Simultaneous high-performance detection and removal of tetracycline with a polyoxomolybdate-based coordination polymer
Ynqiu Zhng, Minrui Sun, Yng Lu, Xi Xu, Erdeng Du, Mingguo Peng,*
a School of Environmental Science and Engineering, Changzhou University, Changzhou, 213164, PR China
b School of Urban Construction, Changzhou University, Changzhou, 213164, PR China
Keywords:Fluorescence detection Adsorption BUC-14 Tetracycline
A B S T R A C T The excessive residues of tetracycline (TC) have been found to cause persistent adverse effects on both human beings and the environment.In this study, a polyoxomolybdate-based coordination polymer (BUC-14) with negative surface and functional group (-NH2) was selected as a multifunctional platform for the detection and removal of TC.The fluorescence emission of BUC-14 at 468 nm was observed to be quenched by TC in a wide concentration range of 7-107 μM attributed to the inner filter effect (IFE) and photo-induced electron transfer(PET)with a detection limit(LOD)of 38.7 nM.The adsorption properties for TC were thoroughly studied through kinetic and equilibrium experiments.And the excellent adsorption performance significantly can promote the preconcentration of TC,thereby enhancing the sensing performance.Possible mechanisms of adsorption including electrostatic attraction, hydrogen bonding and π-π interactions were studied.Furthermore, the practicality of BUC-14 was confirmed by successfully sensing TC in real water samples with satisfactory recovery from 90.61%to 103.53%, and a relative standard deviation (RSD) ≤5.12%.
1.Introduction
Antibiotics,as antibacterial and bactericidal drugs,play a pivotal role in mitigating the occurrence of bacterial infections and associated mortality.However, the improper utilization of antibiotics in both human and agricultural activities lead to their inevitable release into water environments, thereby contributing to significant environmental concerns encompassing ecological risks and detrimental effects on human health[1-4].Recent reports indicated that TCs rank second in global antibiotic production and usage, because of their cost-effectiveness, broad spectrum antibacterial activity, and ease of absorption [5,6].However, the low utilization rate of TC results in more than 70% being released into sewage through human and animal urine and feces [7,8], and further produce residues with its relatively stable structure and durability in environmental water and give rise to serious side effects for human health.Nowadays,TC had been found in the surface water,ground water and drinking water,and the concentration reached to 0.0011-110 μg L-1[9].Thus,the sensing and removal of TC are of great significance to the ecological environment.Until now, the traditional methods for the quantitative detection of TC mainly include the liquid chromatography,colorimetry,fluorescent and electrochemical methods[10-14].Besides,approaches for TC removal including adsorption and photocatalytic degradation, ion-exchange, chemical oxidation/reduction, membrane filtration were used [15-19].However, most of these methods require complex operations with complicated equipment or need expensive chemicals and time-consuming with multiple steps for preparation procedures.Recent days,adsorption and fluorescent detection methods have drawn public attention because of their advantages such as simple operation,good sensitivity.
Coordination polymers (CPs), constructed with assembled organic structures and inorganic nodes, arranged in periodic frameworks, have garnered increasing attention in detection and removal of diverse pollutants in water environment with their customized structures and exposed active sites [20-23].Recently, few studies have succeeded in simultaneously sensing and adsorbing TC using CPs.For instance, Yang et al.[24] combined N, S co-doped carbon dots with a Zr-based metal--organic framework (MOF) for detection and adsorption of TC.Zhao's group[25]reported a europium-based MOF for TC sensing and removal based on the “antenna effect” and host-guest interactions, hydrogen bonding, π-π interactions and electrostatic attraction.Li's group [26]anchored ZIF-8 onto the 2D NH2-MIL-53(Al)nanoplates and realized the adsorption and sensing of TCs.In above methods,one usually used MOF to adsorb TC and further realized the detection of TC by introducing another fluorescent molecule functional groups, which is usually complicated to synthesize.The other is to use the lanthanide MOFs(Ln-MOFs) to simultaneous realize the adsorption and detection of TC.This method with single material is simple to synthesize, but the lanthanide metal ions such as europium is expensive,which makes a high cost in the sensing and removal of TC.Consequently,the construction of a straightforward strategy for the synthesizing CPs with high sensitivity and selectivity is paramount in developing a platform for the simultaneous TC sensing and adsorption.BUC-14 is a polyoxomolybdate-based CP prepared with a facile hydrothermal method in our previous work,which exhibit high uptake efficiency to both cationic dyes and Pb2+because of its negative zeta potential[27].For the TC molecule exists as cationic species in alkaline conditions,BUC-14 with negative surface may have a great potential for the adsorption of TC.In addition, BUC-14 displays a fluorescence emission under the excitation light, the functional group of -NH2bearing long pair of electrons can endow BUC-14 exhibiting strong binding sites toward analytes [20,26], which is conductive to realize fluorescence sensing of the target and enhancing its sensitivity.
