Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain
Dan-Yang Li, Shao-Jie Gao, Jia Sun, Long-Qing Zhang, Jia-Yi Wu, Fan-He Song, Dai-Qiang Liu, Ya-Qun Zhou, Wei Mei
Abstract Nitric oxide (NO)/cyclic guanosine 3′,5′-monophosphate (cGMP) signaling has been shown to act as a mediator involved in pain transmission and processing.In this review, we summarize and discuss the mechanisms of the NO/cGMP signaling pathway involved in chronic pain, including neuropathic pain,bone cancer pain, inflammatory pain, and morphine tolerance.The main process in the NO/cGMP signaling pathway in cells involves NO activating soluble guanylate cyclase, which leads to subsequent production of cGMP.cGMP then activates cGMP-dependent protein kinase (PKG), resulting in the activation of multiple targets such as the opening of ATP-sensitive K+ channels.The activation of NO/cGMP signaling in the spinal cord evidently induces upregulation of downstream molecules, as well as reactive astrogliosis and microglial polarization which participate in the process of chronic pain.In dorsal root ganglion neurons, natriuretic peptide binds to particulate guanylyl cyclase, generating and further activating the cGMP/PKG pathway, and it also contributes to the development of chronic pain.Upregulation of multiple receptors is involved in activation of the NO/cGMP signaling pathway in various pain models.Notably the NO/cGMP signaling pathway induces expression of downstream effectors, exerting both algesic and analgesic effects in neuropathic pain and inflammatory pain.These findings suggest that activation of NO/cGMP signaling plays a constituent role in the development of chronic pain, and this signaling pathway with dual effects is an interesting and promising target for chronic pain therapy.
Key Words: bone cancer pain; chronic pain; cyclic GMP; dorsal root ganglion; inflammatory pain;morphine tolerance; neuropathic pain; nitric oxide; protein kinase G; spinal cord
Introduction
Chronic pain is pain that persists or recurs for more than 3 months, and the global prevalence of chronic pain is more than 30% (Cohen et al., 2021).In contrast to the acute warning effect of physical pain, chronic pain is considered to be a pathological condition that seriously affects quality of life(Zhou et al., 2021a).Currently, nonsteroidal anti-inflammatory drugs, opioids,anticonvulsants, antidepressants, and traditional Chinese therapies such as acupuncture are used to relieve chronic pain (Dale et al., 2016; Zhou et al.,2021b).However, due to the limited effectiveness and/or intolerable side effects of these methods, they often fail to completely relieve chronic pain.Therefore,further investigation of the cellular and molecular mechanisms involved in chronic pain is needed, to provide new therapeutic targets to treat it.
The nitric oxide (NO)/cyclic guanosine 3′,5′-monophosphate (cGMP) pathway regulates axonal growth and neural migration, which are important effectors in neural regeneration.The main process of the NO/cGMP signaling pathway in cells is that NO activates soluble guanylate cyclase (GC), which leads to subsequent production of cGMP.cGMP then activates cGMP-dependent protein kinase (PKG), resulting in the activation of multiple targets such as the opening of ATP-sensitive Kchannels (K) (Schmidtko et al., 2009).It is well established that the NO/cGMP signaling pathway has both nociceptive and antinociceptive effects in various models of chronic pain.However, the mechanisms underlying the NO/cGMP signaling pathway in the spinal cord and dorsal root ganglion (DRG) are still under investigation.The current review summarizes the existing evidence on the mechanisms of NO/cGMP signaling in different pain models, in an effort to provide new therapeutic targets.
Search Strategy
The PubMed database was searched to identify relevant studies published from January 1997 to December 2021 (Figure 1).The terms used in combination were nitric oxide, cyclic GMP, pain, neuropathic pain, bone cancer pain, inflammatory pain, and morphine tolerance.Manual searches were also performed to retrieve important articles.After removing duplicates from the retrieved studies, preliminary screening was performed by reading the title and abstract of each article.Full texts were then read to eliminate studies that did not investigate relationships between NO/cGMP signaling and chronic pain.Only studies published in English and conducted in rodents and non-human primates were included.A total of 64 articles were ultimately included in this review.
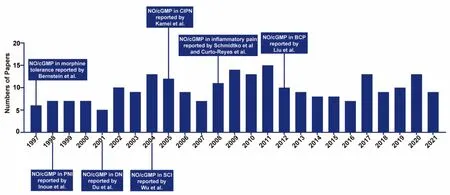
Figure 1|Timeline of research on the NO/cGMP signaling pathway in chronic pain.
Overview of the NO/cGMP Signaling Pathway
NO is a gas synthesized from arginine by nitric oxide synthase (NOS) (Zhou et al., 2016).The NOS family consists of three isoforms; neuronal NOS (nNOS),endothelial NOS (eNOS), and inducible NOS (iNOS) (Fang et al., 2022; Sun et al., 2022).nNOS and eNOS are constitutively expressed in cells, and their function depends on the Ca-calmodulin complex.Physiologically, these two isoforms are inactive until intracellular Caincreases.The Ca-binding protein calmodulin binds to Cato form a complex, which binds and activates NOS.In contrast, iNOS is transcriptionally regulated and Ca-independent.Nociceptive stimuli and inflammatory cytokines stimulate the upregulation of iNOS in microglia, astrocytes, macrophages, and other immune response cells,producing a large amount of NO for several hours to several days that can inhibit or kill pathogens.The three NOS isoforms have different distributions in the spinal cord and DRG.nNOS is mainly distributed in the superficial I–III laminae of the spinal dorsal horn and DRG neurons, iNOS is located on glial cells in the spinal cord and DRG, and eNOS is mostly located in the capillary endothelial cells of the spinal cord (Reuss et al., 2001; Yılmaz et al., 2020).
GC can be divided into two categories, particulate guanylyl cyclase (pGC)on the membrane surface, and soluble guanylyl cyclase (sGC) in the cytosol(Friebe et al., 2020).pGC is a large transmembrane molecule with a receptor domain outside the cell and a catalytic site inside the cell.It has seven forms in mammals, GC-A to GC-G.Previous studies have demonstrated that natriuretic peptides bind and activate pGC to regulate submembrane cGMP microdomains.Both atrial (type A) and brain (type B) natriuretic peptides bind to GC-A (natriuretic peptide receptor-A, NPR-A, or NPR1) to modulate the pressure/volume and energy balance of arterial blood.However, c-type natriuretic peptide activates GC-B (NPR-B or NPR2) to maintain vascular integrity (Kuhn, 2016).Vles et al.(2000) reported that atrial natriuretic peptide can activate pGC, leading to cGMP accumulation in GABAergic structures in laminae I–III of rat cervical spinal cord.However, sGC is a cytosolic heterodimeric enzyme comprised of four different subunits, α1, α2,β1, and β2.The two known catalytic isoforms are sGC1 (submit compositionGCα1/GCβ1) and sGC2 (submit composition GCα2/GCβ1) (Koesling et al.,2016).To date, the role of the β2 subunit in the NO/cGMP signaling pathway has not been clearly defined.The sGC1 isoform is mainly located in inhibitory interneurons in the spinal cord, whereas the sGC2 isoform is mainly expressed in satellite glial cells in DRG.It has been demonstrated that sGC1 activation contributes to neuropathic pain, and that sGC2 plays an inhibitory role in inflammatory pain processing (Petersen et al., 2019).
