinases (MMPs) are major regulators in the initial phase of acute inflammation and the late phase of tissue remodeling (Kuno and Fujii, 2010), and play an important role in chemokine activation (Schwartz and Flynn, 2011).Studies have reported that increased MMP-2 and MMP-9 levels in the vitreous of DR patients contribute to retinal neovascularization(Kompella et al., 2010) and the level of angiopoietin-2, an important modulator of angiogenesis, is significantly upregulated in patients with DME(Campochiaro et al., 2012).
Inflammation-Related Pathways in Diabetic Retinopathy Using Bioinformatics Analysis
We searched datasets in the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) database using the keyword “diabetic retinopathy”,and restricted to human species and retinal tissue.The GSE160306 dataset was the most suitable for this review, and was used for bioinformatics analysis.To identify the possible involvement of inflammation in DR, we analyzed the retinal mRNA expression profiles between DR patients and healthy individuals by downloading the retina gene profiles.Through bioinformatic analysis of GSE160306, 574 up-regulated genes and 225 downregulated genes were enriched with the fold change > 1 andP
< 0.05.Among these genes, the inflammation-related factors, such as TNF-α, NF-κB, IL-1β,IL-17, and transforming growth factor-β (TGF-β) were significantly increased(Figure 1A and B).The gene ontology (GO) terms were primarily enriched in inflammatory response, angiogenesis, signal transduction, and other processes (Figure 1C), and the Kyoto encyclopedia of genes and genomes(KEGG) database indicated that the significant genes were largely enriched in cell adhesion molecules, leukocyte trans-endothelial migration, and NF-κB signaling pathway (Figure 1D).Using Metascape’s functional enrichment analysis, the enriched terms mainly included human TYRO protein tyrosine kinase-binding protein (TYROBP) causal network in microglia, cell migration,regulation of response to external stimulus, glial cell differentiation, and response to growth factor (Figure 1E).Results of the above bioinformatic analysis suggested the involvement of inflammatory signaling pathways and processes in DR (from our unpublished data).
Figure 1 | mRNA transcriptomes analysis in diabetic patient retinas.
Role of Inflammatory Cells in Diabetic Retinopathy
Retinal cell dysfunction may also amplify retinal inflammation at initial stages in DR.Retinal cell dysfunction, including microglial activation, Müller cell gliosis, retinal pigment epithelium (RPE) secretion, endothelial cell proliferation, and pericyte ghost, all involve in inner BRB disruption and macular edema in the progression of DR (Tang and Kern, 2011).
Müller cells
In the healthy retina, Müller cells span the entire neuronal retinal from the internal to external limiting membrane, and are responsible for retinal homeostasis, including structural support, neuronal metabolism, and blood vessel function (Coughlin et al., 2017).In DR, proliferation and reactive gliosis of Müller cells are indicated by increased expression of glial fibrillary acidic protein (GFAP) and increased secretion of IL-1β, IL-6, IL-8, VEGF, as well as ICAM-1 during diabetes progression, confirming their participation in the inflammatory process (Rangasamy et al., 2012; Nagayach et al., 2014).Other inflammatory cytokines, including iNOS, MCP-1, and prostaglandin E, are also produced by Müller cells when exposure to high glucose (Carpi-Santos et al.,2022), confirming that Müller cells are the main source of the inflammatory factors in DR.In addition, Müller cells may also directly influence microglia,enhancing microglial activation and migratory mobilization (Portillo et al.,2017).
RPE
Altered RPE secretion of growth factors and inflammatory cytokines also plays a role in inflammation and angiogenesis during DR (Ponnalagu et al., 2017).One of the most relevant molecules is pigment epithelium-derived factor, a potent anti-angiogenic and anti-inflammatory factor, which is decreased in PDR patients (Zhang et al., 2006).One study showed that sustained pigment epithelium-derived factor expression was able to downregulate VEGF and inflammatory molecules both in diabetic mice retinas and in human RPE cellsin vitro
(Calado et al., 2016).TGF-β plays a role in regulating cell growth, proliferation, apoptosis and differentiation (Hirsch et al., 2015) and platelet derived growth factor (PDGF),secreted by RPE, is angiogenic (Lefevere et al., 2022).Both TGF-β and PDGF promote the proliferation of endothelial cells and induce angiogenesis (Bhattand Addepalli, 2010), and have been detected at high concentrations in the vitreous of PDR patients (Praidou et al., 2011).