Taking inspiration from these considerations,BUC-14 was selected to simultaneously adsorb and detect TC in water.The adsorption/detection performance of BUC-14 towards TC was investigated through batch experiments.According to research findings, BUC-14 can realize the TC sensing through the IFE and PET with fluorescence quenching.Meanwhile, the adsorption of TC through electrostatic attraction, hydrogen bonding and π-π interactions enhanced the sensing sensitivity through the pre-concentration effect.Based on X-ray diffractometer (XRD),Fourier transform infrared (FTIR), X-ray photoelectron spectra (XPS),ultraviolet and visible spectrophotometer (UV-vis) and fluorescence spectrophotometer(FL)characterizations,possible adsorption/detection mechanism was proposed.The practical application of BUC-14 was also validated through the detection of TC in real water samples.This work provided a facile way to simultaneous monitor and remove TC in contaminated water with a multifunctional polyoxomolybdate-based CP.
2.Experiment and method
2.1.Materials
All chemical reagents involved were commercially available and adopted directly without further purification.Ammonium molybdate tetrahydrate (H24Mo7N6O24·4H2O, 99%), cadmium chloride hemi(-pentahydrate)(CdCl2·2.5H2O,99.95%),4-aminopyridine(4-ap,C5H6N2,99%) and ethanol (CH3CH2OH, 95%), tetracycline (C22H24N2O8, 98%)were purchased from Aladdin industrial Co.Ltd.(China).Hydrochloric acid (HCl, 36%), sodium hydroxide (NaOH, 96%), sodium chloride(NaCl,99%),sodium fluoride(NaF,99%),sodium nitrite(NaNO2,99%),sodium sulfite(Na2SO3,99%),sodium hydrogen sulfite(NaHSO3,99%),sodium bisulfate(NaHSO4,99%),sodium bicarbonate(NaHCO3,99.5%),sodium carbonate(Na2CO3,99%),sodium nitrate(NaNO3,99%),sodium sulfate (Na2SO4, 99%), sodium phosphate (Na3PO4, 99%), sodium thiosulfate (Na2S2O3, 98.5%), sodium persulfate (Na2S2O8, 98.5%), magnesium chloride (MgCl2, 99%), potassium chloride (KCl, 99.8%), calcium chloride(CaCl2,96%),Iron(II)chloride tetrahydrate(FeCl2·4H2O,99%),cupric chloride anhydrous(CuCl2,99%),nickel(II)chloride hexahydrate(NiCl2, 98%), cadmium chloride hydrate (CdCl2·2.5H2O, 99%), barium chloride dihyrate(BaCl2·2H2O,99.5%)were purchased from Sinopharm Chemical Reagent Co.Ltd.(China).Deionized water(18.2 MΩ cm at 293 K, pH of 5.5) from a Milli-Q purification system was used in the whole experiment.
2.2.Synthesis of [(4-Hap)4(Mo8O26)] (BUC-14)
BUC-14 was synthesized through the hydrothermal method[28].0.3 mmol CdCl2·2.5H2O, 0.3 mmol 0.028 g 4-ap and 0.6 mmol H24Mo7N6-O24·4H2O were added to 20 mL deionized H2O, and mixed with ultrasound for 10 min and then heated at 160°C for 72 h.After cooled down to room temperature,the resulting product was centrifuged,rinsed with deionized water and ethanol,and finally dried at 60°C for 6 h to obtain BUC-14.
2.3.Characterization of BUC-14
The morphology BUC-14 was examined by a scanning electron microscope (SEM), FEI Quanta 200, Netherlands.The crystalline phase of samples was identified by the powder X-ray diffractometer(XRD,Bruker D8) with graphite monochromatized CuKα radiation (λ = 0.15418 Å)under the operating conditions of 40 mA current and 40 kV voltage with a 2θ scanning ranging from 5°to 50°.The functional groups analyzed with Fourier transform infrared(FTIR)spectra recording from KBr pellets in the range of 4000-400 cm-1on Nicolet iS50 spectrometer.The thermogravimetric analysis (TGA) of samples was conducted in the temperature range of 0-700°C under air atmosphere with a TA SDT Q600.The element composition of BUC-14 and BUC-14 with TC adsorbed was identified by Xray photoelectron spectra (XPS, Thermo Scientific K-Alpha).The fluorescence spectra were measured with a Thermo Scientific Lumina fluorescence spectrometer equipped with a 150 W Xenon lamp.The zeta potential of BUC-14 surface in water solution was assessed with a Malvern zeta sizer Nano ZS and by applying the field strength of 20 V cm-1.