NO binds to a group of prosthetic hemes on activated sGC, then initiates cGMP synthesis.The level of intracellular cGMP is strictly determined by its rate of GC synthesis and the rate of 3′5′-cyclic nucleotide phosphodiesterase(PDE) degradation.Among the PDE families, only PDE5, PDE6, and PDE9 hydrolyze cGMP as specific substrates of inactive 5′-derivatives, and PDE2 can hydrolyze both cAMP and cGMP (de Vente et al., 2006).cGMP can also be produced by NPR-B in DRG neurons.cGMP transmits signals to downstream effector molecules, including PKG, PDEs, and cyclic nucleotide-gated channels(Ghalayini, 2004).
The main effector of cGMP is PKG.PKG has two subtypes, PKG-I and PKG-II.PKG-I is widely distributed in the nervous system, whereas PKG-II is mainly located in serosa, kidney, cerebellum, and mucosal layers (Ding et al., 2017).PKG-I is a homodimeric enzyme with two isoforms, PKG-Iα and PKG-Iβ.Both PKG-Iα and PKG-Iβ are detected in spinal cord and DRG.In DRG PKGIα is mainly expressed in the cytoplasm of small, medium peptidergic, and non peptidergic C-fibers, whereas PKG-Iβ is widely distributed in the nuclei of DRG neurons (Uchida et al., 2018).Both isoforms are also expressed in glutamine synthetase-positive satellite glial cells in DRG.PKG-I evidently mediates presynaptic nociceptive long-term potentiation (LTP) (Luo et al.,2012).Moreover, it can trigger Kopening, resulting in changes in both intracellular and extracellular Kconcentration, leading to depolarization or hyperpolarization inside and outside the membrane (Brito et al., 2006).Cysteine-rich protein 2 (CRP2) is another molecule downstream of PKG-I, and it has an inhibitory effect on inflammatory pain.It has been indicated that NO/cGMP is coordinated such that there is generalized multi-level enhancement rather than a strictly limited step-specific response to noxious stimulation(Schmidtko et al., 2008a; Figure 2).
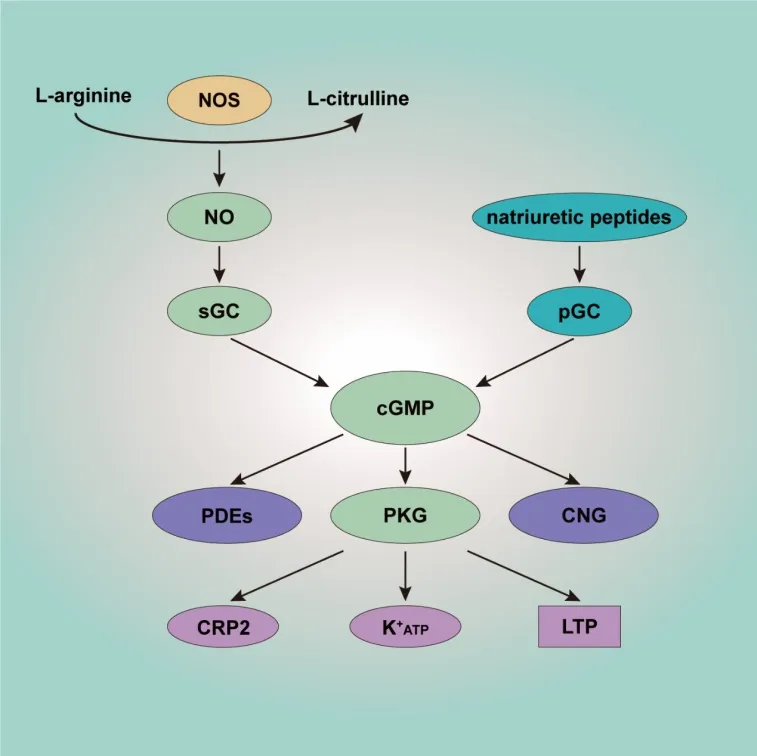
Figure 2| Schematic overview of the NO/cGMP signaling pathway.
NO/cGMP Signaling Pathway and Neuropathic Pain
Neuropathic pain is a chronic condition that is a consequence of nervous system lesions or dysfunction (Zhang et al., 2022).The characteristics of neuropathic pain include enhanced responses to noxious stimuli(hyperalgesia), abnormal responses to non-noxious stimuli (allodynia), and spontaneous pain (Finnerup et al., 2021).Despite multiple studies that have enhanced our knowledge of neuropathic pain by establishing various models of conditions such as peripheral nerve injury, diabetic neuropathy, spinal cord injury, and chemotherapy-induced neuropathic pain, the detailed mechanisms of neuropathic pain remain unclear.A growing body of evidence indicates that activation of the NO/cGMP signaling pathway in the spinal cord contributes to the occurrence and development of neuropathic pain (Figure 3).
