Cardiovascular sequelae in post-COVID-19 patients with moderate to severe CT severity score: A follow-up study
Niharika Agarwal ,Anamika Goyal ,Nikhil Pursnani ,Garima Kanaujia ,Akanksha Semwal ,Prabhat Agrawal✉ ,Abhishek Raj
Department of Medicine1,Department of Paediatrics2,S.N.Medical College,Agra,Uttar Pradesh,India
ABSTRACT Objective: To study cardiovascular sequelae of post-COVID-19 patients with moderate to severe computed tomography (CT)severity score.Methods: A prospective,non-randomized,observational study was conducted on 100 post-COVID-19 patients with moderate to severe CT severity scores from January 2021 to December 2021.Fiftynine were male [mean age (54.1±12.2) years]and 41 were female[mean age (46.9±15.1) years].Patients with previous cardiovascular disease,previous chronic lung disease,and pre-existing primary or secondary pulmonary hypertension were excluded.Patients were examined,and serial electrocardiogram and 2D echocardiography were performed to detect any cardiovascular abnormality.Results: Post-COVID-19 patients had persistent symptoms,the most common being fatigue (59%).Most of these symptoms were relieved on follow-up.A rise in systolic,diastolic blood pressure,and pulse rate was observed.The electrocardiographic evaluation revealed ST-T segment changes,sinus tachycardia,ventricular hypertrophy,and arrhythmias among a considerable number of patients.On echocardiography,left ventricular diastolic dysfunction was most common (43%).Pulmonary hypertension,as evidenced by elevated pulmonary artery systolic pressure,was seen in 15% of patients.Conclusions: The present findings reveal an increased incidence of cardiovascular complications after recovery from COVID-19 infection in those without pre-existing cardiovascular or chronic lung disease.
KEYWORDS: Cardiovascular outcomes;Arrhythmia;Heart failure;Post acute-sequale;Long COVID-19
1.Introduction
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),has emerged as a global public health emergency and has caused unprecedented levels of morbidity and mortality.SARS-CoV-2,belonging to the family Coronaviridae and the genus of coronaviruses B,is a class of enveloped non-segmented single-stranded positive-strand ribonucleic acid viruses[1].It causes multisystemic disease with variable presentation and severity.Besides involving the lungs,COVID-19 also significantly affects the cardiovascular system,especially in the long term.Although most COVID-19-positive patients show full recovery within 3-4 weeks of the onset of infection,some patients develop post-acute COVID-19 syndrome characterized by symptoms and/or delayed or long-term complications persisting beyond 4 weeks from the onset of symptoms[2-4].Myocardial injury incurred during COVID-19 leads to myriad manifestations in the long term,ranging from myocarditis and heart failure to life-threatening arrhythmia,increased risk of ischemic heart diseases,and pulmonary hypertension.The pathogenesis behind these manifestations centers involves the increased expression of angiotensin-converting enzyme 2 which promotes direct tissue damage and a prothrombotic state[5,6].Other suggested pathways leading to long-term COVID-19 infection complications include endothelial injury,immune system dysregulation,cytokine storm-induced proinflammatory state,direct viral attack on myocytes,and hypercoagulability often leading to thrombosis[2,7].In this study,we studied the possible cardiovascular sequelae in COVID-19 survivors,particularly those with moderate or severe disease based on computed tomography (CT) scores.
2.Patients and methods
2.1.Study design and setting
We conducted a prospective observational study at a tertiary care center in North India from January 2021 to December 2021.
2.2.Inclusion and exclusion criteria
COVID-19 patients confirmed by reverse transcription polymerase chain reaction and with moderate to severe CT severity scores(mild 1-8,moderate 9-14,severe 15-25;based on International Nomenclature by Fleischner Society glossary) were included[8-10].All patients with previous cardiovascular or pulmonary disease including pulmonary hypertension were excluded.
2.3.Ethical statement
Ethical approval was obtained from institutional ethical committee of Sarojini Naidu Medical College,Agra (SNMC/IEC/THESIS/2023/176).Informed consent was taken from all patients enrolled in the study.
2.4.Data collection
All patients were followed up for 6 months.Demographic and clinical characteristics were collected.Electrocardiogram (ECG),and 2D echocardiogram (FUJIFILM Sonosite,M-Turbo Ultrasound System SN WK2BVP) were performed at baseline,1 month,and 6 months.
2.5.Outcomes
Clinical symptoms including cardiomyopathy,arrhythmias,ischemic heart diseases,pulmonary hypertension and heart failure,and electrocardiographic abnormalities were primary outcomes.
