Countering microplastics pollution with photocatalysis: Challenge and prospects
Runjing Xu, Lifeng Cui, Shifei Kng,c,*
a School of Environment and Architecture, University of Shanghai for Science and Technology, Shanghai, 200093, China
b College of Smart Energy, Shanghai Jiao Tong University, Shanghai, 200240, China
c Institute of Photochemistry and Photofunctional Materials (IPPM), University of Shanghai for Science and Technology, Shanghai, 200093, China
Keywords:Microplastics Photocatalytic degradation Photocatalysts Bond break mechanism
A B S T R A C T
1.Introduction
Environmental pollution and the lack of clean air and water are serious problems facing the world.Moreover, the fast growth of the global population and energy consumption and rapid increase of plastic production(more than 90%of the world's total production)are results of industrial expansion[1-3].Continuous release of harmful pollutants and waste into the environment leads to major environmental problems,such as global warming,and water and air pollution[4-6].Plastics production and use are expected to further expand in the coming years.Currently there are more than three hundred kinds of plastics are produced, of which more than sixty are popular in daily life.Since the middle of the last century,millions of tons of plastics and plastic-based materials have been produced, with an annual output over 200 million tons, and the plastic industry is continued to grow [7,8].Plastics featured by high durability, inertness and relative cheapness have replaced most of the natural polymers used in daily lives in the early days [9,10].In 2018,enormous amounts of microplastics were discovered in the Marianas Trench, which is the deepest place on Earth, and in 2020, microplastic pollution was discovered near the peak of Mount Everest, the tallest mountain on Earth.Apparently, microplastics have contaminated the whole world.To control microplastic contamination,not only the sources of microplastics but also the transport in the environment and possible solutions shall be investigated [11-13].Plastics are ubiquitous not only in water bodies and lands,but also in the atmosphere,and can travel by wind.Microplastic pollution was even found in remote areas because wind can spread microplastic particles over long distances.Moreover,the microplastics concentration is much higher in densely-populated areas[14,15].
Currently, plastics have been widely used in packaging, housing,construction, automotive, electrics, electronics, agriculture, residential furniture,appliances,sports,health,safety,and other fields.Plastics are organic polymers produced by adding various plasticizers, additives,flame retardants and other organic chemicals to improve their properties[16,17].For example, bisphenol A in plastics, and the polybrominated diphenyl ethers added to textiles are highly bio toxic organic chemicals that can persist in the environment and accumulate through food chains,affecting the growth and development of living organisms [18].The existence of microplastics in the ecological system has become the spotlight of environmental protection all over the world.Microplastics have been distributed throughout the oceans, terrestrial soil and atmosphere, which has become another major environmental issue after"white pollution".Microplastics can release organic toxins and be used as carriers for organic and metal toxins which may accumulate in living organisms,affecting biodiversity through food chains[14].Microplastics are further broken down into plastic nanoparticles after ingestion by organisms,affecting excretion,metabolism,digestion and reproduction.Ingestion of a certain amount of microplastics will cause cell death,allergic reactions and damage to the cell wall.Irregular-shaped microplastics are significantly more likely to induce cell death than their spherical counterparts.The effects of microplastics on health in humans are uncertain because it is unknown how long microplastics remain in the body before being excreted[19].These are not the only hidden dangers of microplastics.Microplastics can adhere to the external membranes of red blood cells and stretch them, significantly decreasing their mechanical stability and impacting the functional oxygen delivery of red blood cells [20,21].
There have been many studies on plastic diversification,performance improvement and degradation since the first synthesis of polystyrenebased plastics in 1839 [9].The number of studies on microplastics has increased exponentially in the past decade, and the main research directions are environmental science, ecology, occupational health, and toxicology.More than half of these directions can be reflected in Fig.1(a).Due to their excellent properties and wide range of applications, the use of plastics has increased nearly 200 times in the last half century[22,23].Such increased use of plastics has led to more research on microplastic degradation.Fig.1(b) illustrates the recent research progress on photocatalytic microplastic degradation.Clearly, many advances have been made in synthesis of photocatalysts, application conditions of photocatalysts[24,25],and evaluation methods of microplastic degradation.The previously reported photocatalysts can effectively degrade microplastics under different conditions and have excellent catalytic stability, examples including such as the noble metal doped TiO2[26,27],metal oxides,and graphite materials[28-30].
Most of the microplastics in daily life are transported through and removed by municipal wastewater treatment plants (WWTPs), but WWTPs do not offer a complete disposal process for removing microplastics [31].When sludge carrying microplastics is used as fertilizer or landfill, the microplastics will pollute natural water bodies during stormwater runoff or due to the disposal of treated wastewater with some remained microplastics to natural water bodies.During their long-term retention in sewage and solids, microplastics leak out or take up many poisonous compounds that may be harmful to natural ecosystems.[3,32].Photocatalytic degradation of microplastics has many advantages: (1)relatively mild reaction conditions and strong oxidizing capability [24,33]; (2) relatively “clean” as it that does not introduce other harmful substances into the original system, avoiding secondary pollution [34];(3)thorough degradation of microplastics into CO2 and H2O;(4)natural sunlight can be used as energy source at large scale with low cost[35-37].
To the best of our knowledge,there are few review papers focusing on modified photocatalysts for the degradation of various kinds plastics.This work aims to comprehensively overview the environmental and ecological impacts of microplastics and the research progress on the degradation of different types of microplastics using various photocatalysts.This review highlights the main mechanisms of microplastic degradation on chemically and structurally modified metal oxides photocatalysts.In addition, this review summarizes and analyzes the photocatalytic degradation of different types of microplastics,aiming to find out the best photocatalysts for each kind of plastic.This work may help researchers to screen for great photocatalysts that have fast degradation rate and low cost of application,and to find novel photocatalysts for new hard-to-degrade pollutants that will emerge in the future.
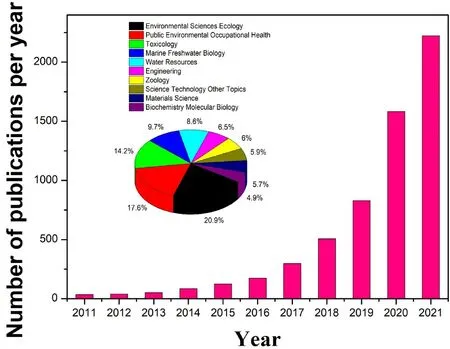
Fig.1a.Published papers in the field of microplastics in recent ten years (data from Web of Science on 1 January 2022).
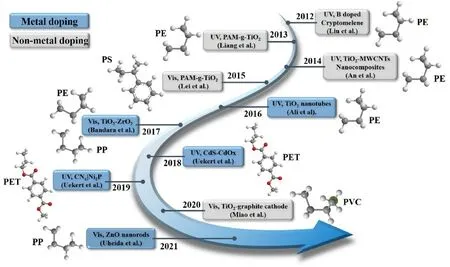
Fig.1b.Research progress of photocatalytic degradation of different types of microplastics (reproduced with permission from reference).
2.Environmental and ecological impacts of microplastics and coping strategies
2.1.Origin and disposal of microplastics in marine and freshwater ecosystems
Because of light weight and persistence,microplastics allow for longdistance transport and accumulation across most ocean environmental matrices and freshwater lakes, covering from urban beaches to sediments, and to distant polar and central regions of oceans[38,39].More than 90% of the microplastics have accumulated in the sediments, and resedimented grains have been observed in smaller plastic fragments in the sand and rocky coastal areas of the intertidal zone[40].Microplastics are present in most trophic groups of marine organisms,and are detected most commonly in the digestive tract.However,growing evidence shows that nanoparticles possibly cross biological obstacles and aggregate in the organs and tissues of multicellular biota[41,42].
Microplastics can affect the nutritional status of food intake and weight loss in fishes.Some fish research demonstrates that modifications in the gut are associated with the introduction of microplastics, such as monomers, polymers and other chemicals that may migrate from microplastics into the environment or transport directly into the ingested organism[43-46].Sources and transport of microplastics in marine and freshwater ecosystems are shown in Fig.2.
Sewage treatment systems are regarded as an essential component of the multifaceted approach required to decrease quantities of microplastic debris arriving in the ocean environment.An increasing amount of crosscountry policy initiatives demand recognition of the requirements for studying and evolving better sewage treatment systems and facilitating and embracing innovation to build economically viable technological approaches [47,48].WWTPs already collect a significant percentage of microplastic grains from wastewater,but because of the large quantity of wastewater,the treated effluent still considerably contributes to aquatic microplastic pollution.In addition, the particles liberated from the treated effluent tend to be relatively small and include a high percentage of fiber, which is harmful to planktonic species and life stages at the bottom of the aquatic food web compared to larger particles [49-51].Finally, it should be emphasized that improving wastewater treatment processes is part of a multifaceted approach to address marine microplastic contamination, and thus environmental microplastic pollution.Part of any effort must be a broader approach to reduce plastic consumption, uncontrolled levels of plastic releases and the amount of plastic waste,and to better manage plastic waste by improving recovery and recycling approaches to the economy[52-54].