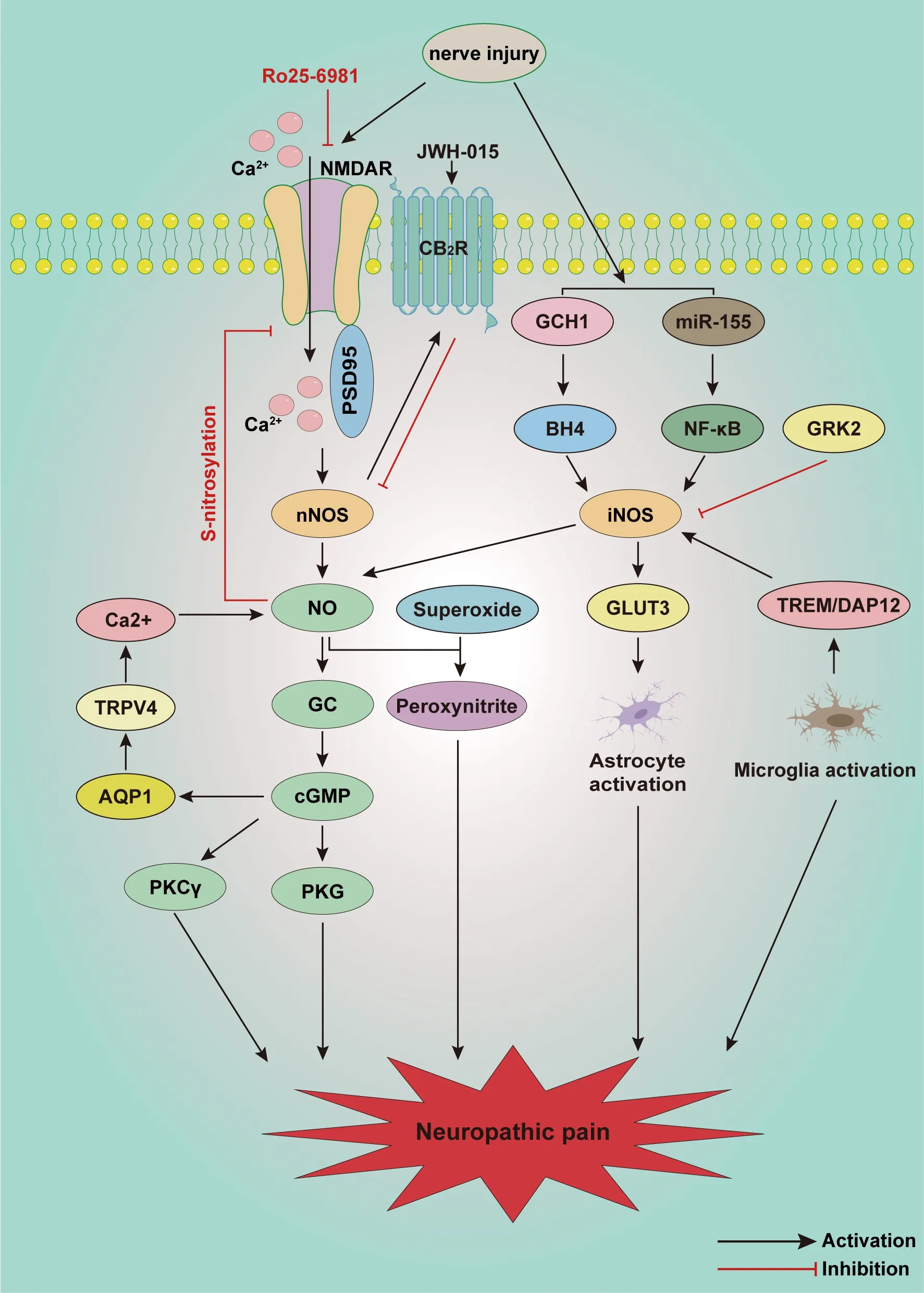
Figure 3 | Schematic illustration of potential mechanisms involved in the NO/cGMP signaling pathway in neuropathic pain.
NO/cGMP Signaling Pathway and Peripheral Nerve Injury
Various studies indicate that the NO/cGMP signaling pathway is activated after peripheral nerve injury.Inoue et al.(1998) first reported that intrathecal injection of a NO-releasing agent promoted the development of thermal hyperalgesia after chronic constriction injury.nNOS and iNOS are expressed in both the spinal cord and DRG, producing NO then activating the NO/cGMP/PKG signaling pathway after nerve injury.It has also been reported that superoxide released from spine can then react with NO to produce peroxynitrite, which appears to mediate neuropathic pain (Tanabe et al.,2009).Therefore NO—which is probably produced via nNOS and iNOS in the spinal cord—promotes the maintenance of neuropathic pain through both the NO/cGMP/PKG pathway and the NO/peroxynitrite pathway.nNOS and iNOS are also closely associated with multiple receptors and signaling pathways.nNOS contributes to δ-opioid receptor downregulation and cannabinoid-2 receptor (CB2R) upregulation in the spinal cord and DRG after chronic constrictive injury (CCI) treatment (Hervera et al., 2010).NMDA, PSD95, and nNOS are also activated in the development of neuropathic pain.PSD95 is the connection between nNOS and NMDA receptors.Disruption of the interaction between nNOS and PSD95 can reverse NMDA-induced thermal hyperalgesia and CCI-induced mechanical allodynia (Florio et al., 2009).However,endogenous NO derived from nNOS decreases the activity of spinal NMDA receptors through S-nitrosylation, attenuating spinal nociceptive transmission and mechanical allodynia in rats that have undergone spinal nerve ligation(SNL) (Chen et al., 2017), indicating negative feedback regulation between nNOS and NMDA receptors.Activation of NMDA receptors in neurons has been shown to induce phosphorylation of JNK in astrocytes through the nNOS-GC pathway in the spinal trigeminal nucleus caudalis, indicating that the nNOS/NO/GC pathway is involved in intercellular communication between neurons and astrocytes in the spinal cord (Lin et al., 2019).Moreover,the expression of NMDA receptors and PKCγ also increases, enhancing neuropathic pain, suggesting that both the activation of NO/cGMP/PKG and the NMDA/PKCγ signaling pathway are involved in neuropathic pain (Zheng et al., 2015).Activation of the GTP cyclohydrolase 1/tetrahydrobiopterin system increases the constitutive expression of iNOS, inducing NO production in the spinal cord of restraint-stressed rats (Huang et al., 2021).iNOS is capable of binding glucose transporter 3, a hexose transporter isoform that is over-expressed in activated astrocytes in the spinal cords of spared nerve injury mice, indicating that iNOS promotes astrocyte activation after spared nerve injury (Giordano et al., 2011).The level of sGC in the spinal cord is greatly increased after sciatic nerve crush.Spinal sGC reportedly regulates neuropathic pain, which is mediated by the NO/cGMP signaling pathway and 5 HT1ARs (Xu et al., 2016).miR-142-5p plays a protective role by targeting the 3′ untranslated region of sGC mRNA, inhibiting sGC protein translation, which further decreases cGMP levels in neuronal cells, alleviating the neuropathic pain caused by Alzheimer’s disease (Xu et al., 2019).
During spinal nociceptive processing, cGMP is generated by the NO/GC system activated in the spinal dorsal horn.However, activation of NPR-B on DRG neurons can also promote cGMP production (Abdelalim et al., 2013).Activation of the cGMP/PKG signaling pathway increases and maintains the hyperexcitability of DRG neurons, with a further decrease in action potential threshold and an increase in repetitive discharge during depolarization.Blocking the cGMP/PKG signaling pathway can markedly reduce the hyperexcitability of DRG neurons, as well as behavior hyperalgesia (Huang et al., 2012b).The level of aquaporin 1 (AQP1), a cGMP-gated channel, is markedly increased in the spinal cord and DRG neurons in rats with chronic compression of the dorsal root ganglia, accompanied by a decrease in the paw withdrawal threshold.Elevated transient receptor potential vanilloid 4 (TRPV4)concentrations in the spinal cord and DRG can be blocked by AQP1 and cGMP inhibitors, suggesting TRPV4 is a downstream mediator of AQP1 (Wei et al., 2020).TRPV4 mediates Cainflux, stimulating NO production, further activating the NO/cGMP/PKG signaling pathway and enhancing hyperalgesia in chronic compression of the dorsal root ganglia (Ding et al., 2010).Oxidantinduced PKG-Iα activation in DRG contributes to the development of CCI-induced neuropathic pain (Lorenz et al., 2014).