2.4.Fluorescence detection of TC
Fluorescence stability was first investigated by adding the powder BUC-14(2 mg)in 2.5 mL deionized water and recording the fluorescence spectra at different time intervals (0-60 min) at room temperature,respectively.The response kinetics was assessed by the emission intensity of BUC-14 at different time with 2 mg powder BUC-14 in 2.5 mL TC aqueous (100 μM) solution at room temperature.To further investigate the sensing performance,BUC-14(2 mg)was further immersed in 2.5 mL TC aqueous solutions with different concentrations at room temperature.After 10 s, the fluorescence response under 350 nm excitation was recorded.
The selectivity and anti-interference performance of BUC-14 in TC sensing was further explored.In detailed, BUC-14 (2 mg) was added in various kinds of anions(3 mL,100 μM,F-,Cl-,NO2-,S2O32-,SO3-,HSO3-,HCO3-,HSO4-,CO32-,NO3-,SO42-,PO43-,S2O82-)and metal ions(100 μM,Na+,Mg2+,K+,Ca2+,Fe2+,Ni2+,Cu2+,Cd2+,Ba2+),and some common antibiotics in water (100 μM, sulfadimidine, sulfadiazine, oxytetracycline, chloramphenicol, nitrofurazone).The emission intensities of the mixture solution with and without TC(100 μM)were measured after 10 s at room temperature.
To evaluate the performance of BUC-14 for TC detection in real water samples, spike and recovery experiments were conducted.Real water samples were collected from Changzhou University in Changzhou,JiangSu, and filtered using a 0.45 μm pore size membrane from Tianjin Jinteng,China.
During experiment, all the mixed solution of BUC-14 and TC was stirred at a constant rate to maintain its uniformity.
2.5.Adsorption performance towards TC
The crystals of BUC-14 were ground to fine powder through a 200 mesh sieve.The TC standard stock solution(1000 mg L-1)was prepared,and diluted to obtain the concentration involved in the adsorption experiment.To prevent photolysis of TC, all of adsorption experiments were carried out in the dark.The adsorption of TC with BUC-14 were performed in the conical flask and oscillated at 150 r·min-1with a constant temperature water bath oscillator.Samples were drawn at a certain time interval with a syringe filter(0.45 μm,Tianjin Jinteng),and the absorbance of TC was obtained by a UV-vis spectroscopy(Shimadzu UV-2550)at 357 nm[29,30].
Different doses of BUC-14 (0, 10, 20, 30, 40, 50, 60 mg L-1) were added to a conical flask containing the TC solution(200 mL,40 mg L-1)at 20°C to study the effect of adsorbent dose on adsorption.For kinetics experiments,4 mg BUC-14 was mixed with 200 mL TC solution(10 mg L-1)at 20°C,and 1 mL solution was collected at specific time intervals for concentration determination.Adsorption isotherm studies were carried out by mixing 0.01 g BUC-14 in 500 mL TC solution with the given concentrations from 10 to 70 mg L-1for 48 h in the dark.The influence of pH was evaluated by adjusting the pH values of TC solution from 3 to 9 with dropwise adding NaOH (0.01 mol L-1) or HCl solutions (0.01 mol L-1).
To investigate the interference of coexisting ions on TC adsorption,4 mg BUC-14 was added in the TC solution(200 mL,10 mg L-1)containing NaCl,KCl,MgCl2,CaCl2,NaNO3and NaSO4(0.5 mol L-1),respectively.In addition, the regeneration of BUC-14 was explored by adding 50 mg BUC-14 in a conical flask with 200 mL TC solutions (40 mg L-1), and vibrated in a constant temperature water bath oscillator at 30°C with a constant speed of 150 r·min-1.After 48 h, BUC-14 was separated from the aqueous solutions by centrifugalizing, and immersed in 200 mL ethanol for 8 h.Subsequently,the desorbed BUC-14 was added into fresh TC solution for six cycles to verify the reusability.
All data related to the sensing and removal of TC with BUC-14 presented in this paper are the averaged values of three parallel experiments.
3.Results and discussion
3.1.Characterization of BUC-14
According to the previous report,the basic structural unit of BUC-14 consist with a dissociative β-octamolybdate anion and four discrete 4-ap ions protonated at nitrogen atom of the pyridine ring.The threedimensional structure of BUC-14 was constructed via the hydrogen bonds (N-H…O) between [Mo8O26]4-and the protonated 4-aminopyridine molecules [28].As demonstrated in Fig.1(a), the XRD pattern of the synthesized BUC-14 matched well with the simulated XRD pattern[28],validating the high phase purity of BUC-14.In(Fig.1(b),the peaks at 3434 and 3231 cm-1were assigned to none-bonded-NH2functions,the band at 1649 and 1000 cm-1were the C--C and C--N respectively,and the peaks of v(Mo--O)and v(Mo-O-Mo)in β-octamolybdate were found at the range of 600-1000 cm-1.The SEM images shown in Fig.1(c)revealed the irregular stone shape of BUC-14.As shown in Fig.S1,BUC-14 has a sharp weight loss at 300°C,indicating that BUC-14 can stay stable in subsequent experiments with temperatures lower than 300°C.
