Revealing the correlation between adsorption energy and activation energy to predict the catalytic activity of metal oxides for HMX using DFT
Xiurong Yng , Chi Zhng , Wujing Jin , Zhoqi Guo ,*, Hongxu Go , Shiyo Niu ,Fengqi Zho , Bo Liu , Hixi M ,**
a Xi'an Key Laboratory of Special Energy Materials, School of Chemical Engineering, Northwest University, Xi'an, 710069, China
b Science and Technology on Combustion and Explosion Laboratory, Xi'an Modern Chemistry Research Institute, Xi'an, 710065, China
c Rocket Force University of Engineering, Xi'an 710025, China
Keywords: Density functional theory HMX Metal oxides Adsorption energy Activation energy
ABSTRACT Traditional selection of combustion catalysis is time-consuming and labor-intensive.Theoretical calculation is expected to resolve this problem.The adsorption energy of HMX and O atoms on 13 metal oxides was calculated using DMol3, since HMX and O are key substances in decomposition process.And the relationship between the adsorption energy of HMX, O on metal oxides (TiO2, Al2O3, PbO, CuO, Fe2O3,Co3O4, Bi2O3, NiO) and experimental T30 values (time required for the decomposition depth of HMX to reach 30%)was depicted as volcano plot.Thus,the T30 values of other metal oxides was predicted based on their adsorption energy on volcano plot and validated by previous experimental data.Further, the adsorption energy of HMX on ZrO2 and MnO2 was predicted based on the linear relationship between surface energy and adsorption energy, and T30 values were estimated based on volcano plot.The apparent activation energy data of HMX/MgO, HMX/SnO2, HMX/ZrO2, and HMX/MnO2 obtained from DSC experiments are basically consistent with our predicted T30 values, indicating that it is feasible to predict the catalytic activity based on the adsorption calculation, and it is expected that these simple structural properties can predict adsorption energy to reduce the large quantities of computation and experiment cost.
1.Introduction
Energetic oxidants and combustion catalysts are important components of solid propellants.One of the main functions of the combustion catalyst is increasing the combustion rate of the solid propellant.Good combustion catalyst can decrease thermal decomposition temperature and reduce activation energy of the energetic components in experiment.At present, the selection of combustion catalysts still relies much on experiments.After the materials were prepared, a series of characteristics and analysis including differential scanning calorimetry (DSC), differential thermal analysis(DTA),thermogravimetric method(TG)and other methods are used to screen out the excellent combustion catalysts.This process inevitably undergoes a lot of trial and error.Now,theoretical calculation instead of traditional experiment as an effective means is used to analyze the catalytic effect of combustion catalysts on energetic oxidants.
Several studies have investigated the adsorption and decomposition of energetic materials(EMs)on the surface of combustion catalysts.The adsorption and electron transfer of various EMs such as 2,4,6-trinitrotoluene (TNT), 1,1-diamino-2,2-dinitroethylene(FOX-7), nitroguanidine (NQ), 5-nitro-2,4-dihydro-3H-1,2,4-triazol-3-one (NTO) on the Al2O3(001) surface were discussed by Manoj K.Shukla et al.[1-3].Ju Xuehai et al.[4-7] studied the adsorption and dissociation of dihydroxylammonium 5,5'-bis(tetrazole)-1,1'-diolate (TKX-50), cyclotetramethylene tetranitramine(HMX), cyclotrimethylenetrinitramine (RDX), hexanitrohexaazaisowurtzitane(CL-20)and FOX-7 on the surface of Al(111).All the results indicated that the more dissociated free radicals originated by EMs,the stronger the adsorption energy.The dissociation mechanism of RDX, HMX, FOX7 and CL-20 on the surface of MgH2were researched by Peng [8] and Zhang et al.[9,10].Moreover,Zhang et al.further compared the computation results of CL-20 and FOX-7 with experiments and found that CL-20, which was easy to dissociate nitro, would observe NO2gas earlier than FOX-7 in the decomposition experiment.This theoretical simulation is consistent with the experimental results.
Additionally, our previous work has demonstrated that the catalytic effect of Bi-Fe2O3on HMX was better that of Fe2O3[11].In subsequent adsorption computations, Bi-Fe2O3not only decomposed HMX but also promoted the reaction of decomposition gas,which was consistent with experiment results [12].It can be inferred that preliminary adsorption calculation is helpful to evaluate the activation energy.The role of intermediates in the decomposition process should not be neglected.Volcanic curve correlates the catalytic activity of the reaction with the adsorption energy of key species, requiring that the adsorption energy of the catalyst for intermediates should not be too high or too low [13].However, it is difficult to determine the intermediates due to the complexity of the HMX decomposition process [14].So the adsorption configurations of HMX on the combustion catalysts was considered emphatically.