Endothelial cells
Under hyperglycemia, endothelial adhesion molecules stimulate leukocyte adherence to the endothelium (Chibber et al., 2007), which in turn induces endothelial activation and leukostasis.Leukocytes release inflammatory cytokines and vascular permeability factors, further disrupting endothelial junctional proteins, prompting leukocytic diapedesis into the retina (Zhang et al., 2011), and aggravating BRB breakdown.In addition to BRB disruption,increased VEGF promotes endothelium sprouting and angiogenesis (Chibber et al., 2007).Many studies have reported that ICAM-1 and VCAM-1 are increased in endothelial cells, mediating leukocyte adhesion in diabetic animals and patients (Kasza et al., 2017), while ICAM-1 deficiency results in significant reduction of adherent leukocytes (Joussen et al., 2004).Cyclooxygenase-2 and MMPs have been shown to co-localize in endothelial cells, and play an essential role in angiogenesis and vascularization.High glucose and cytokines strongly induce the expression of MMP-2, MMP-9,and cyclooxygenase-2 in human endothelial cells (Psaltis and Simari, 2015;Madonna et al., 2016).
Microglia
In view of numerous pro-inflammatory cytokines and chemokines found in the vitreous of diabetic patients, it is evident that microglia play a crucial role in triggering inflammatory response in DR.
In the normal retina, ramified microglia are scattered in the inner retina,while microglia are markedly activated and increased in number at different stages of DR (Zeng et al., 2008).An initial stimulus triggers the ameboid microglia to release anti-inflammatory cytokines, such as IL-4, IL-10, and IL-13, which resolve inflammation and enhance survival of neurons (Tang and Le, 2016).However, sustained hyperglycemia and harmful insults induce the overproduction of pro-inflammatory factors from microglia, such as TNF-α,IL-1β, and iNOS (Scholz et al., 2015), resulting in phagocytosis, neuronal death, and BRB breakdown.These inflammatory molecules also lead to activation of other glial cells such as astrocytes which respond by amplifying neuroinflammation.
Studies have confirmed that activated microglia proliferate and migrate into the plexiform layers in non-proliferative DR (NPDR) patients, whereas microglia gather at ischemic regions in PDR (Zeng et al., 2008; Okunuki et al.,2019; Kinuthia et al., 2020).High numbers of microglia are found throughout the retina, especially in the subretinal space, in DME patients (Zeng et al.,2008).
Similarly, microglial activation has been confirmed in animal models (Zeng et al., 2008; Xie et al., 2021).In our studies, the microglia in diabetic rat retina were activated compared with a normal control, characterized by enhanced migration and proliferation, as well as changed morphologies from ramified to amoeboid (Figure 2).Microglial activation occurs at the beginning of DR, producing plenty of proinflammatory cytokines, such as IL-1β, TNF-α,VEGF, and MMPs, which induce adhesion molecules (ICAM-1 and VCAM-1)secretion, cell apoptosis, leukocytes infiltration and BRB breakdown (Ibrahim et al., 2011).

Figure 2 | Representative images showing the relationship between blood vessels (IB4, green) and microglia (IBA-1, red) in deep capillary plexus of the retinal flatmounts in normal control (N) and 8-week diabetic (D8w) rat retinas.
Astrocytes
Astrocytes, mainly distributed in the nerve fiber layer in the healthy retina,surround the blood vessels and ganglion cells, and are responsible for the integrity of inner BRB and neurotrophic protection.In DR, the astrocytes decrease in number and reduce the expressions of connexin-26/43, glial cell line-derived neurotrophic factor (GDNF), and GFAP with disease progression.In addition, astrocytes amplify the inflammatory process by producing proinflammatory cytokines such as IL-1β, MCP-1 and VEGF, aggravating damage to the retinal neurovascular unit and resulting in neuroinflammation (Nagayach et al., 2014).
Macrophages
The breakdown of the BRB at an early stage of DR facilities blood monocytes entering the retina in significant numbers, and the inflammatory-immune response mediated by macrophages plays an important role in the development of DR.
