Olfactory dysfunction as a common biomarker for neurodegenerative and neuropsychiatric disorders
David Slabik, Olga Garaschuk
The sense of smell supports the identification and the safety of food, warns of danger/predators, and plays a key role in mating (Croy and Hummel, 2017; Kondo et al., 2020; Tzeng et al., 2021).Via connection to the limbic system, it supports behavioral adaption and emotions and can detect fear, tears, and happiness in the body odor of others (Croy and Hummel, 2017;Tzeng et al., 2021).Sensory information is transmitted from the olfactory receptor neurons in the nose via the olfactory bulb (OB) to higher-order olfactory structures including the anterior olfactory nucleus,the piriform and entorhinal cortices, amygdala,hippocampus, orbitofrontal and prefrontal cortices,and nucleus accumbens (Croy and Hummel, 2017;Marin et al., 2018).In the OB, axons from olfactory receptor neurons synapse on the dendrites of OB projection neurons mitral (MCs) and tufted cells in socalled glomeruli (Marin et al., 2018).
Risk factors for olfactory dysfunction (OD) include genetic factors, smoking, alcohol abuse, air pollution,head injuries, exposure to xenobiotics or viruses(e.g.COVID-19), body mass loss, certain kinds of nutrition or medication as well as insulin resistance(Doty, 2012, 2017; Kondo et al., 2020; Tzeng et al.,2021).The most important risk factor is aging, with the incidence of OD increasing at the age of 50–60 years, and more severe dysfunctions being observed in men compared to women (Marin et al., 2018;Murphy, 2019; Kondo et al., 2020; Tzeng et al., 2021).10% of people older than 65 years and 62–80% of those older than 80 years have some form of OD ranging from mild loss to anosmia (Marin et al., 2018;Tzeng et al., 2021).Recent studies have identified OD as an independent risk factor for mortality(Doty, 2012, 2017; Marin et al., 2018; Kondo et al.,2020).Aging is associated with the loss of olfactory receptor neurons as well as reduced regenerative capacity and basal cell proliferation in the olfactory epithelium (Croy and Hummel, 2017; Marin et al.,2018; Kondo et al., 2020).In humans, the OB volume,the thickness of the glomerular layer, the number of glomeruli, and the density of MCs all decrease with age (Kondo et al., 2020).In rodents, there was no age-dependent change in OB volume but the total volume of dendrites and the number of axodendritic synapses in the glomerulus layer decreased with age (Kondo et al., 2020).The olfactory event-related potentials show an age-dependent decrease in the amplitude and processing speed (Kondo et al., 2020).Functional magnetic resonance imaging studies identified the first aging-related OD in higher-order olfactory structures, like the prefrontal, insular and orbitofrontal cortices but not in the primary olfactory(piriform and periamygdaloid) cortex (Marin et al.,2018; Kondo et al., 2020).Low hippocampal volume and entorhinal cortex thickness were also associated with poor odor identification in cognitively normal elderly (Murphy, 2019).
Early OD accompanies many neurodegenerative and neuropsychiatric diseases [i.e., Alzheimer’s disease(AD), frontotemporal dementia, idiopathic rapid eye movement sleep behavior disorder, and idiopathic Parkinson’s disease (iPD); Figure 1].In AD and iPD, OD appears years before the cognitive decline or motor symptoms (Doty, 2017; Marin et al., 2018; Carnemolla et al., 2020; Kondo et al., 2020; Tzeng et al., 2021)and is found in 90% of early-stage iPD patients (Doty,2012; Dan et al., 2021), thus representing a potential clinical marker of early disease stages.Adults with Down syndrome (DS) show significantly poorer odor detection thresholds and odor identification abilities(Figure 1) than age- and cognitively-matched control subjects (Kondo et al., 2020).Odor identification is impaired in all types of schizophrenia (Croy and Hummel, 2017; Carnemolla et al., 2020).Autism,depression, and anorexia are also accompanied by OD, whereas findings in bipolar disorder are controversial (Croy and Hummel, 2017; Carnemolla et al., 2020) and only small deficits in odor identification were found in multiple system atrophy (MSA; Marin et al., 2018).Patients with parkinsonism-associated tauopathies, such as corticobasal degeneration and progressive supranuclear palsy (Figure 1), tend to have a normal olfactory function (Doty, 2012; Fullard et al., 2017; Marin et al., 2018).Thus, olfactory testing might help to differentiate iPD from these tauopathies, as well as from non-degenerative causes of parkinsonism, including drug-induced parkinsonism, vascular parkinsonism, and essential tremor (Fullard et al., 2017).