2.2.Sources and disposal of microplastics in soils and on land
Microplastics are a major problem for not only the aquatic environment but also soil and terrestrial ecosystems.The excessive use of plastics has resulted in potentially harmful contaminants in the environment,as 20-42% of all global plastic is deposited on terrestrial land and will be biodegraded very slowly [55].Although soils are considered to be the main route of microplastic transportation, the biotoxicological determination and disposal of microplastics in soils are neglected[55,56].Recent investigations show that low density polyethylene (LDPE) and polypropylene(PP)utilized for covering are the main sources of microplastics in agricultural soils.Other sources include sewage irrigation, sludge composting, garbage and suburban runoff [57,58].In addition, the landfill of solid waste and the unscheduled disposal of mud fertilizer are the main transmitters of microplastic contaminants.LDPE is decomposed as agricultural mulch and called "white pollution"because it lacks color and is present in large quantities in surface and subsurface soils[59,60].Sources and transport of microplastics on land and in the atmosphere are shown in Fig.1.
Although microplastics can persist in the environment for hundreds of years owing to their specific structural properties, the ability of soil microorganisms to degrade microplastics cannot be ignored.Microplastic degradation by specific microorganisms has attracted wide scientific attention, and may be a good option to mitigate the growing threat of microplastic pollution [61,62].LDPE has long been considered as non-biodegradable and marketed.Bacteria extracted from earthworm guts are highly corrosive to LDPE, and the bacterial community can significantly reduce the particle size of LDPE, providing a promising prospect for developing the LDPE-contaminated soil remediation technology[63].In addition,bacteria isolated from mangrove sediments potentially degrade microplastics.This strategy is very important and the selection of the most suitable degradation strategy requires the optimization of competent microorganisms on top of specific pollutants.Likewise,chemical treatments can be avoided in the microplastic degradation if the optimal use of microbial species produces positive results.The use of microorganisms is also considered to be environmentally safe[64].
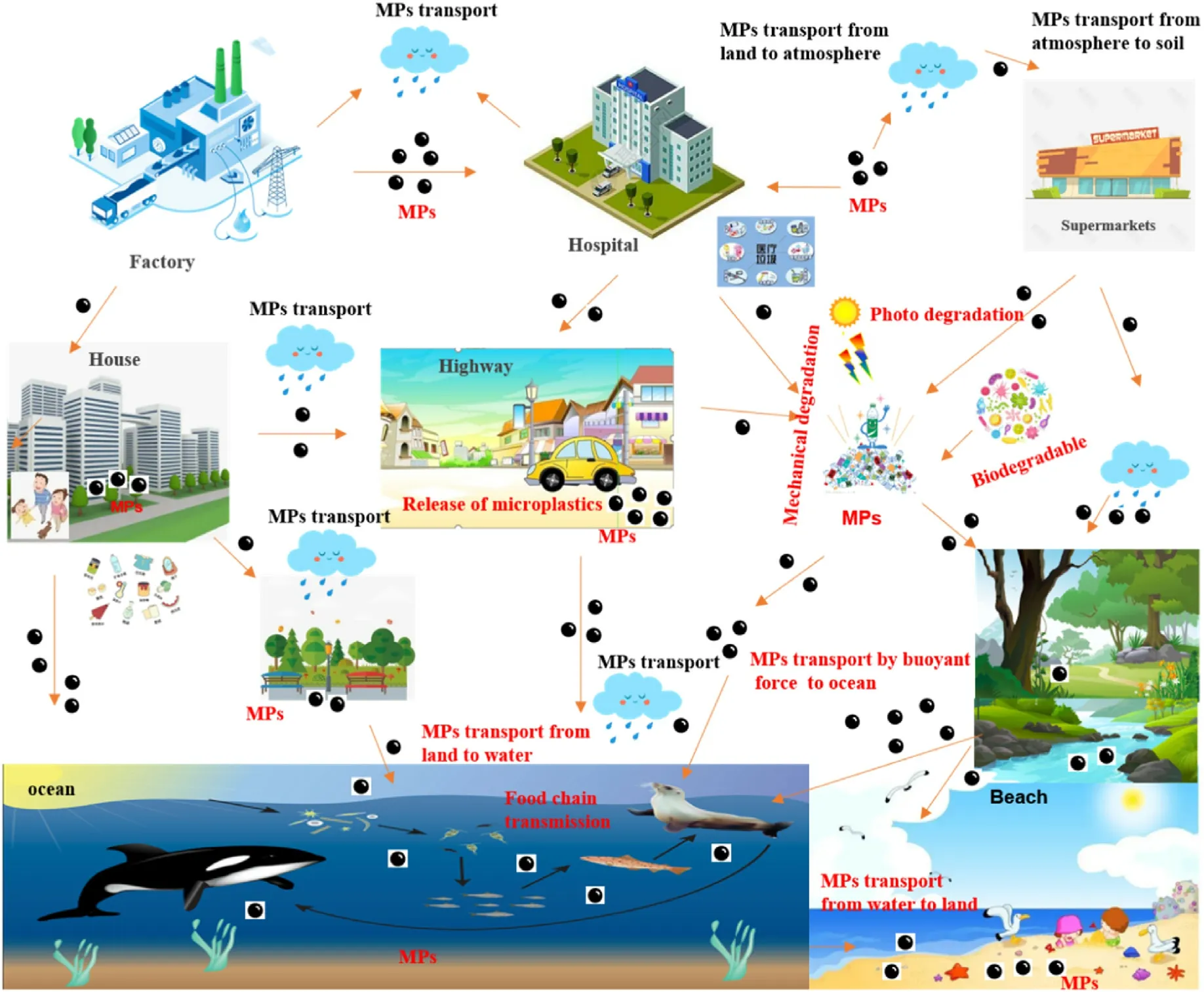
Fig.2.Possible sources and transport routes of microplastics in the environment.
2.3.Strategies for dealing with microplastics
Although there are many sources of microplastics, most come from synthetic materials.Sources of microplastics may be cosmetics and consumer care products, or result from the decomposition or wear and tear of other plastic products, such as fabrics, tires, asphalt and street signage coatings.Owing to their low density and tiny particle size,microplastics are often released into municipal domestic sewage systems.Consequently, municipal WWTPs play an important role in de-treating microplastics in domestic wastewater.Municipal WWTPs in microplastics treatment mainly include the following structures:filter network,primary or secondary sedimentation tank,aeration tank,chlorine contact tank, sand filter tank, sewage sludge, and aerobic tank [65,66].The corresponding structures mainly act as sedimentation, flotation, rapid sand filtration and moderate adjustment of the amount of oxygen required by microorganisms to degrade microplastics [67].
Plastics are the most common organic synthetic polymeric materials used in daily life,and are widely used owing to low prices,light weights,and ease of use.There are many types of plastics, which include polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC), polystyrene (PS), and PET.Given the grim reality of microplastic pollution,corresponding prevention and control measures are proposed.In addition to the above key role played by various ministries of WWTPs in urban domestic wastewater treatment systems, common methods for treating microplastics are shown in Fig.3,including microbial degradation, photocatalytic degradation, advanced oxidation process, thermal degradation, and other physical methods.Research on photocatalytic degradation of microplastics has been slowly appearing in major journals in recent years,and this new technology is widely recognized as a radical advance because of its feasibility and nontoxicity.Photocatalytic degradation of microplastics involves the excitation of electrons in the valence band when the photon energy of light irradiated on the photocatalyst is higher than its band gap,and they jump from the top of the valence band(VB)to the bottom of the conduction band(CB),leaving holes(h+)in the valence band, resulting in electron-hole separation.Then, the photogenerated carriers will be transferred to the catalyst surface for a series of reactions.In the photocatalytic degradation of plastics,the h+generated by photocatalysis can directly oxidize the plastic.On the other hand,some radicals generated by the reaction of e- and h+with O2or H2O,such as hydroxyl radicals and superoxide anion radicals, can also continue to degrade plastics to produce small molecules of organic matter or eventually to CO2and H2O.This new technology is truly a radical advance because of its feasibility and nontoxicity.Research proves that addressing worldwide microplastic contamination with cleaner technologies is a step in this direction[68].

Fig.3.An overview of the strategies for dealing with microplastics.