2.6.Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences version 22.0 (SPSS,Chicago,IL,USA).Normal continuous variables were presented as mean ± standard deviation.Categorical variables were presented as percentages.Data were analyzed using Chi-square test and analysis of variance (ANOVA).Pvalue was considered statistically significant when <0.05.
3.Results
A total of 100 patients were included (Figure 1).
Figure 1.The study flowchart.
3.1.Demographic characteristics
Age and sex distribution are shown in Table 1.The mean age was(51.1±13.9) years,including 59 males [mean age (54.1±12.2) years]and 41 females [mean age (46.9±15.1) years].Most of the patients were aged ≥60 years.

Table 1.Baseline characteristics (n=100).
Among these,33% had moderate CT severity scores (9-14) and 67% had severe CT severity scores (15-25).In our study,51% of patients were hypertensive,29% had dyslipidemia and 29% were diabetic.
3.2.Clinical characteristics
Frequency of symptoms is shown in Table 2.Fatigue was the most common symptom.Other symptoms included dyspnea,palpitations,chest pain,edema,and syncope.
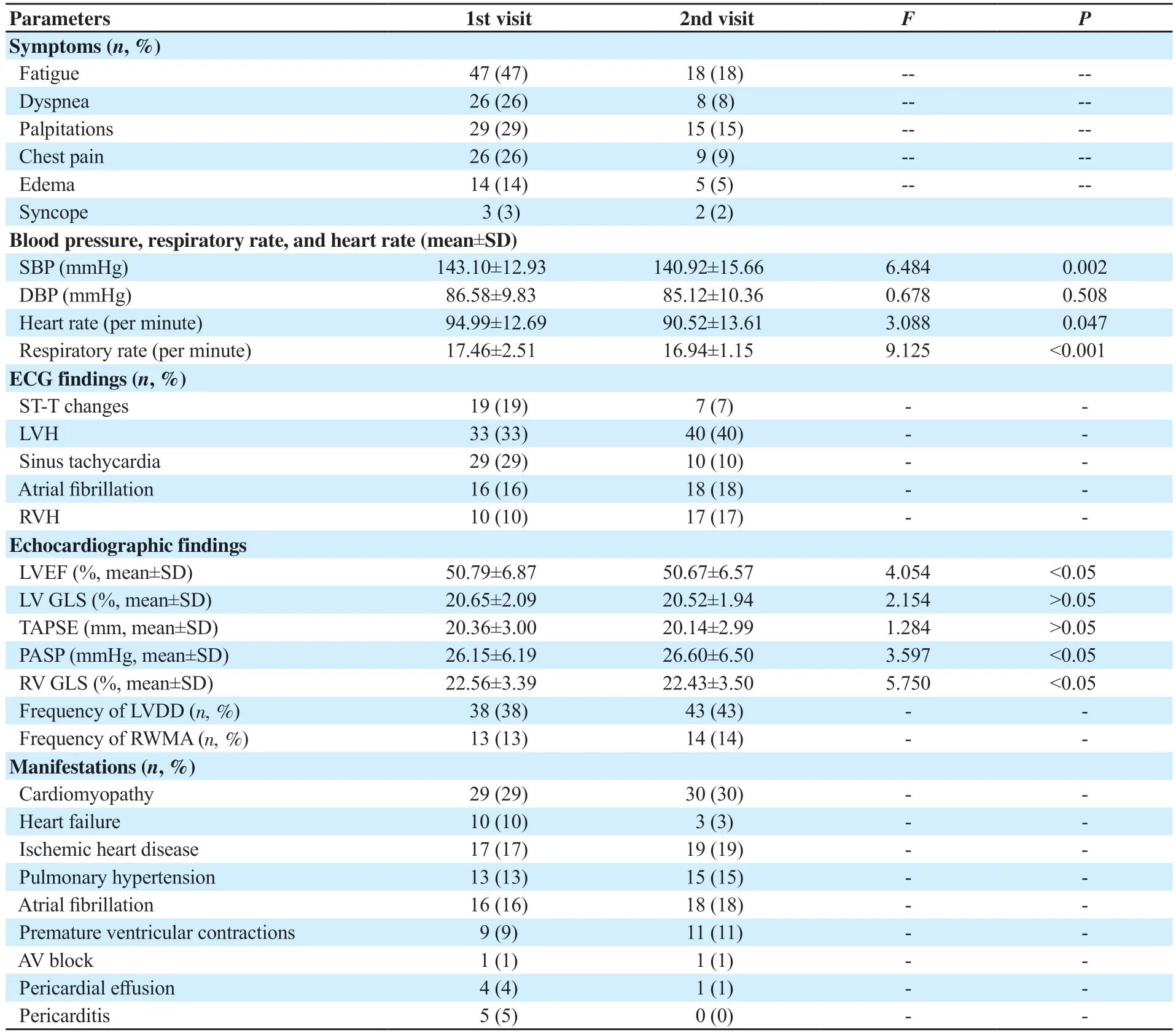
Table 2.Parameters during 1st and 2nd visit (n=100).