Odor detection is the ability to detect an odor at a given concentration wherein the lowest detectable concentration is considered the threshold (Dan et al., 2021).Odor threshold sensitivity is affected in AD(Figure 1) and the degree of impairment correlates with the degree of dementia (Murphy, 2019).Furthermore, cognitively normal Apolipoprotein E4(APOE*ɛ4) carriers, who subsequently develop AD,show odor threshold impairment in the year before an AD diagnosis (Murphy, 2019).Poorer detection thresholds are seen in AD compared to mild cognitive impairment (MCI); and in MCI compared to cognitively normal individuals (Murphy, 2019).Altered odor thresholds in AD or MCI (Figure 1) suggest that the peripheral olfactory system is substantially affected(Murphy, 2019).On the other hand, at a younger age and/or in the early stage of the disease, AD patients seem to exhibit rather normal odor detection thresholds and therefore the impairment of odor detection is probably not the earliest symptom of AD(Tzeng et al., 2021).Odor detection is also impaired in iPD patients (Figure 1; Marin et al., 2018).Whereas the overall severity of OD in AD is similar to that in iPD, iPD patients show more severe impairment of detection thresholds (Marin et al., 2018).It has been revealed that olfactory impairment in iPD is unstable and that marked changes in olfactory threshold correlated with more rapid disease progression (Marin et al., 2018).In depression, results concerning odor detection are mixed.A recent systematic review found no odor detection impairment in half of the studies.The remaining half, however, found significantly lower olfactory thresholds in depressed patients compared to controls (Taalman et al., 2017).Here, the olfactory scores strongly correlated with the depression score on the Beck Depression Inventory.After treatment,both significant differences and correlations were gone (Croy and Hummel, 2017; Taalman et al.,2017), making the odor detection threshold a potential biomarker for monitoring the effects of antidepressant therapy.In depression, the OD might also originate from diminished turnover rates of olfactory receptors and this effect is restorable after remission of depression (Croy and Hummel, 2017).
Odor identification (the ability to detect, identify and name an odorant) and discrimination (the ability to discriminate different odorants) require higher brain functions like cognitive and memory processing (Croy and Hummel, 2017; Fullard et al., 2017; Carnemolla et al., 2020; Tzeng et al., 2021).In healthy young adults,odor identification ability correlates with the grey matter volume of several brain regions, extending from the right insular cortex to the right superior temporal gyrus (Carnemolla et al., 2020).Sex, age,cognitive ability, and smoking were significant predictors of odor identification test scores (Kondo et al., 2020).Age-related OD progressed faster in DS than in normal subjects, with significant differences seen already between children and adults (Kondo et al.,2020).In aging, odor identification ability significantly correlates with a decrease in global cognition and episodic memory.In magnetic resonance imaging studies, odor identification scores correlated with the volume of the right amygdala as well as bilateral grey matter volumes of perirhinal and entorhinal cortices, suggesting that the poor odor identification performance is caused by changes in central olfactory processing (Kondo et al., 2020; Dan et al., 2021).In cognitively normal elderly, worse odor identification was associated with increased cortical amyloid and neurofibrillary pathology in the entorhinal cortex and hippocampus (Marin et al., 2018).Odor identification is impaired in ~85–90% of patients with AD (Figure 1; Murphy, 2019), and the degree of impairment correlates significantly with the loss of grey matter in the right entorhinal cortex and right parahippocampal gyrus (Carnemolla et al., 2020).Ofnote, odor identification and discrimination are more severely impaired than odor detection and low odor identification scores have been associated with higher levels of AD pathology in central olfactory structures(Marin et al., 2018; Kondo et al., 2020; Tzeng et