3.Photocatalytic degradation of microplastics
The widespread testing and build-up of microplastics in the landscape in recent years has raised significant concerns worldwide about environmental safety and long-term ecological impacts.In this segment, we summarize the current developments in removal and transformation technologies for various microplastics and the latest applications of photocatalytic approaches of microplastic degradation and utilization[69,70].The physicochemical properties of various photocatalysts [71,72], such as catalytic performance and stability, were studied to make them suitable candidates for plastic degradation, and the possible mechanisms of photocatalytic degradation of various plastics were also proposed.Photocatalytic degradation of microplastics is divided into two mechanisms: direct degradation and indirect degradation.The indirect mechanism, as the main mechanism, is separated into five steps: excitation,water ionization,superoxide radical,superoxide protonation,and overall reaction.Direct degradation is weaker than indirect degradation[73].At the same time, we try to propose the role played by doping different metal materials in the catalytic process, and point out the development trend of future photocatalyst research.Finally, combining with the previous studies, we reasonably summarize different types of microplastic degradation pathways.
3.1.Research status of photocatalytic degradation of PVC microplastics
Because of excellent material, morphological and chemical properties,PVC and its associated polymers have been used in various industrial applications.PVC products are non-biodegradable,and can be degraded only by a few ways, given their high production volumes [74].Photocatalytic degradation activated by titanium dioxide nanoparticles (TiO2NPs) is considered an attractive and economical solution for the clarification of water and air systems[75].
PVC is less stable for photodegradation because of its high sensitivity to UV light.Overall, light leads to dichlorination, producing HCl and C--C,which tend to degrade under light irradiation and break down the molecular backbone into smaller molecules.PVC is not easily biodegradable because of the presence of halogens,typically chlorine,and its dichlorination during photodegradation facilitates biodegradation [76].
The mechanism of TiO2photocatalytic degradation of plastics is that TiO2will form electron-hole pairs(e-/h+)on the material surface under photon excitation at certain wavelengths, where the holes interact with the surface H2O of TiO2to form strongly oxidizing hydroxyl radicals[77,78].Moreover, the electrons interact with the surface O2adsorbed by TiO2to form strongly oxidizing negative oxygen ion radicals, which oxidize and eventually degrade the microplastic organics[79].TiO2is an ideal photocatalyst carrier for the whole photocatalysis, and when inserted with metal,the electron gain and loss of the metal promotes the whole photocatalytic process [80].Thus, inserting metal into the photocatalyst is a more ideal way to enhance the photocatalytic efficiency(Fig.4(a)).The direct and indirect mechanisms of plastic degradation are presented in Fig.4(b).Under visible light, TiO2also degrades plastics directly, which involves the excitation of plastics from the ground state upon the incidence of visible light photons.The indirect mechanism of plastic degradation is superior over the direct mechanism, and plastic decomposition is more pronounced than the visible-light-induced reaction.In addition, the visible-light-induced reaction is much slower than the UV-based reaction.Polyaniline-modified TiO2is an efficient solid-phase photocatalyst for PVC degradation.In the photocatalytic degradation process,nanoscale TiO2is excited by UV light irradiation to generate electron-hole pairs, which in turn can produce key reactive substances for the degradation of the polymer matrix.Meanwhile, TiO2particles can significantly aggregate to reduce the polymer-TiO2interface area as well as the light penetrating depth into the composite film.Moreover, conducting polymers such as polyaniline can improve the photocatalytic properties of TiO2and optimize the allocation of modified TiO2nanoparticles in polymer matrix[81].
When catalysts such as TiO2are doped with noble metals, the photocatalytic degrading efficiency of microplastics can be significantly improved [82].The Ag/TiO2nanocomposite degrades microplastics much better than TiO2alone,but the Ag nanoparticles doped on TiO2do not provide sufficient topological changes on the catalyst surface,and the Ag/TiO2has a fairly homogeneous morphology [83].Thus, silver can work best as a dopant because the shape of the doped catalyst surface is not significantly modified and no other aggregates affect the photocatalytic performance of TiO2.However, the introduction of a high concentration of silver can improve the amount of silver deposited on the surface of the TiO2photocatalyst.This makes the surface of Ag/TiO2composite slowly turn gray as the silver content increases [83].In addition, a large load of silver can act as a barrier against the TiO2activity to absorb photon energy from the photocatalytic process and can be a complex center.
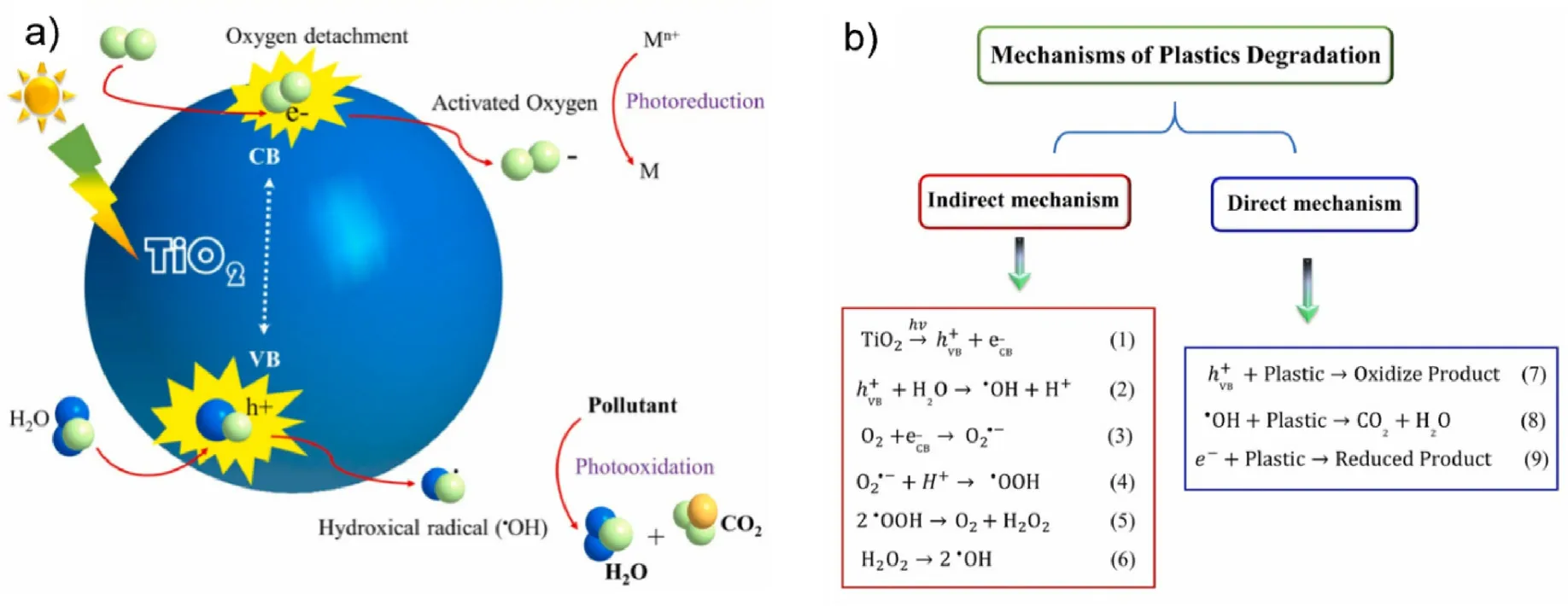
Fig.4.(a) Possible photocatalytic mechanism of TiO2 and intercalated metal ions under illumination; (b) overall photocatalytic mechanism of TiO2 in decomposing plastics under light, (reproduced with permission from Ref.[86], copyright 2021 Elsevier).
In previous studies,calculations using the Kubelka-Munk equation for 1% Ag/TiO2composites indicate that the doping of silver reduces the band gap width to 3.02 eV (3.19 eV in pure TiO2) with an optimum wavelength of 411 nm (381 nm in pure TiO2) [83].Thus, Ag/TiO2nanocomposites are active under both UV and visible light irradiation.Silver as a dopant is more dominant in capturing electrons at lower Fermi energy level and higher energy work functions than TiO2.This electron capture process significantly decelerates the electron-hole complexation rate,leading to a stronger photocatalytic reaction[84].
When the doped Fe elements enter the catalyst, the photocatalytic degradation of PVC is significantly improved.In the photocatalytic degradation of PVC-(FePcCl16-TiO2), superoxide radicals and hydroxyl radicals are two important reactive oxygen species, which attack the adjacent polymer chains to form -CH2CCl- and -CHCHCl-radicals, leading to photocatalytic degradation.Successive reactions in the photocatalytic process produce hydroxyl derivatives and carbonyl intermediates, which break the chain to form carbon dioxide.At the interface of TiO2and FePcCl16is hole injection into the ground-state FePcCl16, effectively separating electrons and holes, while the doped catalyst effectively extends light absorption to the visible light region.All these advantages above largely improve the photocatalytic degradation of PVC[85].In addition,many other photocatalysts have been developed to degrade PVC microplastics(Table 1).