Multiple studies have suggested macrophages as the primary driving force in the pathogenesis of PDR (Pavlou et al., 2018; Al-Rashed et al., 2020; Jia and Zhou, 2020).Macrophages induce chemotaxis and fibroplasia through secretion of leukotrienes and fibronectin.They also influence cellular proliferation via the synthesis of VEGF, PDGF, fibroblast growth factor (FGF),and TGF-β (Pavlou et al., 2018).Torres-Castro et al.(2016) showed that human monocytes and macrophages were activated bothin vitro
andin vivo
,as the levels of CD11c and iNOS were upregulated in high glucose-treated macrophages, and in circulating monocytes of DR patients.Also, activated macrophages secrete excessive inflammatory factors such as IL-1β, TNF-α, IL-6, and IL-12 via the NF-κB signaling pathway (Cheng et al., 2015; Al-Rashed et al., 2020), while their phagocytosis function was damaged, suggesting the dysfunction of macrophages may aggravate the inflammation in DR.Hyperreflective Foci on Optical Coherence Tomography Images Indicate Macrophage/Microglia Activation
Recently, retinal hyperreflective foci (HRF) on optical coherence tomography(OCT) have been considered to indicate active inflammatory cells, involved in the pathogenesis of many retinal diseases (Zur et al., 2018; Chung et al.,2019).HRF were first described by Coscas et al.(2013) as hyperreflective dots(HRD) by spectral-domain OCT in age-related macular degeneration patients.Subsequently, HRF have been identified in DR, DME, retinal vein occlusion, as well as retinal degenerative diseases (Romano et al., 2020).Although there is still no consensus on their origin, HRF may correlate with inflammatory microenvironments and reflect a damaged retinal state.
Typically, HRF on OCT angiography appear as discrete intraretinal spots distinct from hard exudates, and may represent activated microglia or macrophages in DR (Vujosevic et al., 2017).According to OCT angiography images from our research, HRF are mainly distributed in the inner retina of DME patients compared with controls, while HRF were also detected around the subretinal fluid (SRF) in DME with serous retinal detachment (Figure 3).In one study, DME patients with diffuse edema exhibited high level of sCD14 in the aqueous and the inner retina showed increased number of HRF which correlated with level of sCD14, a biomarker of microglia and macrophage,suggesting that the HRF observed on OCT may reflect the activated microglia in DME (Lee et al., 2018).

Figure 3| Representative optical coherence tomography angiography (OCTA) images showing the subtypes of diabetes macular edema (DME) with hyperreflective foci(HRF).
Current and Novel Therapeutic Strategies in Diabetic Retinopathy
Inflammation-related treatments
Since inflammation has been recognized as an important mechanism for DR pathogenesis, significant effort has been made to target the inflammatory process (Table 2).
Corticosteroids
Corticosteroids, such as triamcinolone acetonide (TA), dexamethasone, and fluocinolone acetonide (FA), significantly reduce cellular swelling and prompt fluid reabsorption, thus reducing vascular permeability and restoring BRB integrity in DR (Himasa et al., 2022).As potent anti-inflammatory agents,corticosteroids also target a broad range of mediators, including adhesion molecules, chemokines, and inflammatory molecules (Jeong et al., 2017).
TA, as a potent glucocorticoid, has been shown to inhibit inflammatory factors under hypoxic conditions and decrease central macular thickness and neovascularization by downregulating ICAM-1, TNF-α, and IL-6 (Semeraro et al., 2019).Gillies et al.(2006) showed that intravitreal injection of TA in combination with laser was more effective in improving visual acuity and reducing macular edema than laser photocoagulation.
Several studies have demonstrated that intravitreal dexamethasone is effective in DME treatment, especially for patients unresponsive to anti-VEGF therapy (Lattanzio et al., 2017).Ozurdex, as a sterile, sustained-release implant of dexamethasone, has been implied in the treatment of macular edema.Two phase III pivotal trials of Ozurdex for diabetic macular edema(NCT00168337 and NCT00168389) showed that Ozurdex (0.35 and 0.7 mg)achieved significant improvement in vision and retinal thickness reduction in DME patients, without significant cataract or increased intraocular pressure complication.