3.2.Detection of TC
The fluorescence study showed that BUC-14 displayed a broad emission centered at 468 nm under the excitation at 363 nm(Fig.2(a)),which assigned to the π→π*transitions of 4-ap.It is noteworthy that the intensity of BUC-14 remained stable after 1 h in water (Fig.S2), indicating the high fluorescence stability of BUC-14 in aqueous environment.Additionally, the fluorescence intensity remained stable in a wide pH range of 3-12 (Fig.S3).Thus, the obvious luminescence signal and superior luminescence stability provide a potential possibility for the application of BUC-14 in fluorescence sensing.
Investigations were made into the BUC-14's fluorescence sensing capabilities toward TC in aqueous solution.First,the response time of BUC-14 in TC sensing was tested by immersing BUC-14 in 2.5 mL TC solution(100 μM)at different incubation times(0-10 min).According to Fig.S4,the emission intensity of BUC-14 at 468 nm rapidly decreased in 10 s,and reached fluorescence equilibrium, making an incubation time of 10 s as the ideal experimental setting for TC sensing.Then the fluorescence sensing capabilities of BUC-14 toward TC at various concentration were studied.In Fig.2(b), the emission intensity at 468 nm gradually decreased with the concentration of TC increased from 7 to 107 μM,and the quenching efficiency reached approximately 85% at the TC concentration of 107 μM.Additionally, the Stern-Volmer (S - V) formula [31](Eq.(1))indicated a good linear correlation of 0.9902(Fig.2(c))between the emission intensity and TC concentration.
Where I0and I are the emission intensities of BUC-14 before and after sensing TC, respectively; [M] represents the molar concentration of TC(μM);and Ksvis the Stern-Volmer constant(M-1).
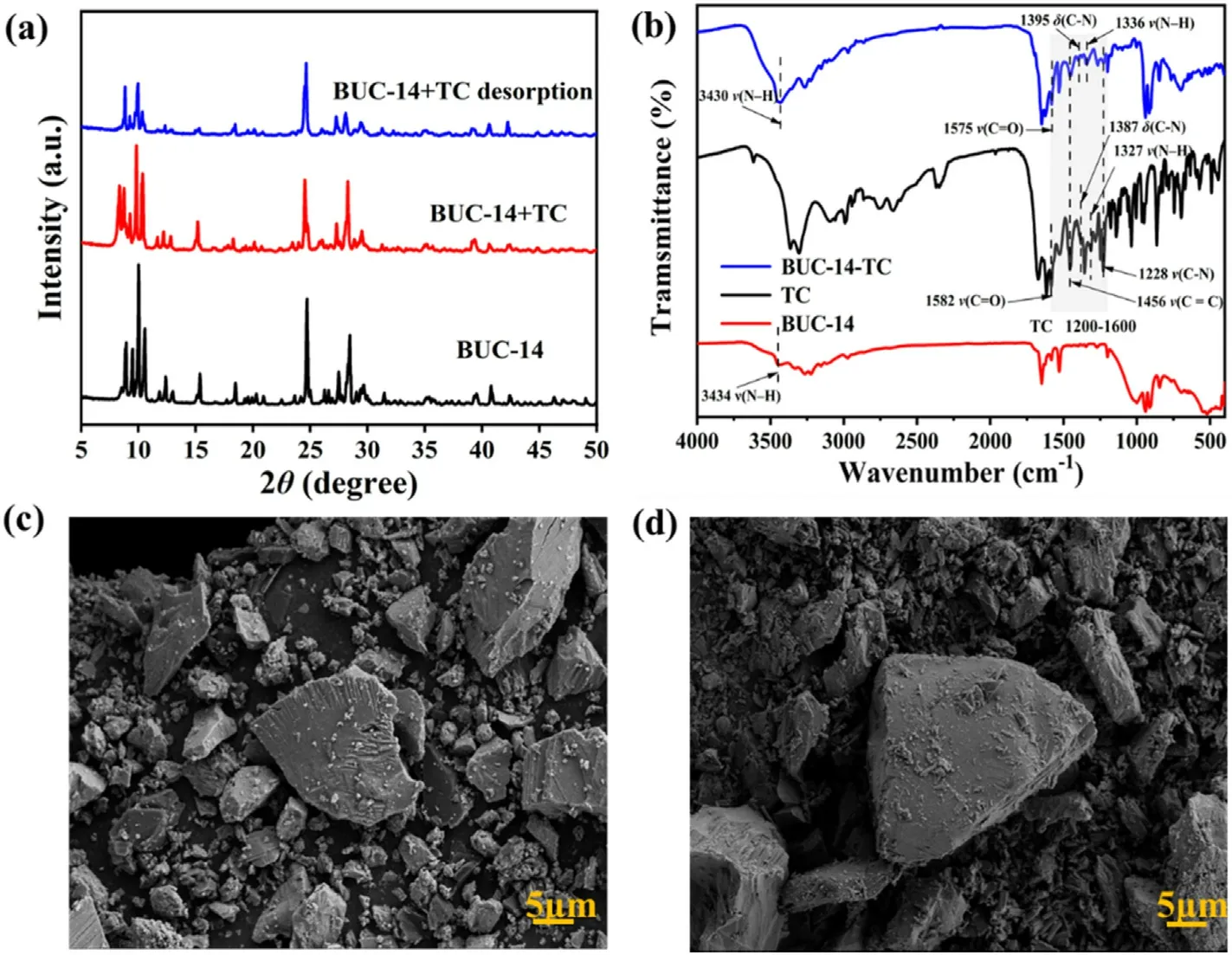
Fig.1.(a)XRD patterns of BUC-14 before,after adsorption and desorption of TC;(b)FTIR spectra of BUC-14,TC and BUC-14 after adsorption TC;SEM images BUC-14(c) before and (d) after adsorption of TC.
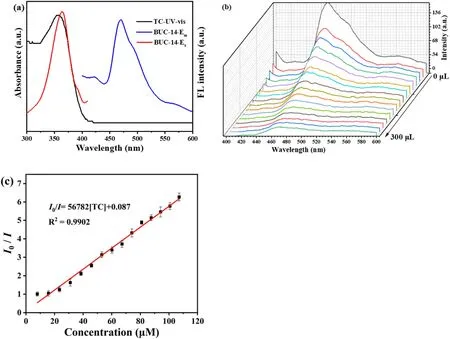