3.2.Research status of photocatalytic degradation of PE and PP microplastics
Polyethylene (PE) and polypropylene (PP), two very undegradable plastics,are used in large quantities,and their wastes are very harmful to ecosystems.Currently, there are more research methods, such as photodegradable plastics, biodegradable plastics, landfill and incineration, high-temperature catalytic thermal cracking, and reuse as fillers(e.g.chemical wood, sample bricks), which are limited in application because of high costs,complicated process,and secondary pollution.PE and PP are vulnerable to light-induced advanced oxidative degradation,which generally occurs in three steps: initiation, propagation and termination.PE and PP are not affected by photo-oxidation at the starting stage, because the main chain does not contain unsaturated colorforming groups that absorb light energy, but a small number of external impurities or some abnormal groups combined into themacromolecular structure can allow light to trigger degradation to certain degree[87].Hydroxyl radicals are formed when light causes the C-H on the main chain of the microplastic polymer to be opened.In the propagation step, hydroxyl radicals react with oxygen to form peroxyl radicals, while a number of complex radical reactions lead to autoxidation and ultimately to chain breakage [88].In the termination step, the free-radical reaction is ended when the two radicals combine into an inert product.

Table 1 Photocatalytic degradation of PVC-type microplastics.
After transition metals(e.g.Cu)are doped into the catalyst to degrade plastics, the photocatalytic effect is significantly improved.In the photocatalytic degradation of PE(TiO2/Cu-Pc),not only superoxide radicals and hydroxyl radicals play an important role, but also cavities are generated in the Cu-Pc ground-state.Although the oxidation capacity of Cu-Pc ground-state pores is lower than the failure band of TiO2, they energetically participate in the oxidation of PE polymers.TiO2/Cu-Pc in PE plastics can only be partially excited by solar UV light.TiO2/Cu-Pc photocatalysts have higher charge separation efficiency, so more active oxygen is produced on the film surface and inside the film,which makes TiO2/Cu-Pc photocatalysts mineralize faster and more completely than TiO2photocatalysts [89].When the noble metal Pt is incorporated into the catalyst, we find better results in photocatalytic degradation of microplastics, especially low-density polyethylene (LDPE)films.
In the photocatalytic degradation of LDPE/(ZnO-Pt), the plasma absorption in Pt nanoparticles enhances the visible light absorption of the catalyst, which in turn leads to the diffusion of photoelectrons from the ZnO nanointerface into the Pt nanoparticles, resulting in a reduction in the number of electron-hole complexes and thus enhancing photocatalytic efficiency.As a result, the visible-light-driven photocatalytic performance is improved more than 15%,and the oxidation potential of the modified ZnO-Pt catalyst for LDPE films increases by about 13%due to the combined effect of the above plasma catalysts[90].ZnO nanorods exhibit relatively strong UV absorption due to their intrinsic semiconductor properties, but weak visible light absorption (Fig.5(a)).However, the visible light absorption is significantly enhanced after depositing Pt nanoparticles on the surface of ZnO nanoblocks because of the surface plasmon resonance effect of Pt nanoparticles at 385 mm.Specifically,the presence of the deposited noble metal reduces the band gap energy, and the adsorption threshold of the composite catalyst is extended to the visible light region.At 362 nm,the interband excitation of ZnO decreases, which may be due to the increased defect density within the structure of ZnO nanorods [91,92].Fig.5(b) shows that the photodegradation efficiency is improved in the presence of ZnO nanorods containing Pt nanoparticles.On average, the carbonyl and vinyl indices increase by 13%and 15%respectively compared to the unmodified ZnO nanorods,indicating the degradation efficiency of the ZnO-Pt plasma photocatalyst is significantly improved.At the ZnO-Pt interfacial energy band,Pt nanoparticles function as electron traps to decrease the exciton complexation rate, which will contribute to the photodegradation reaction(Fig.5(c)).Researchers have designed a generalized light-induced C-C bond cleavage and coupling pathway for converting waste plastics (PE, PP, etc.) into C2 fuels with relatively high energy density under conditions modeling various natural environments.(Fig.6(a)).Researchers Jiao et al.synthesized Nb2O5atomic layers,which showed a significant increase in the photodegradation rate of PE and PP within different time periods.In addition,the researchers found that the photodegradation of PE to CH3COOH actually undergoes the following two consecutive carbon-carbon bond breaking and linkage stages.Fig.6 (b) and (c) show well these 2 steps:(1) Step 1 is mainly a C-C bond breaking process: the plastic is oxidized and mineralized to CO2by ROS(·OH)resulting from the valence bands of O2and Nb2O5;(2)Step 2 is mainly a carbon-carbon bond conjugation process: the formation of CO2is catalyzed by light-induced electrons in the Nb2O5conduction band and reduced to CH3COOH through the coupling of·COOH intermediates [93].Therefore, it is suggested that the carbon-carbon bond breaking and conjugation processes can be optimized when enhancing photodegradable PE as well as PP microplastics.In addition,there are many other photocatalysts for the degradation of PE microplastics(Table 2).
3.3.Research status of photocatalytic degradation of PS microplastics
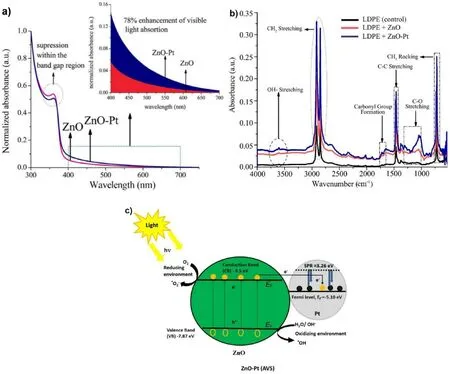
Fig.5.(a)UV-vis absorption spectra of ZnO nanorods and Pt nanoparticles deposited on ZnO nanorods;(b)Emission spectra of ZnO nanorods and ZnO-Pt under 320 nm excitation; (c) Schematic diagram of the mechanism of ZnO-Pt enhanced photocatalytic activity (reproduced with permission from Ref.[94], copyright 2019 MDPI).
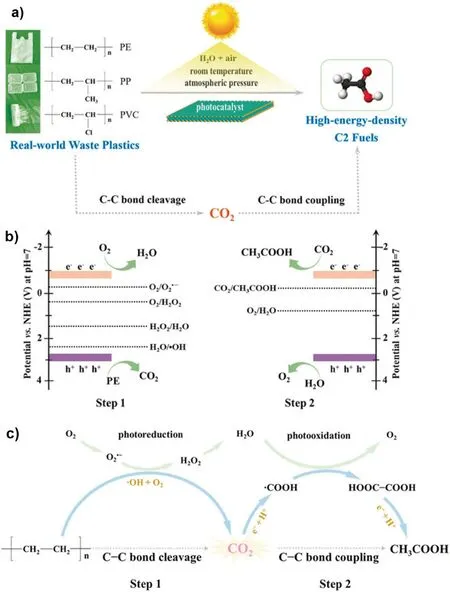
Fig.6.(a)Schematic diagram of the conversion of various waste plastics into C2 fuel under simulated natural environmental conditions;(b)Schematic diagram of the band edge positions of Nb2O5 atomic layers and the potentials of CO2,H2O,H2O2 and O2 redox couples;(c)C-C bond breaking and coupling mechanism of pure PE to CH3COOH under simulated natural environmental conditions (reproduced with permission from Ref.[93], copyright 2020 Wiley.
Polystyrene (PS) has low cost, high quality, low heat transfer coefficient, excellent mechanical properties, easy coloring, acid and alkali resistance, moisture and shock resistance, high stability, and good transparency.It is mainly used in outer packaging, heat insulation,moisture-proof, shock-absorbing materials and quick meal industries.Many polystyrene plastics are discarded after single use, and become serious "white pollution" because of undegradability.Polystyrene, a plastic with high light transmission, can transmit all wavelengths of visible light, and its compounding with a photocatalyst can effectively enhance the light absorption effect of the photocatalyst.When polystyrene is exposed to UV light, the benzene ring tends to be less stable,and this energy is transferred to the adjacent carbon-hydrogen bond,leading to hydrogen cleavage and the formation of polymer radicals.[95]studied the photocatalytic degradation of polystyrene plastics in ambientair was under UV light irradiation using TiO2as a photocatalyst, and found the PS-TiO2composite showed more weight loss,higher intensity of carbonyl peaks and more CO2release compared with pure polystyrene[95].
Table2PhotocatalyticdegradationofPE-typeandPP-typemicroplastics.