NO/cGMP Signaling Pathway and Diabetic Neuropathy
Multiple studies indicate that the NO/cGMP signaling pathway plays an important role in the onset and maintenance of diabetic neuropathy (DN).Hyperglycemia induces the upregulation of nNOS and iNOS but inhibits eNOS activity in the spinal dorsal horns of diabetic rodents (Du et al., 2001;Li et al., 2020).The S-methylisothiourea sulfate and β-lactamase inhibitor clavulanic acid relieves DN by inhibiting the expression of spinal iNOS(Ahlawat et al., 2018; Kolahdouz et al., 2021).iNOS activation secondary to NF-κB elevates the level of NO in the spinal dorsal horn, then triggers the cGMP/PKG signaling pathway.Both iNOS and NF-κB are downstream of miR-155.In one study, spinal miR-155 was upregulated in a rat model of DN, but this could be inhibited by L-arginine and ibuprofen (El-Lithy et al., 2016).Simultaneous injection of nNOS or iNOS inhibitors and CB1R or CB2R agonists has synergistic effects in the alleviation of diabetic hyperalgesia (Bujalska-Zadrożny et al., 2015).The CB2R agonist JWH-015 dose dependently inhibits hypersensitivity induced by diabetes by inhibiting nNOS (Castany et al., 2016).In other studies, activation of the NO/cGMP signaling pathway alleviated DN.Inhalation of hydrogen sulfide gas attenuates DN by activating the NO/cGMP/PKG signaling pathway, and increases the expression of nNOS and PKG-I in the spinal cord (Li et al., 2020).NCX1404, a novel NO donating pregabalin, also exerts anti-allodynia effects in STZ-induced DN (Varani et al., 2016).Taken together, these observations indicate that activation of central and peripheral NO/cGMP signaling has dual effects in DN rats.
NO/cGMP Signaling Pathway and Spinal Cord Injury
It has been estimated that 250,000 to 500,000 people suffer spinal cord injuries worldwide each year (Quadri et al., 2020).Spinal cord injury disrupts communication with the brain, irreversibly damaging motor and sensory functions (Solstrand Dahlberg et al., 2018).Injured spinal cord tissue is extensively deacetylated.Deacetylated proteins also participate in the occurrence and development of spinal cord injury via the cGMP/PKG signaling pathway (Liu et al., 2020).It has been reported that Kcurrent decreases in DRG after spinal nerve ligation.NO activates Kchannels in large DRG neurons via direct S-nitrosylation of cysteine residues in the SUR1 subunit,one of the four subunits of K+ATP channels.The capacity of NO to activate K+ATP channels via this mechanism remains intact after spinal nerve ligation,thus selective pharmacological enhancement of Kcurrent may be another way to relieve neuropathic pain (Kawano et al., 2009).This may also be one of the mechanisms associated with Wu et al.’s (2004) observation that intrathecal administration of NO-releasing drugs alleviates pain induced by spinal cord injury.Moreover, inhibition of PDE5 can increase the level of cGMP, then activate downstream PKG, leading to a reduction in intracellular Calevels.The oral PDE5 inhibitor lodenafil carbonate reverses mechanical and thermal hyperalgesia in the SNL model via activation of cGMP/PKG signaling.Immunofluorescence analysis also indicates that lodenafil reduces GFAP-stained area densities in ipsilateral dorsal horns, suggesting that increased cGMP concentration may be involved in the activation of astrocytes in the SNL model (Vieira et al., 2021).Taken together, these results indicate that the activation of NO/cGMP/PKG signaling has algesic and analgesic effects in multiple models of spinal cord injury.
NO/cGMP Signaling Pathway and Chemotherapy-Induced Peripheral Neuropathy
Chemotherapy-induced peripheral neuropathy is a common side effect of anticancer treatment with vinca alkaloids, platinum drugs, and taxanes (Hu et al., 2019; Zhou et al., 2020).It has been reported that unpleasant pain or paresthesia remains or even intensifies in approximately 30–40% of patients after the termination of chemotherapy, which severely affects quality of life(Staff et al., 2017).Multiple studies have demonstrated the dual role of the NO/cGMP signaling pathway in chemotherapy-induced peripheral neuropathy.L-arginine can increase the mechanical pain threshold in vincristine-induced mice and improve the contents of NO metabolites and cGMP the in the spinal cord (Kamei et al., 2005).Upregulation of nNOS expression is detected in DRG after intraperitoneal injection of vincristine, as is NF-κB activation.As a partial agonist of 5-HT1A and dopamine D2 receptors, aripiprazole can reverse vincristine-induced neuropathic pain by reducing NO levels and inhibiting NF-κB in DRG neurons (Khalilzadeh et al., 2020).NO and peroxynitrite in the spinal cord are involved in oxaliplatin-induced oxidative stress injury of spinal dorsal horn neurons (Azevedo et al., 2013).Spinal NR2B-containing NMDA receptors are evidently involved in oxaliplatin-induced mechanical allodynia (Mihara et al., 2011).NR2B mRNA and protein are increased in the spinal dorsal horn in the late phase after intraperitoneal oxaliplatin injection.nNOS, a downstream target of NMDA receptor, is also activated in oxaliplatininduced neuropathy due to the inhibitory effect of nNOS inhibitor 7-NI on oxaliplatin-induced hyperalgesia.As a histochemical marker of NOS, the intensity of NADPH diaphorase staining also markedly increases in the spinal dorsal horn, and this can be reversed by intrathecal injection of the selective NR2B antagonist Ro25-6981.Furthermore, overexpression of spinal neuronal G protein-coupled receptor kinase 2 can downregulate iNOS levels in cisplatin-induced peripheral neuropathy (Ma et al., 2021).Triggering receptor expressed on myeloid cells 2/DNAX-activating protein of 12 kDa signaling in spinal cord microglia is enhanced by cisplatin, and this is accompanied by iNOS upregulation (Hu et al., 2018).
NO/cGMP Signaling Pathway and Bone Cancer Pain
Advanced prostate, breast, and lung cancers metastasize to the bone causing excruciating pain and severely affecting quality of life (Ge et al., 2019).Studies have shown that the NO/cGMP signaling pathway is involved in tumor proliferation, differentiation, and invasion (Yarla et al., 2019; Zhou et al., 2019; Lv et al., 2020).In this regard, we focused on the key role of NO/cGMP in bone cancer pain (Figure 4).In a mouse model of osteosarcomainduced hyperalgesia, levels of spinal nNOS and iNOS mRNA markedly increase on day 10 after osteosarcoma inoculation.nNOS decreases to normal on day 14 however, whereas iNOS remains at high levels on day 14.Immunohistochemical analysis suggests that nNOS and iNOS are mainly distributed in the superficial dorsal horn and around the central canal of the L3–L5 spinal cord.Intrathecal injection of the NOS inhibitor NG-monomethyl-L-arginine attenuates pain behaviors on day 14, indicating that the upregulated nNOS and iNOS contribute to the development of osteosarcomainduced hyperalgesia (Yang et al., 2016).It has also been demonstrated that iNOS as a pro-inflammatory factor released by M1 microglia is significantly increased in the spinal cord in a bone cancer pain model (Huo et al., 2018).In another study sulforaphane, an isothiocyanate bioactive metabolite in cruciferous vegetables reportedly had anti-inflammatory, anti-oxidant,and anti-cancer effects.Intrathecal injection of sulforaphane reduces the upregulation of iNOS in the spinal cord, and reverses hyperalgesia induced by tumor cell implantation (TCI) (Fu et al., 2021).Moreover, inhibition of NADPH oxidase 4 (NOX4) decreases the production of ROS and the expression of NOS in spinal dorsal horn, indicating that NOS also contributes to oxidative stress in the spinal dorsal horn induced by bone cancer pain (Long et al., 2020).