Fig.2.(a)The UV-vis spectra of TC and the excitation and emission spectra of BUC-14;(b)Emission spectra of BUC-14 in water with the addition of TC(1 mM,20 μL addition each time); (c) Corresponding Stern - Volmer fitting curves of BUC-14 toward TC.
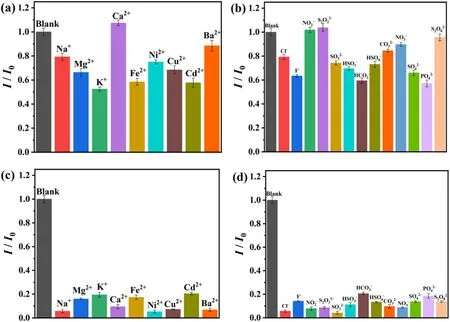
Fig.3.The luminescence intensity at 468 nm of BUC-14 in 100 μM different metal ions(a)and different anions(b) aqueous solution; The luminescence intensity at 468 nm of BUC-14 with different coexistent metal ions (100 μM) (c) and anions (100 μM) (d) in 100 μM TC solution (Ex = 363 nm).
The Ksvvalue of TC was 5.68 × 104M-1, suggesting that TC had a good quenching effect on the fluorescence of BUC-14.Meanwhile, the LOD of BUC-14 toward TC was estimated to be 38.7 nM based on the 3σ IUPAC criteria (3σ/slope, σ is the standard deviation of three repeated fluorescence measurements of a blank solution) [32].It is worth noting that this LOD is significantly below the allowable concentration(225 nM)stipulated by the European Union [33].In addition, as summarized in Table S1, the sensitivity of BUC-14 demonstrates competitiveness when compared to other detection methods.
Selectivity of BUC-14 in TC sensing was studied.Compared to the detection of TC,those metal ions and anions showed negligible effects on the fluorescence emission of BUC-14(Fig.3(a)-(b)).The anti-interference is also a critical index to determine whether BUC-14 can be used in practical application.Thus, the sensing performance for TC in the solution coexist with some metal ions and anions were further studied.Results in Fig.3(c)-(d) showed that only slight fluorescence emission intensity variations were found compared to the solution containing only TC,indicating the great application potential of BUC-14 in the field of TC detection.Furthermore, the effect of other five common antibiotics on the fluorescence of BUC-14 was tested.As shown in Fig.S5,all of the five antibiotics did not have significant quenching effect on the fluorescence of BUC-14,indicating that BUC-14 can identify TC specifically.