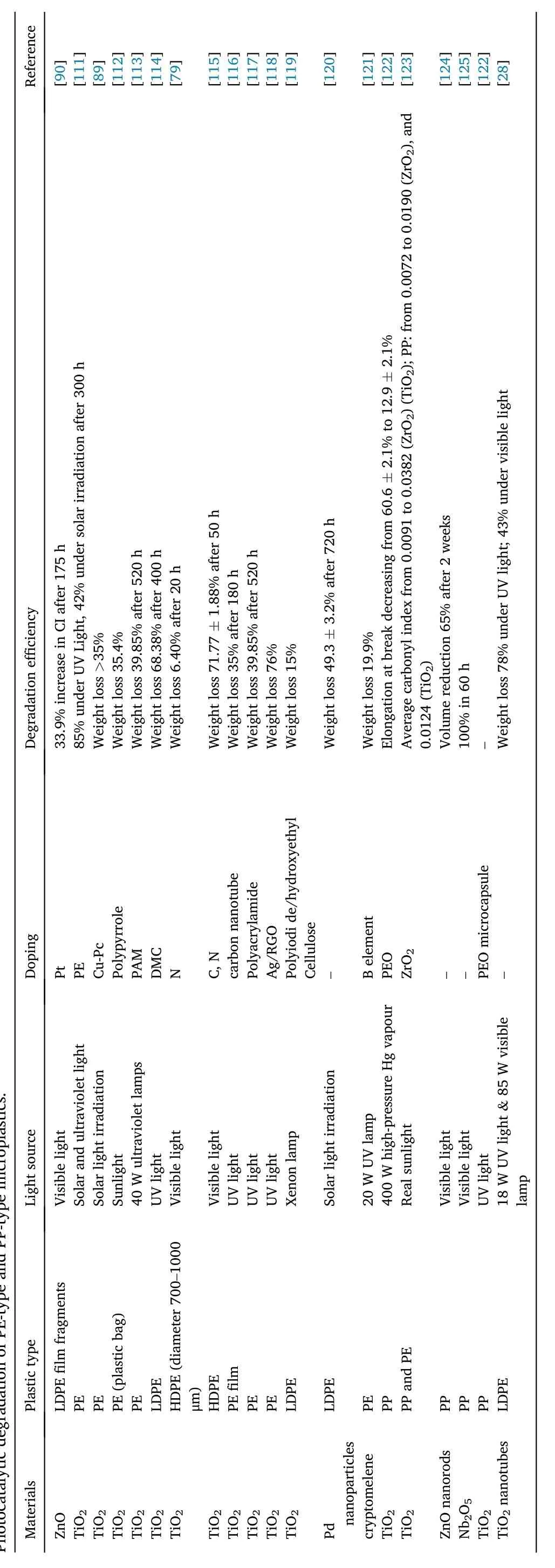
Reference[90][111][89][112][113][114][79][115][116][117][118][119][120][121][122][123][124][125][122][28]under solarirradiationafter300h 2.1%to 12.9 ±2.1%Degradation efficiency 33.9% increase in CI after175h 85% underUV Light, 42%3.2%after720h Weightloss >35%Weightloss 35.4%Weightloss 39.85% after520h Weightloss 68.38% after400h Weightloss 6.40%after20 h Weightloss 71.77±1.88%after50 h after 180h under UV light;43%undervisiblelight Weightloss 35%Weightloss 39.85% after520h Weightloss 76%Weightloss 15%Weightloss 49.3 ±Weightloss 19.9%Elongationat breakdecreasing from 60.6 ±Average carbonyl indexfrom 0.0091 to 0.0382(ZrO2)(TiO2);PP:from 0.0072 to 0.0190(ZrO2),and 0.0124(TiO2)Volumereduction65%after2weeks 100%in 60 h-Weightloss 78%Doping Pt PE Cu-Pc Polypyrrole PAM DMC N C, N carbonnanotube Polyacrylamide Ag/RGO Polyiodide/hydroxyethyl Cellulose-B element PEO ZrO2--PEO microcapsule-Visible light Light source Solar andultravioletlight Solar lightirradiation Sunlight 40Wultravioletlamps UVlight Visible light Visible light UVlight UVlight UVlight Xenon lamp Solar lightirradiation 20WUV lamp 400 Whigh-pressureHg vapour Realsunlight Visible light Visible light UVlight 18WUV light&85 Wvisible lamp 000 Plastic type LDPEfilmfragments PE PE PE(plasticbag)PE LDPE HDPE(diameter700 1-μm)HDPE PEfilm PE PE LDPE LDPE PE PP PPandPE PP PP PP LDPE Materials ZnO TiO2 TiO2 TiO2 TiO2 TiO2 TiO2 TiO2 TiO2 TiO2 TiO2 TiO2 Pdnanoparticles cryptomelene TiO2 TiO2 ZnO nanorods Nb2O5 TiO2 TiO2nanotubes
When doped with copper, the polystyrene degradation by a CuPcsensitized TiO2photocatalyst (TiO2/CuPc) under light radiation is well improved in efficiency compared to TiO2alone due to the light expansion to the visible light range[96].When transition metal irons are inserted,the light absorption is extended again to the visible light region,enhancing the photocatalytic efficiency.The FePc-TiO2nanoparticles were uniformly distributed in polystyrene, while the untreated TiO2particles are embedded in the polymer and aggregate to form flocs of several microns.Consequently, the presence of aggregates reduces the polymer and photocatalyst interfacial area, resulting in lower photocatalytic degradation efficiency.The formation of a bulk heterojunction between the composite and polystyrene polymer chains results in high stability and homogeneous distribution in the TiO2-FePc composite[97].The mass change curve of the polymer film under UV irradiation is shown in Fig.7(a) and (b).Among the three films, the weight loss rate of the PS-FePc-TiO2film is the highest, increases steadily with the irradiation time and reaches 65%and 82%after irradiation at 1 and 2 mW/cm2UV for 480 h, respectively.Under the same experimental conditions, the degradation rates of polystyrene films and PS-TiO2films are much lower than those of polymeric films.The mechanism studied by the authors is that successive reactions in the photocatalytic process produce free radical precursors and carbonyl intermediates that lead to carbon chain breakage and carbon dioxide release from plastic polymers.Therefore,O2and H2O on the catalyst surface play a key role in the reaction process.Nanoscale TiO2absorbs light with energy above 3.2 eV and generates electrons and holes in the conduction band(CB)and valence band(VB),respectively.Electrons can combine with oxygen molecules to form superoxide radicals,while light-generated electrons combine with water on the catalyst surface to form hydroxyl radicals[97].
The results of the researcher Nabi et al.can be shown:where the PS particle size, photodegradation efficiency and content of inorganic carbon during the photocatalytic reaction are shown in Fig.7 (c)(d),respectively.On(without catalyst)FTO,the particle size variation of 400 nm PS was small,while on(Water-based TiO2)WT films,(Ethanol-based TiO2) ET and (Triton X-100 based TiO2) TXT films showed a clear reduction of PS particle size and the photodegradation efficiency of PS increased with the increase of light exposure time.The highest degradation efficiency was observed for TXT after 12 h of UV irradiation.TXT showed higher performance due to its relatively low band energy and excellent charge separation.TXT produced more electron-hole pairs under illumination and broadened the charge separation, thus significantly increasing the rate of degradation of PS.The photodegradation efficiency of PS was observed to increase with increasing irradiation time.During the photodegradation process, PS is separated into many kinds of monomers,and the results of the separated m/z from Fig.7 (e)can be found in styrene,benzene,and toluene,which generate CO2and H2O with further photodegradation[75].
Reportedly, a new photodegradable polystyrene composite was prepared by linking aromatic polyamide dendrimers with TiO2to form HADPG-TiO2, which was embedded in polystyrene.HADPG-TiO2has better dispersion and visible light absorption ability in the polystyrene polymer and has better tensile properties.HADPG-TiO2forms a donoracceptor type p-conjugated complex on the surface of TiO2, which is easily excited by sunlight [98].From Fig.7 (f) we can see that: Since PS-HADPG-TiO2films have a substantial amount of amine groups on the surface of the HADPG-TiO2composite photocatalyst, however, these active substances can also combine with amine groups to form well-stabilized nitrogen-oxygen radicals, which then combine with R*radicals and ROO* radicals to form carbonyl and ether compounds that are not easily decomposed and relatively stable.During the irradiation of PS-HADPG-TiO2 films by visible light for 250 h,a large amount of amine groups react in combination with reactive radicals.Therefore,PS-HADPG-TiO2films exhibited strong photodegradability under visible light irradiation.After the PS-HADPG-TiO2films were irradiated by sunlight for 250 h,the reacting amine groups no longer combined easily with oxygen radicals.Meanwhile, the formed carbonyl and ether compounds can easily absorb UV light and act as light collecting molecules for HADPG-TiO2.As a consequence,more aggressive oxygen free radicals readily occur to attack neighboring PS chains,resulting in the formation of carbon-centered free radicals and carbonyl intermediates [98].SEM and AFM after 90 h of light radiation confirm the irradiated PS/BiOCl composite films have better absorption properties than the unirradiated films.The tensile strength clearly greatly declines after degradation[99].In addition, many photocatalyst degradation processes of polystyrene microplastics are listed in Table 3.