3.3.Blood pressure,respiratory rate,and heart rate
Variation in blood pressure,respiratory rate,and heart rate is shown in Tables 1&2.Results showed significant difference in mean systolic blood pressure (P<0.05) and respiratory rate (P<0.05)compared with baseline,but the difference in mean diastolic blood pressure (P>0.05) and heart rate (P>0.05) was not significant.
3.4.ECG findings
ECG findings are presented in Tables 1&2.At baseline,sinus tachycardia and left ventricular hypertrophy (LVH) were the most common finding.The case number of LVH,atrial fibrillation,and right ventricular hypertrophy (RVH) were increased for each visit.
3.5.Echocardiographic findings
Echocardiographic findings are shown in Tables 1&2.At baseline,left ventricular (LV) diastolic dysfunction was the most common finding.Left ventricular ejection fraction,right ventricular global longitudinal strain and pulmonary artery systolic pressure (≥35 mmHg) showed significant variation (P<0.05).
3.6.Cardiovascular manifestations
As shown in Tables 1&2,at baseline,cardiomyopathy was the most common sequelae followed by heart failure and ischemic heart diseases.The case number of cardiomyopathy,ischemic heart diseases,pulmonary hypertension,atrial fibrillation,and premature ventricular contractions was increased for each visit.
4.Discussion
The COVID-19 pandemic has alarmed health systems all over the world.Long-term follow-up of recovered patients revealed a significant rise in cardiovascular diseases.Our study aimed at cardiovascular sequelae of post-COVID-19 patients who had moderate to severe CT severity scores.A total of 51% of patients were hypertensive,29% had dyslipidemia and 29% were diabetic.The majority of these patients had severe comorbidities indicating that COVID-19 was more common and more severe in those with comorbidities.This is also observed in a study conducted by Saeedet al.,in which hypertension was the most prevalent comorbidity(56.6%),followed by obesity (41.7%) and diabetes (33.8%)[11].Ghosnet al.found that women were more likely to experience three or more symptoms six months after recovering from a COVID-19 infection[12].In our study,fatigue was the most common symptom.A total of 18% of patients reported fatigue at 6 months follow-up.Dyspnea,chest pain,palpitations,and pedal edema were found in up to 40%,31%,38%,and 23% of patients,respectively at baseline.Carfiet al.also found an increased prevalence of fatigue (53.1%),dyspnea (43.4%),and chest pain (21.7%) among patients who recovered from COVID-19 in their study[13].
In our study,systolic blood pressure was found to be raised in a significant number of patients.Similar results were found in the study by Akpeket al.in which,new onset hypertension was observed in 12% of patients after COVID-19[14].Tachycardia was also found in a significant number of patients.It is consistent with the study by Radinet al.in which an increase in resting heart rate by≥5 beats per minute was seen in 13.7% of patients[15].Respiratory rate was elevated in 30% of patients at baseline,13% at 1st visit,and only 1% at 2nd visit.This suggests a relief of respiratory distress and recovery following COVID-19.
Electrocardiography was done in patients at each visit.The most common finding was sinus tachycardia.Atrial fibrillation was the most common pathological arrhythmia.Other arrhythmias included premature atrial contraction,premature ventricular contractions,sinus bradycardia,and AV blocks.ST-T changes were found in a significant number of patients.This demonstrated myocardial injury incurred during COVID-19 infection.LVH and RVH were commonly observed for each visit.The findings suggested ventricular dysfunction following COVID-19 infection and were consistent with a review by Longet al.on electrocardiographic abnormalities in COVID-19 patients[16].Sinus tachycardia was the most common finding in their study.Other findings included supraventricular tachycardias such as atrial fibrillation or flutter,ventricular arrhythmias like ventricular tachycardia or ventricular fibrillation,bradycardia,interval and axis changes (QT prolongation),and ST segment and T wave changes.