3.3.Adsorption of TC
The adsorption capabilities of BUC-14 towards TC were further probed.As the morphology of the TC molecule in aqueous solution can be affected by pH[29,34],we adjusted the pH value in the range of 2-9 to examine the effect of pH on the adsorption of TC.As shown in Fig.S6,the removal efficiency of TC decreased with the increase of pH, and the highest adsorption capacity was observed at the pH=2.The adding dose of adsorbent usually affects the adsorption performance, so the experiments using TC solution(40 mg L-1)with different dose of BUC-14 under the temperature of 20°C were conducted.Results in Fig.S7 showed that with increasing dose of BUC-14,the adsorption capacity increased from 75.2 to 85.9%and then decreased which may be caused by the aggregate of the adsorbents.However, as the dosage of BUC-14 increased, the adsorption capacity decreased and the maximum adsorption capacity was obtained with 2 mg BUC-14, demonstrating a high utilization efficiency of adsorption sites.Conversely, with the increase of BUC-14 dosage, the utilization of active sites decreased and thus resulted to the decrease of the adsorption amount per gram of BUC-14.
Additionally,we investigated the influence of contact time on the TC adsorption,and the results are presented in Fig.4(a).BUC-14 exhibited rapid TC removal in the initial 10 h because of the abundance of adsorption sites.Subsequently,as most of the active sites on the surface became occupied,it gradually reached equilibrium between 20 and 48 h.Pseudo-first-order model [6] and the pseudo-second-order model [35]were used to study the adsorption kinetics processes, which can be expressed in linear form by Equations(2)and(3).
where k1(min-1) and k2(g·mg-1·min-1) are the rate constant for the pseudo-first order model and the pseudo-second order model, respectively.Qe(mg·L-1)and Qt(mg·L-1)are the adsorption capacity at time t and equilibrium time,respectively.
As shown in Fig.4(b), the experimental data were better fitted the pseudo-first-order model (R2= 0.994) than the pseudo-second-order model (R2= 0.984).This suggests that the control mechanism of TC adsorption on BUC-14 is not the chemisorption [36].As listed in Table S2, the adsorption capacity calculated by the first-order kinetics(440.32 mg·g-1) were in good accordance with the experimental Qevalue(423.17 mg·g-1).
In addition,Langmuir and Freundlich isotherm were used to study the adsorption isotherms and further depict the interaction between BUC-14 and TC.Langmuir isotherm model[7]has the following assumptions:(i)Adsorption occurs as a monolayer on the surface; (ii) The adsorption activity sites on the surface of adsorbents are uniform; (iii) The adsorption of particles onto the adsorbent is not influenced by the presence of other particles.The adsorption isotherm models of Langmuir expressed as Equation(4):

Fig.4.(a)Adsorption kinetics curve of TC on BUC-14;(b)Pseudo-first-order and pseudo-second order kinetic of BUC-14;Fitting adsorption isotherm with Langmuir(c) and (d) Freundlich.
Where Ceis equilibrium concentration of adsorbate (mg·L-1); Qeis the adsorption amount (mg·g-1); Qmis maximum adsorption capacity(mg·g-1); KLis Langmuir constant(L·mg-1or L·mol-1).
Freundlich isotherm[37] can be used to estimate the adsorption capacity of the adsorbent towards the adsorbate.This model is empirical in nature and does not make any specific assumptions, and suitable for describing multi-layer adsorption processes.It can be represented by Equation(5):
KFis the adsorption amount in per unit adsorbate;n is the Freundlich constant.
As depicted in Fig.4(c),Langmuir isotherm model is more inclined to describe the adsorption process, indicating the adsorption of TC onto BUC-14 is affected by the precise positioning of the adsorbent surface.Moreover,it was observed that once TC molecules occupied a particular position,they cannot be further adsorbed[7].The calculated parameters from both models are summarized in Table S3 and the maximum capacity for TC of BUC-14 in this study(2404.64 mg·g-1)is satisfactorily fitted to the Langmuir maximum capacities, which is higher than other adsorbents listed in Table S4.