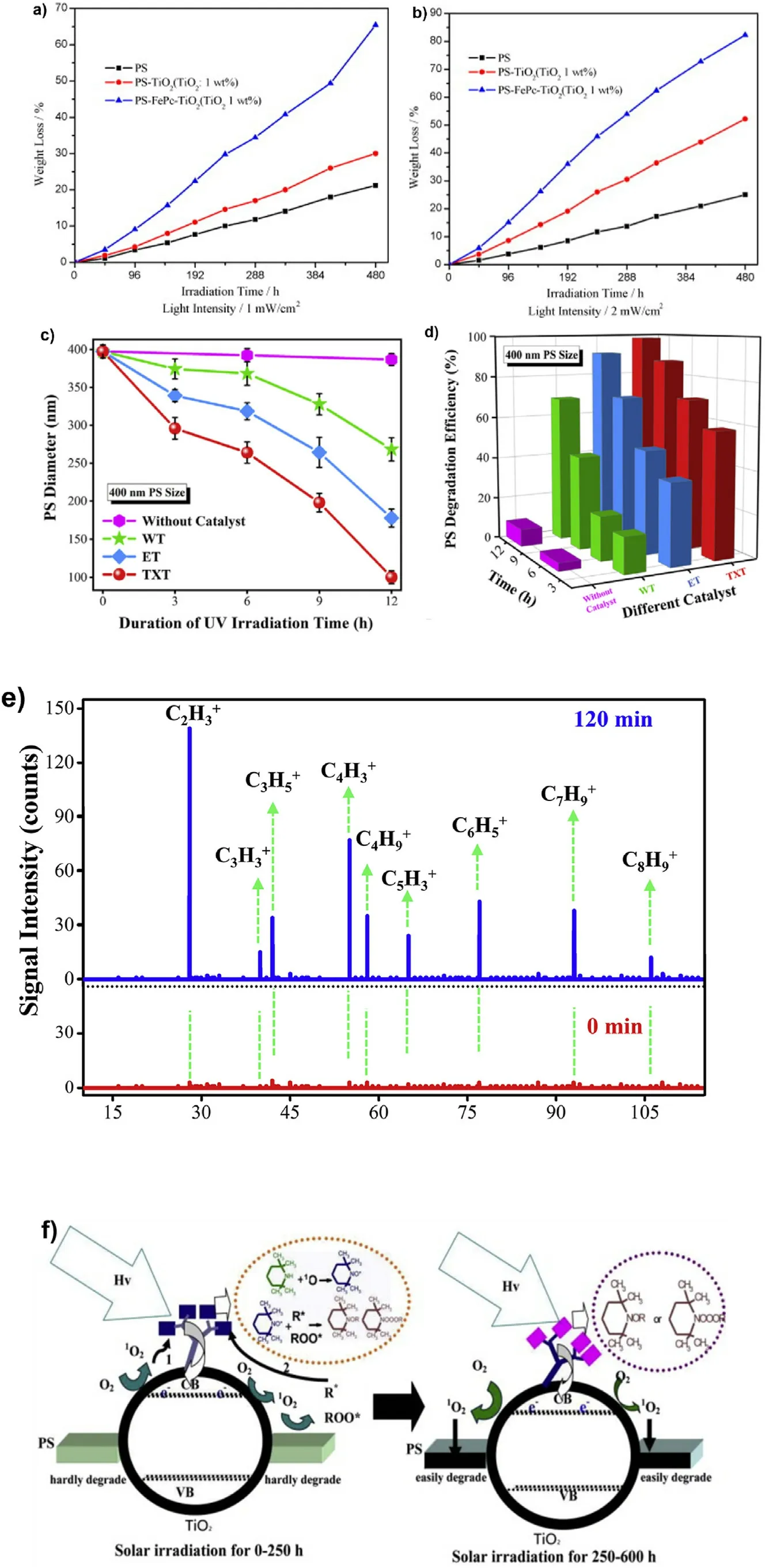
Fig.7.(a) and (b) Weight loss of pure and composite films under UV irradiation of different light intensities(reproduced with permission from Ref.[97],copyright 2008 Elsevier);(c)Diameter change of 400-nm PS on TXT, ET, WT films, and without catalyst under different irradiation time intervals;(d)PS degradation efficiency with (TXT, ET, WT) and without catalyst; (e) Mass spectra obtained by high-pressure photon ionization (HPPI)-TOFMS during the photodegradation of PS(reproduced with permission from Ref.[75], copyright 2020 Elsevier); (f) Proposed photodegradation mechanism of the PS-HADPG-TiO2 films (reproduced with permission from Ref.[29], copyright 2015 Elsevier).
3.4.Research status of photocatalytic degradation of PET microplastics
PET, an odorless and non-toxic semi-crystalline linear thermoplastic polyester with excellent performance and high transparency, can maintain excellent physical and mechanical properties in a wide temperature range.As one major member of packaging wastes, PET can be applied extensively.However,its waste is undegradable in the nature,which will cause severe white pollution.Under marine conditions, photocatalysis and photo-oxidative hydrolysis are important pathways for PET degradation.Photocatalytic degradation induces the breakage of the ester bond in the polymer, which may lead to the direct formation of carboxylic acid end groups and vinyl end groups,or to the formation of free radicals, which are similar to the changes in carbon-carbon backbone plastic polymers (PP, PS, PE, PVC).PET can proceed to photo-induced autoxidation by free radical reaction[87].
When transition metal Cd is used to photodegrade PET, the degradation effect is obvious.Efficient photocatalysis using cheap CdS/CdOx dopants in alkaline aqueous solutions can effectively hydrolyze PET plastics to terephthalate,ethylene glycol,and isophthalic acid esters,and photocatalytically oxidize PET to formate, ethanoate, ethanol, acetate,and lactate.PET degradation occurs mainly through photocatalytic oxidation and hydrolysis processes, and hydrolysis also results in a decrease in polymer molecular weight and an increase in carboxylic acid end groups.Thus,light-induced free radical reactions and PET hydrolysis can form carboxylic acid radicals[100].
An economical, clean carbon nitride/nickel phosphide (CNx|Ni2P)photocatalyst with superior performance in degrading PET when nickelbased catalysts are used.The role of CNx|Ni2P is due to the relatively intense combination of Ni2P catalyst with CNx, which facilitates charge separation and improves the capacity and reliability of photocatalytic degradation.The optimal Ni2P loading is 2 wt% because at less Ni2P loading there is too little co-catalyst available for electron extraction,and parasitic light blocks in higher loadings for further improvement[101].Under solar irradiation, the photocatalyst makes the organic substrate and water produce H2under light radiation conditions.the substrate acts as an electron donor and the excited photocatalyst produces more active substances to degrade the substrate into other organic molecules.(Fig.8(a)).Then, photo-generated electrons are moved from the photocatalyst to the co-catalyst which will reduction of water to H2.The catalyst has a significant effect on PET plastic and polyester microfiber under the light condition (Fig.8(b)) [101].In addition, many photocatalyst degradation processes of PET microplastics are listed in Table 3.