Mas oncogene-related gene C receptors (MrgCs), Gi protein, NR2B, and nNOS are reportedly upregulated in the spinal cords of mice with bone cancerassociated pain.After intrathecal injection of the MrgC agonist bovine adrenal medulla 8–22, activated MrgC causes the activation of Gi protein and then reduces the expression of spinal NR2B and nNOS, leading to attenuated painbehavior (Sun et al., 2016).Additionally, phosphor-Tyr1472 NR2B, PSD-95, and nNOS are upregulated in the spinal cord after TCI.Intrathecal administration of Myr-NR2B9c can disrupt the connections between NR2B and PSD-95,relieving mechanical and thermal hyperalgesia.Spinal nNOS expression is also decreased after Myr-NR2B9c treatment.Therefore, interaction between NR2B and PSD-95 increases spinal nNOS activity, which is also involved in the process of bone cancer pain (Liu et al., 2014b).Moreover, the chemokine C-C motif receptor 2 antagonist RS102895 reportedly inhibits the expression of NR2B and nNOS in the spinal cord, and exerts analgesic effects during the development of bone cancer pain (Ren et al., 2015).Downstream of NO the cGMP/PKG pathway is upregulated in the spinal cord and DRG in a rat bone cancer model.Liu et al.(2014) reported that cGMP and PKG activity in the spinal cord and DRG were significantly increased after TCI.The cGMP activity in DRG begins to increase on day 4 after TCI, peaks from days 7 to 14,and decreases slightly on day 21.In contrast, PKG activity in DRG increases from day 3 and remains high for 21 days, and increases from days 10 to 14 in the spinal cord.Intrathecal injection of RP-8-pCPT-CGMPS, an inhibitor of the cGMP/PKG pathway, significantly alleviates thermal hyperalgesia and mechanical allodynia after TCI as well as reducing the activity of PKG.The cGMP/PKG pathway can also increase the hyperexcitability of DRG neurons induced by TCI, as evidenced by reduced action potential threshold current and increased repetitive discharges (Liu et al., 2014a).
NO/cGMP Signaling Pathway and Inflammatory Pain
Inflammatory pain is usually caused by an inflammatory response to tissue damage (Conaghan et al., 2019; Sun et al., 2022).Chemical inflammatory mediators released by damaged tissue such as histamine, prostaglandin E2 (PGE2), leukotriene, and cytokines act on nociceptive nerve endings,reducing neuronal excitation thresholds and sensitive afferent discharge rates,leading to the development of hyperalgesia and/or allodynia (Muley et al.,2016).The NO/cGMP signaling pathway reportedly plays a crucial role in the development of inflammatory pain (Figure 5).
Schmidtko et al.(2008a) reported reduced paw licking time in the second phase of the formalin test in GC-KO mice compared with wild-type mice,and the paw withdrawal latency time of GC-KO mice was longer than that of wild-type mice in a zymosan-induced inflammatory pain model.These data showed that sGC contributed to exaggerating sensitivity to inflammatory pain.Petersen et al.(2019) demonstrated that sGC2 primarily contributed to the development of inflammatory pain, whereas sGC1 was primarily involved in neuropathic pain.It has been suggested that the actin-destabilizing protein cofilin is a downstream target of the NO/cGMP/PKG signaling pathway.The PKG-I activator 8-Br-cGMP induces phosphorylation of cofilin in a timedependent manner and markedly increases neurite outgrowth in the spinal cord, which contributes to zymosan-induced inflammation and nociceptivity.The underlying mechanism is mediated by the Rho-GTPases RhoA, Rac1, and Cdc42, and their corresponding downstream targets Rho-kinase and p21-activated kinase.Both NOS inhibitor and Rho-kinase inhibitor could reduce the phosphorylation of cofilin, relieving inflammatory hyperalgesia (Zulauf et al., 2009).Actin reportedly exhibits rapid and maximal S-nitrosylation in the spinal cord in formalin-induced inflammatory pain (Lu et al., 2011).That study suggests that as well as the NO/cGMP signaling pathway, S-nitrosylation is involved in spinal pain transmission through disinhibition of inhibitory neurons.In contrast, targeted activation of the NO/cGMP signaling pathway in the spinal cord plays a protective role in inflammatory pain.Cyclic nucleotidegated channel subunit 3 (CNGA3), a target of NO/cGMP signaling, is increased in the superficial dorsal horn of the spinal cord and DRG after intraplantar injection of zymosan.CNGA3-KO mice exhibited increased inflammatory pain behavior, and also exhibited pain hypersensitivity after intrathecal injection of cGMP analogs or NO donors (Heine et al., 2011).CRP2, another downstream effector of NO/cGMP/PKG-I signaling, also has an analgesic effect during inflammatory pain.It is expressed in laminas I and II of the spinal cord and DRG, and colocalized with PKG.After zymosan or complete Freund’s adjuvant injection into the hind paw, CRP2-deficient mice exhibited increased pain behaviors compared with wild-type mice (Schmidtko et al., 2008b).Taken together, these observations indicate that activation of NO/cGMP/PKG signaling has dual effects during inflammatory pain.
Activation of multiple receptors in the hind paw can further activate the NO/cGMP signaling pathway, suppressing the development of inflammatory pain.Kusuda et al.(2020) investigated the analgesic effect of subcutaneous injection of choline on inflammatory pain, and reported that it acted on 7-nicotinic acetylcholine receptor, further activating the NO/cGMP/Ksignaling pathway and attenuating PGE2-induced hyperalgesia.Notably,choline exerted this analgesic effect without affecting neutrophil migration or inflammatory cytokine production in planter paw tissue.Peripheral activation of A1 adenosine receptors can also activate the NO/cGMP/PKG/Ksignaling pathway, directly blocking PGE2-induced hyperalgesia (Lima et al., 2010).In another study, intraplantar injection of the CB2R agonist N palmitoylethanolamine increased nNOS, inducing NO release in the hind paw, thus activating the NO/cGMP signaling pathway which can markedly reduce the inflammatory pain induced by PGE2 (Romero et al., 2012).Another CB2R agonist JWH-015 exerts peripheral analgesic effects that activate local NO/cGMP/PKG/Ksignaling and increase the transcription of nNOS in DRG(Negrete et al., 2011).Loperamide acts on μ opioid receptors, activating the NO/cGMP/Ksignaling pathway and relieving complete Freund’s adjuvantinduced pain (Curto-Reyes et al., 2008).Cunha et al.(2012) reported that local plantar injection of the κ opioid receptor agonist U50488 could significantly relieve PGE2-induced inflammatory hyperalgesia, and that this effect could be antagonized by selective nNOS inhibitor and PI3Kγ/AKT antagonists.These data indicated that peripheral anti-nociceptive effects of excited κ-opioid receptors were dependent on PI3Kγ/AKT activation, and PI3Kγ/AKT may further activate the nNOS/NO signaling pathway.