Two FA implants have been applied in clinical trials for DME treatment.Retisert (0.59 mg) is superior at improving best-corrected visual acuity(BCVA) in DR patients especially with persistent DME (Wang and Lo, 2018).However, considering the high incidence of complications, such as intraocular pressure elevation and cataract, Iluvien (0.19 mg) has been designed as an alternative which successfully increases BCVA in persistent DME patients with a significantly low rate of intraocular pressure elevation in a two-year study(Studsgaard et al., 2022).
Antagonists of inflammatory factors
Several studies have demonstrated the role of inflammatory inhibitors in DR treatment.Canakinumab, a selective IL-1β antibody, can stabilize retinal neovascularization and reduce macular edema in PDR patients (Stahel et al.,2016).In addition, capillary degeneration is prevented in mice lacking the IL-1β receptor (Vincent and Mohr, 2007).
TNF-α, as an up-regulated proinflammatory factor in the vitreous of DR patients, has been identified as a promising target for DR (Demircan et al.,2006).Consistent with the role of IL-1β, mice genetically deficient in TNF-α show alleviated leukostasis, vascular leakage, and loss of pericyte and endothelial cells (Joussen et al., 2009; Huang et al., 2011).Infliximab, as a monoclonal antibody against TNF-α to treat Crohn’s disease, was also applied in DME patients and showed positive results, including improvement in visual acuity and reduction in macular thickness (Markomichelakis et al., 2005).Infliximab also alleviates retinal capillary degeneration and pericyte loss (Behl et al., 2009), thus preventing BRB breakdown in diabetes.