3.5.Possible pathways for photocatalytic degradation of different microplastics
In recent years,polymeric materials have been developed rapidly and are widely available,but when these polymeric materials are exposure to
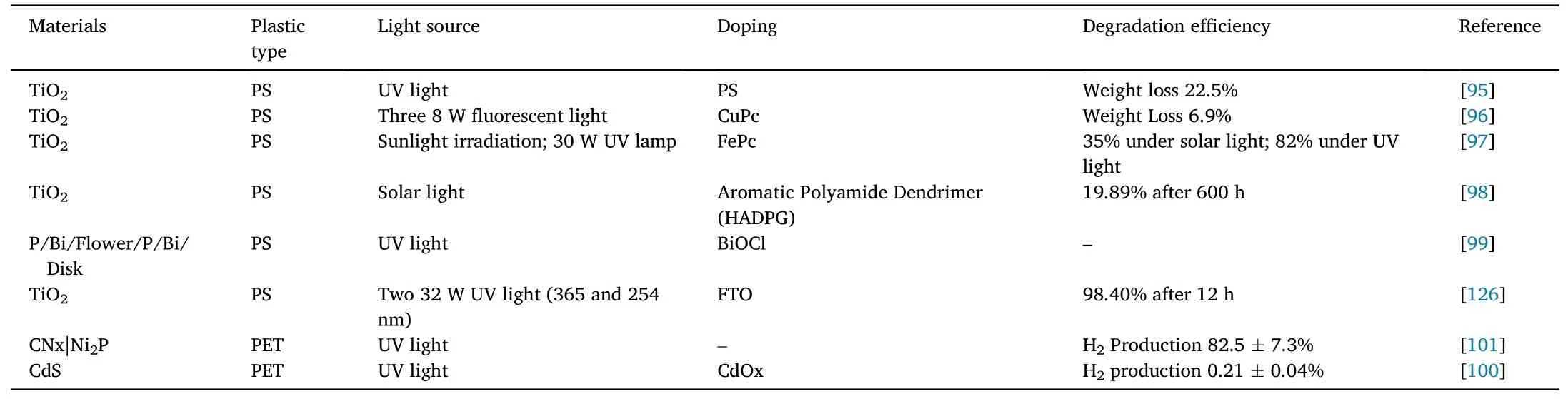
Table 3 Photocatalytic degradation of PS-type and PET-type microplastics.
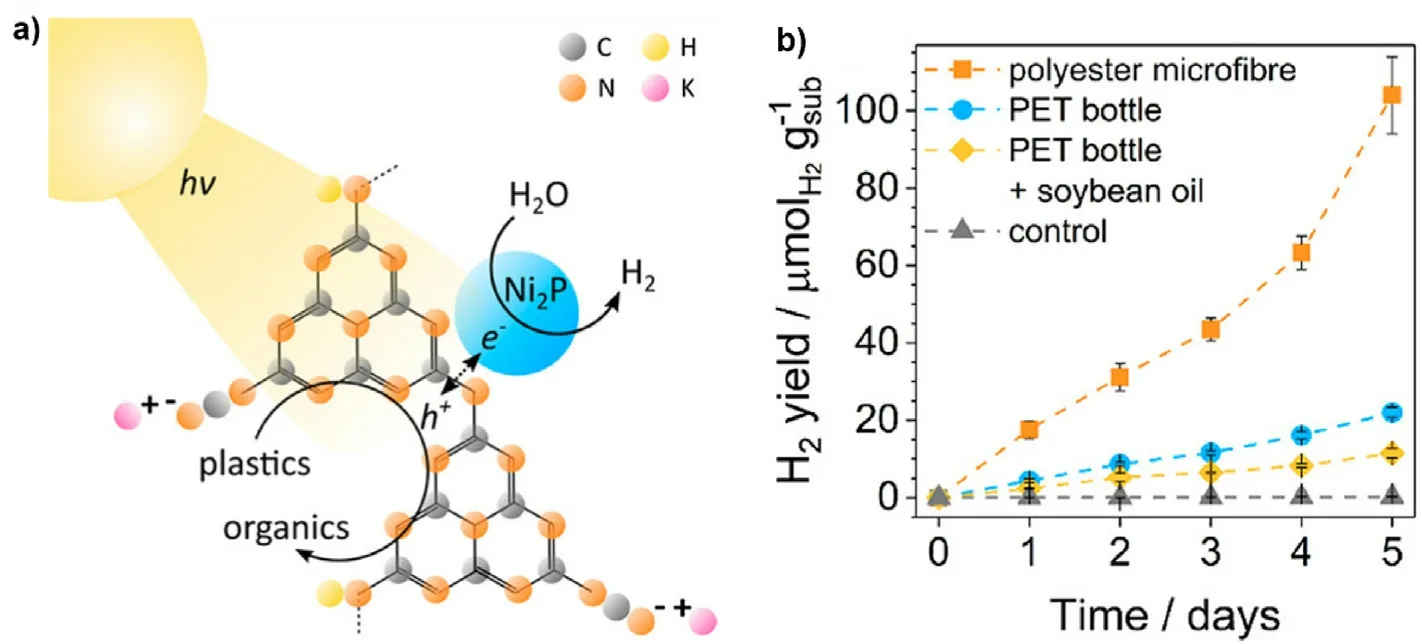
Fig.8.(a)Schematic diagram of mechanism of CNx|Ni2P photocatalytic degradation of microplastics;(b)Hydrogen production rates from photocatalytic degradation of different target pollutants (reproduced with permission from Ref.[101], copyright 2019 American Chemical Society).
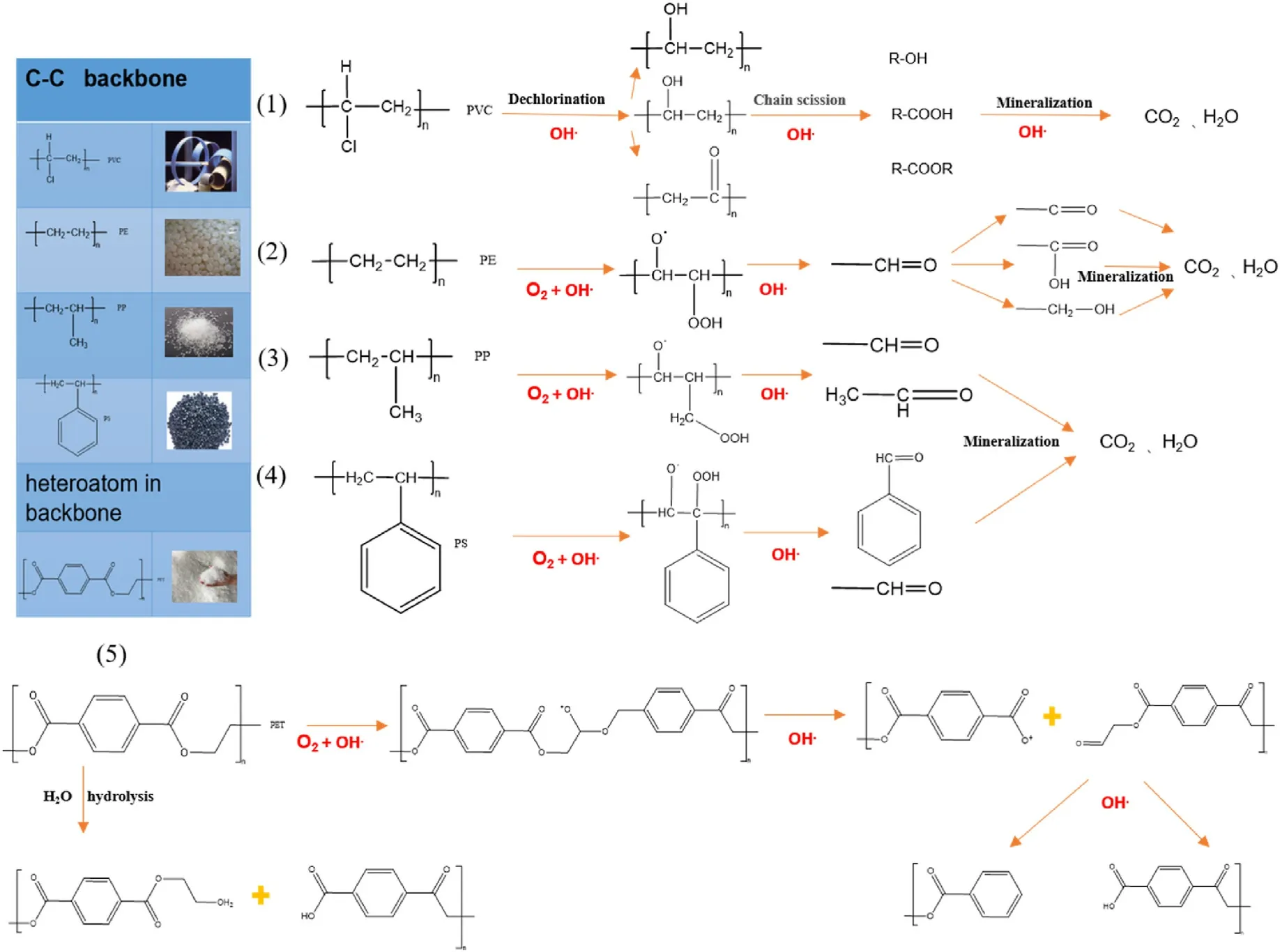
Fig.9.Possible degradation pathways of different microplastics.