NO/cGMP Signaling Pathway and Morphine Tolerance
Among the many pain treatments available, morphine is the most commonly used to treat severe and chronic pain.Long-term use of morphine has undesirable side effects however, including tolerance and hyperalgesia,which hinders the maximization and maintenance of its use for clinical analgesia (Roeckel et al., 2016; Liu et al., 2018).Morphine tolerance refers to diminution of its analgesic effects after prolonged use, and the need for larger doses to achieve the same analgesic effects (Liu et al., 2019).
Morphine primarily acts on μ opioid receptors, not κ and δ opioid receptors,to induce morphine tolerance and opioid-induced hyperalgesia (Zhang et al., 2019).Xu et al.(2014) reported that repeated intrathecal injection of morphine activated the mammalian target of rapamycin (mTOR) in rat spinal dorsal horn neurons, which was triggered through μ-opioid receptors and mediated by intracellular PI3K/Akt.The elevated spinal mTOR activity increased spinal nNOS expression, which is involved in the development of morphine tolerance.It has been demonstrated that the NO/cGMP signaling pathway contributes to the development of morphine tolerance.Ozdemir et al.(2011) reported that intraperitoneal injection of NO independent sGC activators (YC-1 and BAY 41-2272) promoted morphine tolerance, whereas a NOS inhibitor (L-NAME) attenuated it.Similarly, atorvastatin (a competitive inhibitor of 3-hydroxy-methyl-glutaryl coenzyme A reductase) and thalidomide(a glutamic acid derivative) could also alleviate morphine tolerance, and both were inhibited by nNOS, iNOS, and GC inhibitors (Hassanipour et al.,2016).Liang et al.(2004) also found that after morphine exposure multiple sites of NO/cGMP signaling in the spine were upregulated, and that this was involved in morphine tolerance.The iNOS inhibitor aminoguanidine can reportedly block the activation of NMDA receptors, which is followed by reduced NO production in the brain and plasma, leading to reduced morphine tolerance (Abdel-Zaher et al., 2006).Bernstein et al.(1997) reported that intracerebroventricular and intrathecal injection of PKA inhibitor KT5720 or PKG inhibitor KT5823 reduced morphine tolerance.Morphine-3-glucuronide is a major metabolite of morphine that has no analgesic effects but is related to morphine tolerance and hyperalgesia.It can evidently evoke ERK activation following NO/cGMP/PKG signaling in the spinal cord, inducing nociceptive responses (Komatsu et al., 2009).
NO/cGMP signaling contributes to the activation of glial cells in morphinetolerated rodents (Liu et al., 2006).Intrathecal injection of the selective nNOS inhibitor 7NINA attenuates morphine tolerance by reducing the activation of p38 MAPK in microglia.Moreover, morphine activates the μ-opioid receptor-PKCɛ signaling pathway, enhancing IL-1β, TNF-α, IL-6, and NO release in M1 microglia (Merighi et al., 2013).Astrocytes are activated, accompanied by the appearance of stellate soma and hypertrophic processes in chronic morphine-infused rats.The activation of astrocytes could be suppressed by the tricyclic antidepressant amitriptyline, by downregulating the expression of spinal PSD-95/NMDAR NR1/nNOS/PKCγ (Huang et al., 2012a).A collective of evidence indicates that nNOS/NO is involved in the activation of microglia and astrocytes in the process of morphine tolerance.Hence, the NO/cGMP signaling pathway is likely to be a new target for the treatment of chronic morphine tolerance (Figure 6).
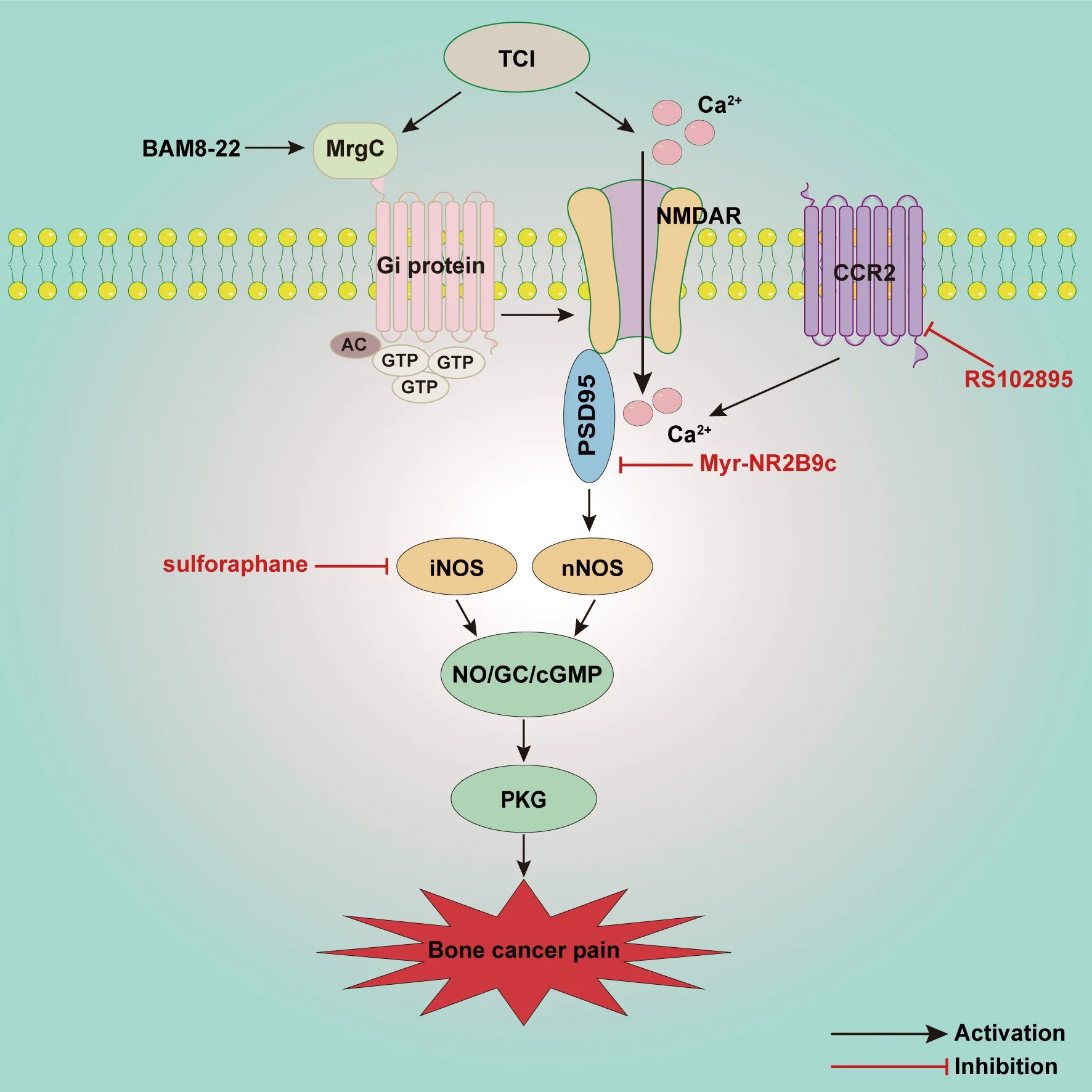
Figure 4 | Schematic illustration of potential mechanisms involved in the NO/cGMP signaling pathway in bone cancer pain.