Antagonism of the adhesion molecule and integrin is also under clinical investigation.Leucocyte function-associated antigen-1, which binds to ICAM-1, is responsible for leucocyte-endothelial cell interaction.One study showed that lifitegrast, an antagonist of leucocyte function-associated antigen-1,decreased leukostasis and retinal vascular leakage in diabetic rat retina (Rao et al., 2010).Similarly, anti-CD49 antibody, blocking interactions between VCAM-1 on endothelial cells and very late antigen-4 on leucocytes, significantly attenuates diabetes-induced leukostasis and vascular leakage (Iliaki et al.,2009).Moreover, the anti-CD49 antibody reduces levels of VEGF and TNF-αby inhibiting NF-κB activity, indicating that leucocyte recruitment provides positive feedback to the DR inflammatory pathway (Zhang et al., 2011).
Luminate (ALG-1001), the integrin inhibitor, has promising effects on visual acuity and alleviating macula edema for DME by binding to the integrin receptors (Shaw et al., 2020).
Other treatments
As the VEGF level is significantly elevated in DR patients and increases with disease progression, anti-VEGF treatment becomes the first-line therapy in treatment of DR and DME.Meanwhile, anti-angiogenesis as well as other none-inflammatory treatment have been under development (Table 3).
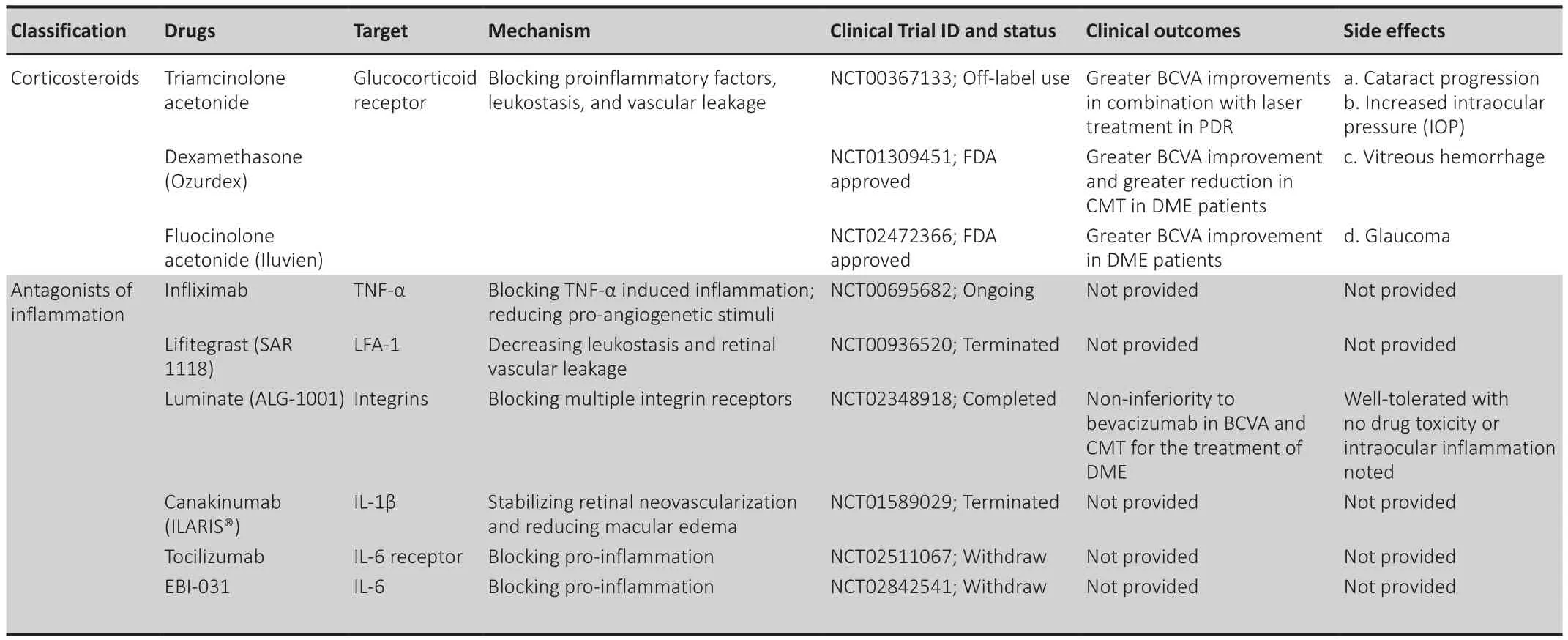
Table 2 |Mechanism-based treatments targeting inflammation in diabetic retinopathy and diabetic macular edem
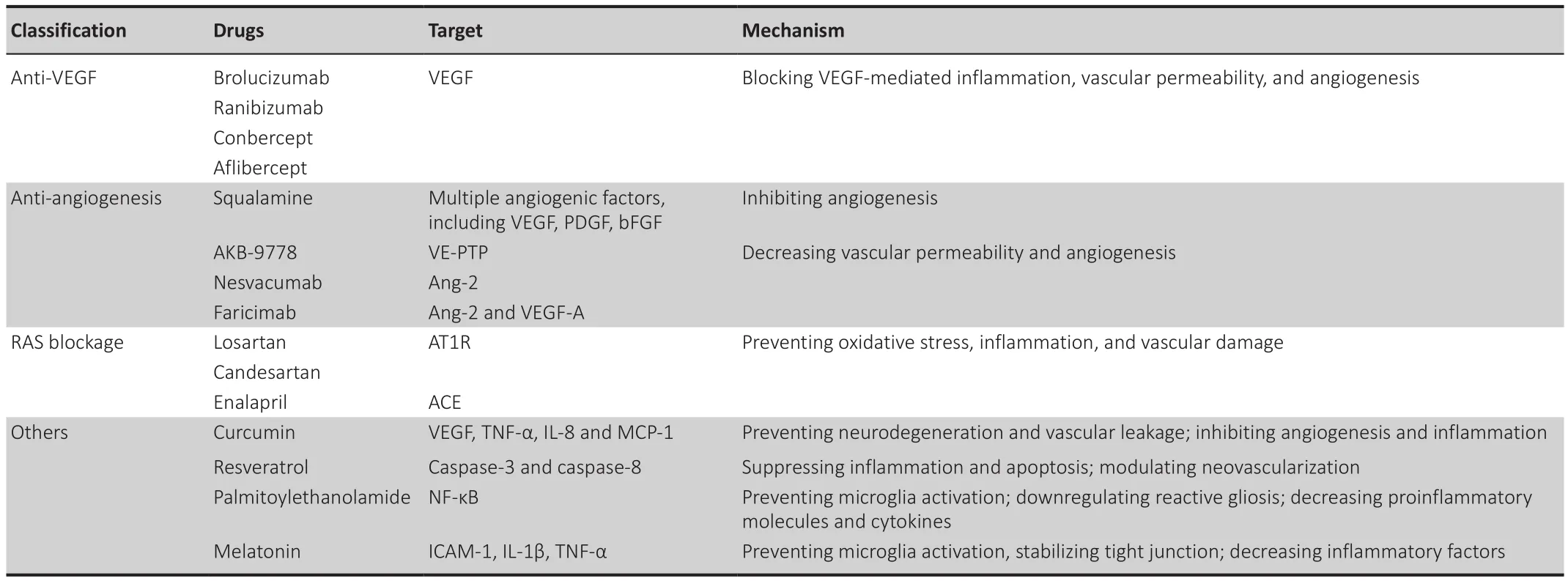
Table 3 |Other treatments for diabetic retinopathy and diabetic macular edema
Anti-VEGF agents
Currently, anti-VEGF agents, including brolucizumab, bevacizumab,ranibizumab, conbercept, and aflibercept, benefit millions of DME patients with improved visual function and decreasing macular edema.