light the phenomenon of fast photodegradation may appear.Among them, PE, PP, and PS microplastics which are mainly from packaging materials are more susceptible to oxidative degradation by light,which is also a better way of plastic degradation.Photodegradation is not effective for PE and PP that have saturated bonds in their polymer backbones,but under UV irradiation, the plastics can be effectively broken down into fragments containing low oxygen molecular weight, such as alcohols,aldehydes,and ketones.PS plastics are more vulnerable to weathering in the natural environment.When plastic polymers are exposed to UV irradiation, the benzene ring is triggered and the activation energy is diverted to the nearest carbon-hydrogen bond, leading to hydrogen cleavage and forming polymer radicals,which further degrade to styrene and acetophenone.PVC plastics are relatively unstable compared to other plastics and are therefore sensitive to UV light.Dechlorination is one of the most important procedures in the photodegradation of PVC,which causes the creation of conjugated double bonds in polyolefins and the formation of unsaturated C--C that are less reliable to photodegradation,so that the polymer backbone degrades into tinier fractions.The visual result of degradation is polymer discoloration.The rate of photo-induced PVC dechlorination is enhanced under aerobic conditions.In addition,air pressure, impurity ratio,polymer surface water content,degree of polymerization and temperature can also affect the degradation efficiency of PVC.Photo-oxidation and hydrolysis are possible pathways for PET degradation.Photodegradation directly results in the breakage of ester bonds to form carboxylic acid end-groups and vinyl end-groups,or leads to breakage of free radicals to eventually form carboxylic acid endgroups.PET can proceed to photo-induced autoxidation through free radical reactions.However,the hydrolysis reaction of PET is very slow at room temperature, during which carboxylic acid and alcohol functional groups are formed.In acidic or alkaline conditions,the hydrolysis rate is faster when the carboxyl end group is formed.Hydrolysis of PET is an autocatalytic reaction[102-104].
Plastic polymers containing carbon chains (PE, PP, PS and PVC) are degraded in the environment.Photo-triggered oxidative degradation of PE, PP and PS results in the breakdown of the backbone into smaller molecules and the formation of carboxyl groups.However, UV light is more effective in dechlorinating PVC, and the lower-molecular-weight polymer fragments formed by light-induced chain breakage can be biodegraded.Plastics that include heteroatoms in the primary chain,such as PET, can form smaller fragments and carboxyl end groups through hydrolysis, photo-oxidation and biodegradation.Several possible degradation pathways are shown in Fig.9.
4.Conclusions and prospects
4.1.Conclusions
Plastics are persistent man-made pollutants that become a global problem due to their prevalence and unknown threat to living organisms.With the increase in global plastic manufacturing and use, microplastic pollution is expected to cause serious harm to various ecosystems.In recent years, photocatalysis has been successfully applied, bringing significant progress in the degradation of macroplastics and microplastics.Photocatalytic materials can be modified in various ways.This review focuses on the introduction of metallic elements to modify catalysts to obtain better catalytic effects, which is the leading research direction.Then the explorations of researchers in recent years are reviewed and analyzed.
When doping noble metals or transition metals, the efficiency of the photocatalyst in degrading microplastics is improved by four main mechanisms.
(1) Noble metal in suitable nanoparticle size doped into the catalyst does not produce sufficient morphological structural changes on the catalyst surface,so the noble metal nanoparticles can be well dispersed in the catalyst.Too large or too small noble metal particle size is not conducive to improving the photocatalytic activity.The presence of the deposited noble metal reduces the band gap energy of the catalyst, and the composite catalyst shows an adsorption threshold extended to the visible light region.
(2) Surface plasmon resonance of photoexcited nanometals enhances surface electron activity and interfacial electron transfer efficiency.The transferred electrons may then be combined with dissolved oxygen in solution to form various ROS.(e.g.hydroxyl radicals,singly linear oxygen)and thus promoting photocatalysis.
(3) Under light conditions,the valence band electrons of the catalyst are activated to the conduction band,while valence band cavities are formed.The conduction band electrons are rapidly transferred to the metal, and the precious metal with good electron storage capacity effectively extends the lifetime of the photoelectrons.Photoelectrons react with oxygen on the metal surface to generate superoxide radicals, and the valence-band holes can absorb the electrons on the hydroxyl radicals to generate hydroxyl radicals.The active substances including superoxide radicals, hydroxyl radicals and photogenerated holes can degrade the target pollutants.
(4) When the metal nanoparticles are excited by visible light, the photogenerated hot electrons have enough energy to be transferred to the conduction band by overcoming the Schottky barrier or tunneling effect at the catalyst and metal interface.The heterogeneous structure can effectively enhance the separation of electron-hole pairs.
4.2.Prospects
Plastics are chronic industrial pollutants that have emerged as a worldwide problem due to their ubiquitous presence and unknown threat to living organisms.In recent years, the photocatalytic technology has been successfully applied into sustainable decomposition of microplastics.However, recent studies have shown that photodegradable microplastics in the aqueous environment have poisonous effects on living organisms due to the production of long-term free radicals on the polymer surface.These microplastics that age in the process of photodegradation emerged as pollutants with undervalued environmental impacts.Researchers should be concerned about microplastics and nano plastics, which have the potential to be released into the environment and cause unspecified environmental effects.Although they are not categorized as microplastics, which are chemically similar to synthetic polymers, they may result in unintended harmful impacts.The specific source of microplastics is difficult to trace because the properties of plastics change during long-term use and transportation and undergo complex biochemical reactions in the environment.The mechanical and chemical characteristics of plastics can change dramatically over time as they age in the natural environment.Therefore,studies on the detection and presence of microplastics should cover the type, size and shape of microplastics, which may offer insights for root tracing.Analytical validation with appropriate QA/QC will also be required for future analyses.Moreover, since different studies may describe the same fragments differently, making the results incomparable, criteria for microplastic shape definition and classification shall be established.
Although microplastics were first introduced in 2004, specific approaches to moving,degrading,or scavenging them have not been widely studied.To date, most methods for repairing plastics are based on biodegradation.Until recently,advanced oxidation techniques have been effective methods for microplastic degradation and recovery,but related studies are still in the initial steps and require deeper progress.To establish suitable control and decontamination tactics for microplastics and to improve their adaptability,future research should consider three aspects.
(1) Degradation processes under fewer extreme conditions are needed and some other advanced oxidation systems such as ozonation,comprehensive approaches (heat, photocatalysis and other peroxides) should be investigated to explore green, simple and stronger systems.Enhancing and optimizing currently available water treatment technologies in wastewater treatment plants can offer a more economical way to avoid the emission of microplastics than developing new methods and equipment to remove them.
(2) In addition to mass loss,there are several methods to identify and assess the efficiency of degradation of microplastics.Nevertheless,they all have strengths and weaknesses.In order to get more dependable results, different assessment methods should be combined or other feasible methods should be proposed.
(3) The suitability of specific remediation techniques for various categories of plastics should be measured and provided.The natural degradability differs among various polymers.Awareness of the applicability of a technology to a variety of plastics can help in the appropriate design, enhancement and adaptation of the system.In addition, plastics in real life are mixed with various additives during the process of manufacturing for a variety of applications.Currently most of researches on MPs are concerned with the plastic itself only.Therefore, research should be conducted on the effect of additives on the purification process and their potential impact.Such research will provide meaningful information for the rational selection and use of additives in the plastics industry.
(4) Future research directions allowed more studies on the toxicity of photocatalytic decomposition of nanoplastics and their reaction intermediates.The research to elucidate and figure out the main components is still crucial because the photodecomposition of macroscopic and microscopic plastics is complex.In contrast to microplastic degradation in aquatic environments,it is difficult to collect degradation products from soil or other environments.Therefore,the development of extraction methods for degradation products should attract more attention.Due to the excessive complexity of the products, accurate HPLC-MS or GC-MS analytical methods are required to be established.
(5) Although many efforts have been made in macro and micro plastic degradation, it should be acknowledged that there are still knowledge gaps that need to be investigated, such as the optimization of photocatalysts in terms of degradation performance,reusability,cost and environmentally friendly.So far,TiO2-based photocatalysts in the UV region have been clearly highlighted as potential materials.Therefore, it is essential to develop new and efficient visible light-driven photocatalytic materials.In addition,most of the current research has focused on specific types of macro- and microscopic plastic degradation.In future studies,photocatalysts should be able to evolve from effectively decomposing a single type of polymer to decomposing multiple types of polymers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Natioanl Natural Science Foundation of China (Grant No.51671136) and Shanghai Natural Science Foundation(Grant No.23ZR1444100).
 Progress in Natural Science:Materials International2023年3期
Progress in Natural Science:Materials International2023年3期
- Progress in Natural Science:Materials International的其它文章
- Research progress of composite cathode materials for Solid oxide fuel cells
- A review on solidification of alloys under hypergravity
- Improving mechanical properties of Mg-Sn alloys by co-addition of Li and Al
- Multi-stimuli bilayer hydrogel actuator for remotely controllable transportation of droplets
- The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant
- Towards high-efficiency of hydrogen purification in metal hydride