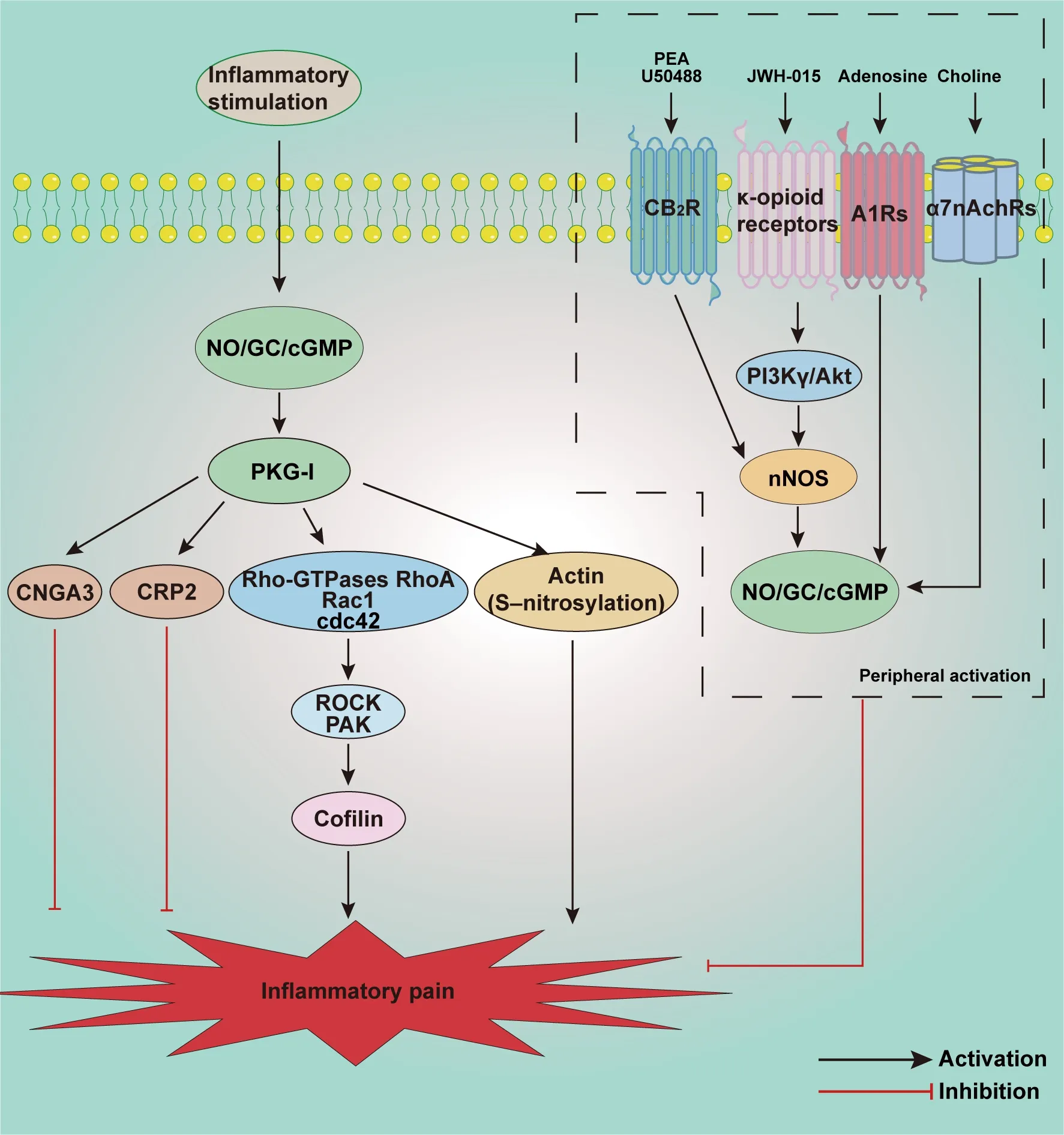
Figure 5 | Schematic illustration of potential mechanisms involved in the NO/cGMP signaling pathway in inflammatory pain.