There are many kinds of ions in complex environmental water,among which the cations may compete for binding sites of adsorbents, and the anions may show strong complexing ability on the surface of various adsorbents [38].Therefore, the interference of coexisting ions on the adsorption of TC with BUC-14 was investigated,and seven ions(K+,Na+,Ca2+, Mg2+, Cl-, SO42-, NO3-) widely distributed in water environment were selected for the experiment.As shown in Fig.S8, all of the adsorption capacities showed decreasing trend caused by the competition of coexisting ions expect of K+and Cl-.The adding of Na+,SO42-and NO3-showed negligible effect on the adsorption with only reduced by less than 5.3%.The presence of Ca2+and Mg2+significantly influenced the adsorption of TC, leading to a decrease in removal efficiency to 41.5%and 37.3%, respectively.This may be due to the larger hydrated radius and higher occupation of adsorption sites by alkaline-earth cations(e.g.,Ca2+and Mg2+)compared to alkali-metal cations(e.g.,K+and Na+)and H+, thus inhibiting the TC adsorption with greater electronic screening effect [34].To tackle this issue, Na2CO3solution was tested to remove Ca2+and Mg2+ions, and the adsorption performance of BUC-14 in the presence of TC and excess Na2CO3was evaluated.Results in Fig.S9 demonstrated that with the addition of excess of Na2CO3(0.5 M), no obvious removal efficiency was decreased of BUC-14 in TC solution(200 mL, 10 mg L-1) with the coexist of Ca2+and Mg2+.The above interference experiments provide evidence that BUC-14 had superior selectivity for TC removal,highlighting its high feasibility in practical applications.
3.4.Possible mechanism of the adsorption and detection toward TC
A variety of characterization experiments were conducted to investigate the mechanism of BUC-14 in adsorption and sensing TC, including zeta potential,SEM,XRD,FTIR and XPS of BUC-14 before and after adding TC.As decribed in Fig.1(a)and(d),the XRD patterns and morphology of BUC-14 before after the addition of TC showed no significant change,indicating that the structure and morphology of BUC-14 remained unchanged during the adsorption.In addition,the zeta potential for BUC-14(Fig.S10) decreased with increasing pH,which can be attributed to the protonation of N-H…O groups on its surface.In the pH range from 2 to 9,BUC-14 demonstrates an overall negative surface charge.As shown in Fig.S11-12,TC molecule existed in four distinct species at different pH.At the pH lower than 3.3,the main species was TC cationic species(TCH3+),which leads to favorable electrostatic attractions between TCH3+and the negatively charged surface of BUC-14,thereby enhancing the adsorption performance.In the pH ranges from 3.3 to 7.7, the nearly neutral or zwitterionic species(TCH2±) was the dominating species,thus the electrostatic repulsion between BUC-14 and ions with negative charge resulting to the decline of the adsorption performance.When the pH is higher than 7.7, TC existed in negative species (TCH-).The adsorption capacity decreased dramatically caused by the partially mineralization of TC in alkaline media[29]in addition to the electrostatic repulsion.
Compared to the spectrum of virgin BUC-14,new bands around 1200-1600 cm-1corresponding to the characteristic of absorption bands of TC appeared in the spectra of BUC-14-TC(Fig.1(b)),which confirmed the TC adsorbed on the BUC-14.The adsorption bands at 1228,1456 and 1582 cm-1were respectively corresponded to the vibrations of the bonds ν(C-N) of the amide group, the skeletal vibrations ν (C--C), and the vibrations of the bonds ν (C--O)oftheAringsoftheTCmolecular(Fig.S11), and the bands located between 1430 and 1230 cm-1were assigned to the bonds of ν(C-C),δ(C-C),ν(amine,N-H),δ(amine,C-N)and ν (C-O) of the TC molecular [29].Compared to the spectrum of virgin TC,the bands of the δ(C-N)(1387 cm-1)and ν(N-H)(1327 cm-1)bonds of the dimethylamino group shifted towards longer wavelengths in BUC-14-TC related to the interaction between the TCH3+species with the negative surface of BUC-14.In addition, both the C--O vibration(1582 cm-1) of TC and the -NH2vibration (3434 cm-1) of BUC-14 shifted to lower wavenumber of 1575 cm-1and 3430 cm-1, indicating the formation of hydrogen bonding between-NH2of BUC-14 and-COOH of TC[26].Furthermore, the adsorption/detection mechanism were verified by XPS measurements.As can be seen from the XPS spectra,the banding energy of O 1s, N 1s and Mo 3d of BUC-14 did not change significantly after TC added(Fig.5(a)),indicating that no chemical reaction occurred between BUC-14 and TC.Moreover,the increase of the C-N and-CONHcontent(Fig.5(b)-(c))which respective from the dimethylamino and the acylamino of the TC molecule,indicating that TC molecules were tightly bound to BUC-14.Compared to the C 1s spectra of BUC-14(Fig.5(d)),the characteristics peak of C-O/C--O emerged at 288.19 eV,and the content of C-C/C--C,C-N decreased from 66.31%to 63.91%and from 33.69%to 29.57% after adsorption (Fig.5(e)), indicating the electron distribution may be affected by π-π interactions during TC adsorption [26].In summary, the adsorption mechanism of TC by BUC-14 can be summarized as follows:the positively charged TC species could be adsorbed on negatively charged BUC-14 through electrostatic interaction, and hydrogen bonding,π-π interactions also play a crucial role in promoting the adsorption of TC onto the BUC-14 surface.