Brolucizumab has a low molecular weight and is the first single-chain antibody fragment designed specifically to target VEGF-A via intraocular use in humans(Markham, 2019).Two phase III pivotal trials of brolucizumab for DME showed that at final visit, brolucizumab (6 mg) was not iinferior to aflibercept in BCVA improvement in patients with DME, while more subjects achieved anatomical improvements such as central subfield thickness (CSFT) < 280 μm,and decreased subretinal and intraretinal fluid compared with aflibercept(Brown et al., 2022).
Ranibizumab has been comprehensively evaluated in clinical trials, and the results show that it is effective in BCVA improvement (Spooner et al., 2020).In terms of OCT results, bevacizumab effectively reduced diffuse macular edema (Fazel et al., 2020).However, DME patients with subretinal fluid (DMESRF) may not respond well to anti-VEGF treatments due to external limiting membrane and RPE impairment.The randomized controlled trial of DRCT.NET, Protocol T (NCT01627249) showed that aflibercept was superior to ranibizumab in improving BCVA of DME patients with poorer baseline visual acuity.
Anti-angiogenic therapy
Currently, several anti-angiogenic agents are undergoing clinical trials.Squalamine presented better visual improvement in DME patients by inhibiting multiple angiogenic factors, including VEGF, PDGF and bFGF(Wroblewski and Hu, 2016).AKB-9778, a small molecule targeting Ang/Tie2 signaling pathways, activates Tie2 by inhibiting VE-PTP and decreases vascular permeability (Campochiaro et al., 2016).Nesvacumab, an inhibitor of Ang-2,also decreases vascular permeability by activating Tie2.Faricimab, a bispecific antibody targeting both Ang-2 and VEGF-A, was comparable to aflibercept in DME treatment, with a longer treatment period (Sahni et al., 2019).
Targeting the renin-angiotensin system (RAS) and AGEs
RAS could be a promising target for DR treatment as it participates in diabetes or hypertension-induced retinal inflammation.Specific blockade of the RAS with angiotensin converting enzyme (ACE) inhibitor and angiotensin type-1 receptor blocker has been shown to prevent inflammation, oxidative stress,and vascular injury in diabetic animal models (Zhang et al., 2007).
AGEs and the receptor for AGEs, involved in inflammatory mechanisms, may also be promising targets for DR (Zhang et al., 2011).Studies have shown that inhibiting AGE formation improves neuronal function and prevents pericyte loss and acellular capillaries in diabetic mice (Barile et al., 2005).
Other potential treatments
Recently, increasing attention has been paid to supplemental therapies for DR.Agents, such as curcumin, resveratrol, palmitoylethanolamide and melatonin have regulated pathophysiological changes in DR via their anti-inflammatory and anti-proliferative properties.
Curcumin prevents neurodegeneration and vascular alterations, including restoration of blood vessels and repair of choroidal microvascular, by decreasing TNF-α and VEGF, and extracellular matrix production in DR (Yang et al., 2021).
Resveratrol, an antioxidant and neuroprotective agent, is particularly effective in the improvement in DR (Seong et al., 2017).Resveratrol suppresses apoptosis of retinal ganglion cells by downregulation of caspase-3 and caspase-8 expression in diabetes-induced mouse retinas (Chalke and Kale,2021).