Serial echocardiographic evaluation of patients revealed significant ventricular dysfunction.LV systolic dysfunction as evidenced by reduced LV ejection fraction (<50%) was seen in 18% at baseline,23% at 1st visit,and 24% at 2nd visit.Reduced LV global longitudinal strain (GLS) (<18%) was seen in 11% of patients at baseline,14% at 1st visit,and 16% at 2nd visit.LV diastolic dysfunction was found in 30% of patients at baseline,38% at 1st visit,and 43% at 2nd visit.Right ventricular (RV) dysfunction as evidenced by decreased tricuspid annular plane systolic excursion(TAPSE) (<17 mm) was seen in 19% at baseline,23% at 1st visit,and 24% at 2nd visit.Decreased RV GLS (<20%) was seen in 8% at baseline,11% at 1st visit,and 13% at 2nd visit.These findings were comparable to that found by Duttaet al.in their study in which LV systolic dysfunction,LV diastolic dysfunction,and RV dilatation and/or dysfunction were found in 27.7%,23.1%,and 37% patients respectively[17].Sharmaet al.in their study on post-COVID-19 patients found LV diastolic dysfunction in 48 out of 63 patients,though LV systolic dysfunction (<50%) was seen in only 6.3% of patients[18].
COVID-19 also results in an increased incidence of ischemic heart disease.Echocardiography revealed pericardial effusion,pulmonary fibrosis,right ventricular dysfunction,and pulmonary hypertension on long-term follow-up.In our study,pulmonary artery systolic pressure was elevated (≥35 mmHg) in 4% of patients at baseline,13% at 1st visit,and 15% at 2nd visit.The findings were similar to that found in the study by Erdemet al.in which 16.4% showed evidence of pulmonary hypertension[19].
Sustained tissue damage during acute illness accounts for the great majority of cardiovascular issues that continue after COVID-19.Our study showed an increase in cardiovascular morbidity among COVID-19 survivors due to resultant ventricular dysfunction,myocarditis,and pulmonary hypertension.The prevalence of heart failure,pericarditis,and pericardial effusion decreased in patients on follow up while arrhythmias,ischemic heart diseases,cardiomyopathy,and pulmonary hypertension showed a substantial rise on follow-up.Increased prevalence of cardiovascular disease was found in many studies.Xieet al.found an increased risk of incident cardiovascular diseases,including cerebrovascular disorders,arrhythmias,ischemic and non-ischemic heart disease,pericarditis,myocarditis,heart failure,and thromboembolic disease in post-COVID-19 patients at 30 days follow up[20].
Yarlagaddaet al.compiled data on effectiveness of antiinflammatory agents,anti-coagulants,and non-steroidal antiinflammatory drugs in preventing development of cardiovascular sequelae of post-COVID-19 based on current trials[21].Gloecklet al.observed beneficial effect of pulmonary rehabilitation techniques in improving the quality of life in post-COVID-19 patients[22].No effective management strategy has been devised so far.The possible role of cardiovascular drugs in slowing the progression of disease needs further research.
Our study revealed an increased incidence of cardiovascular complications in post-COVID-19 patients on long-term follow-up.Many patients experienced persistent symptoms,most commonly fatigue.The common cardiovascular manifestation included cardiomyopathy and heart failure,followed by ischemic heart disease and arrhythmias.Most of these were relieved during subsequent visits.The most common ECG abnormalities were sinus tachycardia and ST-T changes.Echocardiographic findings suggested an increased incidence of LV systolic and diastolic dysfunction and RV dysfunction.
However,there are some limitations to our study.The sample size of this study is small.Baseline cardiac function was not known in the majority of patients.For the study in future,we need to collect a larger sample size and advanced non-invasive and invasive cardiac evaluation needs to be done to quantify myocardial damage.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgements
The authors thank Dr Deependra for helping with collection of data.The authors also acknowledge Dr Ajeet Singh Chahar and Dr Ashish Gautam for their valuable contribution.
Funding
This study received no extramural funding.
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’ contributions
AN,PN,GA,and KG: conceptualization,formal analysis,resources;AN,SA,and GA: validation,formal analysis;PN and RA: writing original draft,data curation,methodology,resources;AP and GA: formal analysis,writing,review.
 Journal of Acute Disease2023年5期
Journal of Acute Disease2023年5期
- Journal of Acute Disease的其它文章
- Efficacy of Willis covered stent of intracranial pseudoaneurysms in the internal carotid artery: A systematic review and meta-analysis
- Intention and hesitancy to receive a booster dose of COVID-19 vaccine among pregnant women using a health belief model: A cross-sectional study
- Mechanical ventilation and outcomes in COVID-19 patients admitted to intensive care unit in a low-resources setting: A retrospective study
- Diagnostic value of ABCD2 and ABCD3-Ⅰ risk scoring systems in determining one-month risk of stroke in patients with transient ischemic attack: An observational study
- Pathological and immunohistochemical findings of lungs,heart,liver,and kidneys,and unexpected findings of fungi and parasites in lungs of deceased COVID-19 patients: A case series
- Penetrating soft palate injury by lollypop candy stick