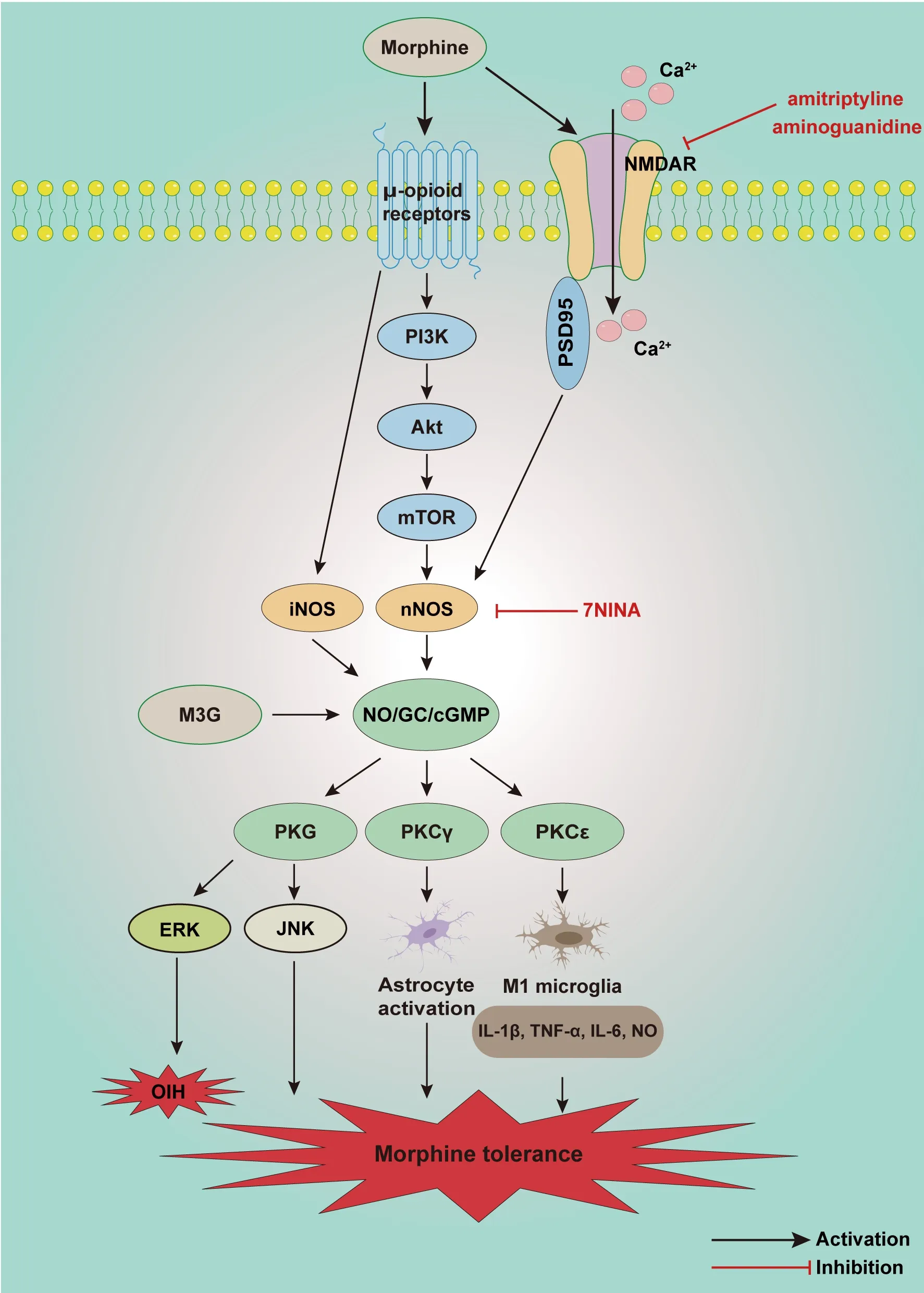
Figure 6 | Schematic illustration of potential mechanisms involved in the NO/cGMP signaling pathway in morphine tolerance and opioid-induced hyperalgesia.
Discussion
Summary and future perspective
In this review we summarized and discussed mechanisms of the NO/cGMP signaling pathway involved in various types of chronic pain.NO/cGMP signaling in spinal dorsal horn and DRG evidently contributes to the development of chronic pain, and also plays an analgesic role by upregulating downstream molecules such as CNGA3 and CRP2.Activation of multiple receptors in localized tissue injuries may activate NO/cGMP signaling however, which can play an effective analgesic role in inflammatory pain.Many effective analgesic methods involving manipulation of NO/cGMP signaling have been described,and they provide new ideas for the treatment of chronic pain (Table 1).Nevertheless, there are still some problems that need to be addressed via further research.Recent studies have mainly focused on the application of NO/cGMP signaling inhibitors to confirm its involvement in chronic pain, but underlying changes in NO/cGMP signaling in the spinal cord and DRG have generally not been thoroughly investigated; which has led to an inadequate understanding of the NO/cGMP signaling pathway.Peripheral changes in NO/cGMP signaling have protective effects on chronic pain, and there is an urgent need to understand the specific mechanisms underlying the NO/cGMP signaling pathway in order to better target drug delivery for pain relief.It has been reported that activated nNOS may be crucial to inducing the spinal LTP of Aδ and C fiber evoked responses after peripheral nerve injury (Bahari et al.,2014).Studies have also shown that in DRG, activated NO in neurons stimulates sGC, promoting cGMP expression in satellite glial cells, which establishes the connection between neurons and satellite glial cells during the development of chronic pain (Belzer et al., 2019).Therefore, the specific mechanism of NO/cGMP signaling in the spinal cord requires comprehensive investigation.

Table 1 | Summary of pharmacological treatments for chronic pain via NO/cGMP signaling pathway
Study limitations and strengths
Unlike previous publications, this review comprehensively summarizes and discusses the role of the NO/cGMP signaling pathway in the spinal cord and DRG by classifying chronic pain into the four most common types.It will assist the investigation of similar neural mechanisms in the development of various types of pain.However, there are some limitations to this review.The search strategy was largely restricted to screening the PubMed database, thus some relevant articles may have been omitted.The search period only extended to December 2021, so no subsequent publications were included in the review.Lastly, because the spinal dorsal horn is the primary center of pain transmission modulation and processing, and primary sensory neurons in the DRG are critical for initiating chronic pain, we mainly reviewed the activation of NO/cGMP signaling in the spinal cord and DRG during pain.There are few studies on the NO/cGMP signaling pathway in the brain region, and they were not included in the current review.
Conclusion and prospects
This review provides possible directions with respect to targeting the NO/cGMP signaling pathway for chronic pain treatment.The dual role of NO/cGMP signaling is an interesting and promising therapeutic target to investigate.Building on previous results, the specific mechanisms involved in the signaling pathway in the spinal cord need to be characterized in more detail.Further investigation of the brain regions related to chronic pain,pain encoding, and pain modulation such as the anterior cingulate cortex(Bliss et al., 2016), dorsolateral prefrontal cortex (de Santana et al., 2013),and amygdala (Neugebauer et al., 2020) is also critical.A previous study has shown that NO release is involved in NMDAR-mediated inhibition of LTP in the hippocampus (Izumi et al., 2008).Moreover, although the therapeutic effects of drugs that act on the NO/cGMP signaling pathway reported in animal studies are inspiring, clinical trials have yielded few results.Several drugs targeting the NO/cGMP signaling pathway have been used in the clinic.For example, linaclotide activates the type I transmembrane receptor guanylate cyclase-C (GC-C), with intrinsic GC activity expressed on mucosal epithelial cells.This results in the release of cGMP, which then acts on and inhibits nociceptors, reducing abdominal pain (Castro et al., 2013).Sildenafil, a PDE5 inhibitor, significantly alleviates acute menstrual pain in patients with primary dysmenorrhea (Dmitrovic et al., 2013).Thus, currently underway preclinicalstudies are expected to translate into clinical trials investigating the protective role of targeting NO/cGMP signaling in chronic pain.In summary, although there are more challenges ahead, the development of treatments for chronic pain based on the NO/cGMP signaling pathway is a promising concept.
Author contributions:
YQZ and WM conceived the idea.DYL, SJG and JS retrieved literature.LQZ, JYW and FHS analyzed the literature and summarized the results.DYL drafted the original draft and drew the figures.YQZ and WM revised the draft and figures.All authors read and approved the final manuscript.
Conflicts of interest:
The authors declare that there are no conflicts of interest.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Patient-specific monocyte-derived microglia as a screening tool for neurodegenerative diseases
- Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases
- Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy
- Neurosteroids as stress modulators and neurotherapeutics: lessons from the retina
- Myelinosome organelles in pathological retinas:ubiquitous presence and dual role in ocular proteostasis maintenance
- Anti-IgLON5 disease: a novel topic beyond neuroimmunology