The fluorescent spectra of BUC-14 and absorption spectra of TC were measured to further discussed the possible sensing mechanism.As illustrated in Fig.2(a),there is negligible overlap between the absorption spectra of TC and the fluorescence emission spectrum of BUC-14, suggesting that no fluorescence resonance energy transfer (FRET) occurred[39].However,the absorption spectra of TCs at about 360 nm showed a significant overlap with the excitation spectrum of BUC-14, indicating that the UV light can be absorbed by TC,and results to the fluorescence quenching through the IFE.Moreover,analysis of FTIR and XPS revealed the presence of π-π interaction, hydrogen-bonding and electron donor acceptor interactions between the-NH2of BUC-14 and the-COOH/-OH of TC.This enhanced the electron transfer between BUC-14 and TC,potentially leading to the quenching of the fluorescence emission through PET [26].Moreover, BUC-14 with ultra-high adsorption performance can pre-concentrated TC on BUC-14, thereby enhancing the sensitivity of TC detection[40].
3.5.Application in real samples
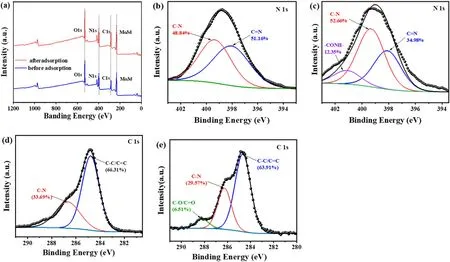
Fig.5.(a)XPS spectra of BUC-14 before and after the adsorption of TC;The N 1s spectra of BUC-14 before(b)and after(c)the adsorption of TC;The C 1s spectra of BUC-14 before (d) and after (e) the adsorption of TC.

Table 1 Recovery results of the determination of TC in river water and tap water.
The reliability of BUC-14 used in practical applications was validated by the standard addition method.The aqueous samples with different TC concentrations(25,50,100 μM)were respective prepared with tap water and river water.Took 3 mL of the above solutions, added 2 mg BUC-14 respectively, and measured the fluorescence response after 10 s at room temperature.Recoveries of BUC-14 in TC sensing were calculated by Eq.(5).
where Cd(mg·L-1) represents the detected concentration of TC, Cs(mg·L-1) is the spiked concentration of TC.Results in Table 1 showed that the relative standard deviation(RSD)is around 5.12%of the spiked samples.Additionally, the recovery values range from 90.61% to 103.53%.These findings indicate that the reliability of BUC-14 for TC sensing in real samples.
3.6.Regeneration of BUC-14
To assess the regeneration capability of BUC-14, several rounds of adsorption-desorption experiments were performed.As depicted in Fig.S13, after six cycles, the TC removal efficiency decreased from 77.93% to 71.95%, suggesting that BUC-14 exhibits good regeneration performance.In the cyclic experiment,BUC-14 can be separated from the aqueous solution, indicating its potential for future engineering applications.
4.Conclusion
A bifunctional platform based on a polyoxomolybdate-based coordination polymer (BUC-14) which exhibits exceptional performance in both the detection and removal of TC was reported in this work.By exploiting the IFE and PET between BUC-14 and TC,BUC-14 can achieve sensitive detection of TC within a wide concentration range of 7-107 μM with a LOD of 38.7 nM.Moreover,BUC-14 showed excellent selectivity for sensing TC in aqueous solutions containing common anions and metal cations, which was further validated by the high recoveries (90.61%-103.53%, RSD ≤5.12%) for sensing TC in practical application.Additionally,BUC-14 exhibits remarkable TC adsorption capabilities owing to the synergistic effects of the electrostatic attraction, hydrogen bonding and π-π interactions.In all, this work presents a reliable and straightforward method for the detection of TC, offering high sensitivity, selectivity and rapid response.At the same time,it can efficiently adsorb TC in aqueous solution with excellent reusability.This versatile approach for the design of multifunctional platform holds great promise for simultaneous sensing and removal of organic pollutants from polluted water.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20210856), the Science and technology Project of Changzhou city (CJ20210117) and Science and Technology Project of China Petroleum and Changzhou University Innovation Consortium(Research on key supporting technologies for multi-element thermal fluid heavy oil production).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.pnsc.2023.08.015.
 Progress in Natural Science:Materials International2023年3期
Progress in Natural Science:Materials International2023年3期
- Progress in Natural Science:Materials International的其它文章
- Research progress of composite cathode materials for Solid oxide fuel cells
- A review on solidification of alloys under hypergravity
- Improving mechanical properties of Mg-Sn alloys by co-addition of Li and Al
- Multi-stimuli bilayer hydrogel actuator for remotely controllable transportation of droplets
- The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant
- Towards high-efficiency of hydrogen purification in metal hydride