al.,2021).In non-demented elderly, low olfactory test scores aligned with future cognitive decline and an increased probability of AD diagnosis during the next 2–5 years (Doty, 2017).In APOE*ɛ4 carriers,impairment of odor identification occurred before the impairment of cognition; and in patients with AD, left hippocampal volume was highly correlated with odor identification performance (Murphy, 2019).One study showed a correlation between poorer odor identification scores and the ratio between the total level of tau (or tau phosphorylated at threonine 181) to the level of Aβin the cerebrospinal fluid(Murphy, 2019).In iPD, deficits in odor identification are essentially similar to that of early-stage AD and are found in 90% of early-stage patients (Doty, 2012).Odor identification is more frequently impaired than odor discrimination and has higher sensitivity and specificity in distinguishing iPD patients from controls(Figure 1; Dan et al., 2021).The risk to convert to iPD was higher in subjects, who had problems with odor identification, a significantly reduced thresholddiscrimination-identification score, and/or idiopathic rapid eye movement sleep behavior disorder diagnosis (Dan et al., 2021).Moreover, in a recent study a combination of odor identification testing and dopamine transporter imaging predicted the conversion from prodromal to full-blown iPD within 4 years in 67% of cases (Dan et al., 2021).Another study showed that hyposmia is associated with a stable 4-fold risk of iPD (Mahlknecht et al., 2020).Of note, this risk remained stable for up to 10 years of follow-up, whereas other iPD-associated risk factors,like substantia nigra hyperechogenicity on transcranial sonography, declined within the first 5 years(Mahlknecht et al., 2020).However, with a predictive accuracy of 64%, hyposmia cannot be used as a stayalone clinical marker.Likely, a combination of several markers (e.g., hyposmia, dopamine transporter imaging, and substantia nigra hyperechogenicity)is required.iPD-induced impairment of olfaction is sex-specific, with males having significantly stronger deficits than females (Dan et al., 2021).Asymptomatic individuals having both a first-degree relative with iPD and an olfactory deficit have at least a 10% chance of developing clinically defined iPD (Doty, 2012).Mild deficits in odor identification have been found in PSP and MSA (Figure 1; Marin et al., 2018).For all subtypes of frontotemporal dementia, patients showed severely impaired odor identification but preserved odor discrimination (Carnemolla et al., 2020).In contrast, both odor identification and discrimination were impaired in all types of schizophrenia, with the degree of impairment depending on age and duration of illness, as well as in some types of depression (Croy and Hummel, 2017;Taalman et al., 2017; Carnemolla et al., 2020).
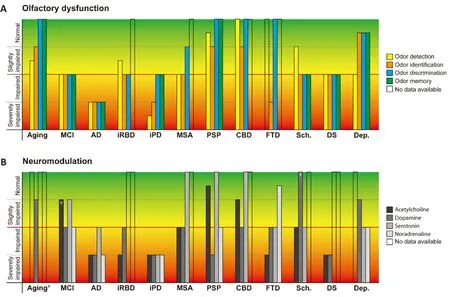
Figure 1 | Characteristics of OD in aging and different neurodegenerative and neuropsychiatric disorders along with the respective impairments of the neuromodulatory systems.
Data on odor memory in the aforementioned diseases are sparse.Processing of tasks that combine odor naming with odor memory involves the hippocampus and seems to lateralize to the left hemisphere(Murphy, 2019).Early AD-related neuropathological changes in the entorhinal cortex disrupt the flow of olfactory information to the hippocampus and are expected to affect odor memory and odor identification performance (Murphy, 2019).Indeed,odor recognition memory was profoundly impaired in so-called questionable (a term previously used for MCI) and full-blown AD, particularly in APOE*ɛ4 carriers (Murphy, 2019).In APOE*ɛ4 carriers, deficits in odor recognition memory developed early during disease progression, with males showing greater memory impairment than females (Murphy, 2019).In iPD, a few studies have noted associations between olfactory test scores and measures of verbal learning,memory, or executive function (Doty, 2012).Patients with schizophrenia performed worse in all odor tests and their odor discrimination deficits might arise from the additional demands on already depleted attentional and working memory capacities(Carnemolla et al., 2020).