Palmitoylethanolamide, as an endogenous cell-protective lipid, inhibits oxidant stress and reactive gliosis between glial cells, thereby decreasing the levels of proinflammatory molecules and cytokines (Rajamani and Jialal,2014).
Melatonin, produced in pineal gland and retina, regulates redox reactions and dopamine metabolism.Our previous work demonstrated that melatonin maintained the inner BRB by up-regulating the expressions of tight junction proteins and decreasing the production of inflammatory factors such as ICAM-1, IL-1β, and TNF-α (Tang et al., 2021).
Discussion
DR, characterized by microangiopathy and neuronal degeneration, is the main cause of visual impairment in diabetes patients.Increasing data confirm that chronic, low-grade inflammation plays an important role in the pathogenesis and development of DR.The inflammation-related cells, together with secreted inflammatory factors, promote microinflammation and aggravate destruction of the BRB.Thus, this review highlights the role of inflammatory cells and proinflammatory cytokines in the pathogenesis of DR, as well as anti-inflammatory treatments for DR and DME.
Although anti-VEGF therapies remain the first-line treatments for PDR and DME, their limitations including frequent injections, increasing financial burden, poor compliance of patients, and a lack of effect, are of major concern.The etiology of DR is multifactorial, and in addition to BRB breakdown pathogenesis involves inflammation.In DR patients, increased levels of serum C-reactive protein (Yang et al., 2016) and intraocular inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and MCP-1 (Tamura et al.,2012), have been detected.In addition, hyperglycemia-induced activation of retinal microglia, together with infiltration of immune cells such as neutrophils and macrophages, may contribute to the pathogenesis of DR (Xu and Chen,2017).For example, patients with persistent DME who are not responsive to intravitreal bevacizumab, intravitreal methotrexate, synthetic folic acid analogue with anti-proliferative and anti-inflammatory properties, showed anatomical improvement in a significant proportion of eyes with a significant visual improvement in 16.6 % of eyes (Falavarjani et al., 2016).For this reason, it is important to explore immunosuppressive or immunomodulating therapies for the treatment of DR (Xu and Chen, 2017).
In this review, we explored the role of inflammatory factors and related signaling pathways with bioinformatics analysis in DR patients from public databases.Currently, in addition to anti-VEGF drugs and steroid hormones(such as dexamethasone, TA and FA), many drugs targeting inflammationrelated molecules, such as infliximab, lifitegrast (SAR 1118), luminate (ALG-1001), canakinumab, tocilizumab and EBI-031, have already entered the clinical trial stage.However, most of the trials have been terminated or withdrawn, and have not achieved the primary endpoint.Limitations of the trials may include the fact that numerous cytokines are involved in inflammation, oxidative stress and apoptosis in DR pathogenesis, so it is difficult to treat DR by antagonizing a single molecule.The retina is a complex structure and DR involves interaction of all retinal cell types, so potential therapeutics targeting multiple molecules or pathways, instead of only one should be the focus of future research.
In conclusion, inflammation plays a pivotal role in the pathogenesis of DR and DME.Elucidation of the key inflammatory factors in DR would aid the development of mechanism-based therapies targeting inflammation as well as combination therapy (such as anti-VEGF combined with anti-inflammatory drugs) the latter having potential for comprehensive and personalized treatment in DR patients.
Author contributions:
LT, GTX and JFZ contributed to the conception, design and definition of intellectual content.LT contributed to the data analysis and manuscript preparation.GTX and JFZ revised the article critically for important intellectual content and are guarantors of this work, who had full access to all the data in this study and take responsibility for the integrity and accuracy of the data.All authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare that there is no conflict of interest regarding the publication of this paper.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others
to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Patient-specific monocyte-derived microglia as a screening tool for neurodegenerative diseases
- Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases
- Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain
- Neurosteroids as stress modulators and neurotherapeutics: lessons from the retina
- Myelinosome organelles in pathological retinas:ubiquitous presence and dual role in ocular proteostasis maintenance
- Anti-IgLON5 disease: a novel topic beyond neuroimmunology