Neuromodulation in OD:
Mechanistically, OD might be caused by a dysfunction of the signal processing pathway (see above) or neuromodulatory (e.g.,cholinergic, adrenergic, or serotonergic) inputs.Indeed, 68–77% of cholinergic cells in the nucleus basalis of Meynert are lost in AD, 50–77% in iPD,and 24–50% in young adults with DS (Figure 1; Doty,2012).Interestingly, a small study has found an improvement in odor identification abilities in some AD patients after treatment with the cholinesterase inhibitor Donepezil (Marin et al., 2018; Murphy,2019).In contrast, little loss of cholinergic cells occurs in diseases with minor smell loss (e.g., multiple sclerosis or PSP).The loss of dopaminergic cells in the ventral tegmental area is considerable in AD (40–60%),middle-aged adults with DS (53%), and iPD (39–65%;Figure 1).As for OB neurons, iPD patients show a loss of MCs, but not dopaminergic periglomerular neurons, and the OD is not altered by levodopa or dopamine agonist therapy (Doty, 2012, 2017; Fullard et al., 2017; Marin et al., 2018).In those neurodegenerative diseases for which data are available, the relative damage to the locus coeruleus (LC), the main source of noradrenergic projections to the OB, is similar to that seen for the nucleus basalis of Meynert (Doty, 2017).There is early impairment of LC in AD (Murphy, 2019), and in iPD, the loss of noradrenergic neurons in LC is even larger than the loss of dopaminergic neurons in the substantia nigra (Figure 1; Doty, 2012).In post-mortem studies, 70% of iPD patients exhibited an α-synuclein pathology in LC, 40% in the anterior olfactory nucleus, and 50% in OB (Doty, 2012).In rats, it has been shown that a pharmacological blockade of α- and β-adrenergic receptors disrupted the initial learning of an odor discrimination task,but had no effect after the task was learned (Doty,2017).A depletion of bulbar noradrenaline by 6-hydroxydopamine also did not alter a learned odor discrimination task, and in patients with dopamine β-hydroxylase (the enzyme converting dopamine to noradrenaline) deficiency the smell function was intact (Doty, 2012, 2017).
Serotonin (5-HT) is intimately associated with brain circuits related to smell function (Doty, 2012).For instance, activation of 5-HT fibers originating in the raphe nuclei can alter the activity of MCs, the main projection neurons of the OB (Doty, 2012;Marin et al., 2018).Serotonin affects the activity of dopamine, glutamate, and gamma-aminobutyric acid and, depending on the 5-HT receptor subtype,regulates the release of dopamine from nerve terminals (Doty, 2012).Serotonergic signaling plays an important role in the formation of short-term memory and associative conditioning in olfaction(Fomin-Thunemann and Garaschuk, 2021) and deafferentation of 5-HT projecting fibers to the OB produces anosmia in rats (Doty, 2012).In PD,depletion of 5-HT in the OB, entorhinal and frontal cortices, hippocampus, and thalamus is accompanied by accumulation of Lewy bodies and loss of 5-HT-positive neurons in the raphe nuclei.A similar loss of 5-HT-positive neurons is found in AD but not in PSP or MSA (Figure 1; Doty, 2012).
In summary, dysfunction of the neuromodulatory pathways, such as cholinergic and, to a lesser extent,dopaminergic, adrenergic, and serotoninergic OB inputs, might represent a common mechanism for OD in neurodegenerative and neuropsychiatric disorders(Figure 1).Detailed knowledge about the role of each of these modulatory systems in olfaction, as well as their disease-specific dysfunctions, is needed to facilitate the understanding, prevention, and/or future treatment of these devastating diseases.
We thank A.Fallgatter (Eberhard Karls University of Tübingen) for comments on the manuscript.
This work was supported by the DFG grant GA 654/14-1 to OG and the PATE program of the Faculty of Medicine, Eberhard Karls University of Tübingen to DS.
David Slabik, Olga Garaschuk
Department of Psychiatry and Psychotherapy,Tübingen Center for Mental Health, Eberhard Karls University of Tübingen, Tübingen, Germany(Slabik D)
Institute of Physiology, Department Neurophysiology, Eberhard Karls University of Tübingen, Tübingen, Germany (Garaschuk O)
*Correspondence to:
Olga Garaschuk, PhD,olga.garaschuk@uni-tuebingen.de.https://orcid.org/0000-0001-7400-5654(Olga Garaschuk)
Date of submission:
May 20, 2022Date of decision:
June 29, 2022Date of acceptance:
July 21, 2022Date of web publication:
October 10, 2022https://doi.org/10.4103/1673-5374.355756
How to cite this article:
Slabik D, Garaschuk O(2023) Olfactory dysfunction as a common biomarker for neurodegenerative and neuropsychiatric disorders.Neural Regen Res 18(5):1029-1030.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Concepció Marin, Institut d’Investigacions Biomediques August Pi i Sunyer,Spain; C.Vicario-Abejón, Consejo Superior de Investigaciones Cientificas (CSIC), Spain.
Additional file:
Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Patient-specific monocyte-derived microglia as a screening tool for neurodegenerative diseases
- Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases
- Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy
- Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain
- Neurosteroids as stress modulators and neurotherapeutics: lessons from the retina
- Myelinosome organelles in pathological retinas:ubiquitous presence and dual role in ocular proteostasis maintenance