Since metal oxides are widely used as combustion catalyst, the adsorption configurations of HMX on 15 metal oxides was calculated.The influence of the O atom on the catalytic performance was revealed by analyzing the adsorption configuration.A volcano plot was established to clarify the relationship between adsorption energy of HMX, O and T30 values based on the existing experimental data, and this plot was used to predict T30 values of other metal oxides.To further save computation resources, the relationship between intrinsic properties of metal oxides and adsorption energy was analyzed.Finally,DSC technology was used to verify the predicted values.The consistent tendency between the predicted values and the experimental values shows the feasibility of predicting activation energy based on the adsorption energy.
2.Computational detail
All computations were performed using the DMol3 module of Material studio (MS) software based on the DFT [15].Electron exchange and correlation effects were described by the generalized gradient approximation(GGA)proposed by Perdew-Burke-Ernzerh(PBE)[16].The DFT-D method proposed by TS was used to describe the van der waals (VDW) interaction [17].All core electrons of metal with element number greater than 21 were represented by DFT Semi-core Pseudopots(DSPP)method,and those with element number less than 21 were represented by All Electron [18,19].Double-numeric quality plus polarization functions were applied to all atoms during the calculations[20].The spin polarization was set in consideration of the magnetic properties of partial metal oxide.Given the efficiency and accuracy of calculation,the Brillouin zone was integrated using a grid of 3 × 3 × 1 Monkhorst-Pack scheme.Smearing value was 0.01 Ha.The convergence criteria for structure optimization and energy calculation were set as follows:(a)The SCF tolerance is 1.0 × 10-6Ha; (b) The energy tolerance is 1.0 × 10-5Ha; (c) The maximum force tolerance is 2 × 10-3Ha/Å; (d) The maximum displacement tolerance is 5×10-3Å 15 Å is taken as the thickness of vacuum layer to avoid the interaction between the plates.The surface energy of metal was defined as Eq.(1).
whereEslabis the total energy of the selected slab supercell,Ebulkis the energy of the bulk crystal per atom,n is the number of atoms in the slab, andAis the area of the slab.When the number of n has little influence onEsurf,the repeat units slab was selected as model.
The adsorption energy of gas molecules on the surface was defined as Eq.(2).
whereEadsdenotes the adsorption energy,EHMX/surfaceis the total energy of HMX adsorbed on metal oxides,EHMXandEsurfacerepresent the energy of HMX and metal oxide, respectively.
3.Results and discussion
3.1.Adsorption configuration
There are many experimental studies of metal oxides as combustion catalysts, the decomposition temperature and apparent activation energy are often measured as well.The adsorption configurations of HMX on the metal oxides (TiO2, ɑ-Al2O3, PbO,CuO,ɑ-Fe2O3,Co3O4,Bi2O3,NiO,ZnO,Cr2O3,γ-Al2O3,γ-Fe2O3)was calculated referring to these existing experimental data,which was convenient for comparing the theoretical and experimental values.The adsorption configurations of HMX on other metal oxides(CdO,MgO, SnO2) were also computed.The slab model of metal oxides mainly selected the various common stable crystal face [21-33].For example,the(001),(012)surface of ɑ-Fe2O3and Cr2O3were all selected.Here the nitro of HMX is vertical with surface ensuring that more atoms interact with the metal sites, as the computation of β-HMX on many surfaces shows that the ring of HMX parallel to the metal oxide usually has strong adsorption [6,9], and the metal of metal oxide are often regarded as active site[21,31,32].
Fig.1 shows the stable adsorption configurations of HMX on the surface of metal oxides.Table 1 lists the bond length of HMX and Table 2 lists the adsorption energy.HMX has strong chemical adsorption energy on the surface of Co3O4,Cr2O3.The adsorption of HMX on Co3O4is the strongest with an adsorption energy of 4.35 eV.As the N-O bond of HMX is elongated by 0.13 Å,the nitro groups of HMX are connected closely with the surface Co to form five Co-O bonds with an average bond length of 1.94 Å, which is close to that of metal oxide.When HMX interacts with Cr2O3,four Cr-O bonds and two hydrogen bonds are formed with an adsorption energy of 4.05 eV on Cr2O3(001),and the N-O bond of HMX is stretched by 0.18 Å.The adsorption of HMX on Cr2O3(012) only forms three Cr-O bonds with an adsorption energy of 3.38 eV.
For the adsorption of HMX on ɑ-Fe2O3(001),there are only three O atoms of HMX interact with Fe atoms to form Fe-O bonds and three H atoms form hydrogen bonds with surface O.Its adsorption energy of 3.57 eV is weaker than that of Co3O4and Cr2O3(001).However, the adsorption energy of HMX on ɑ-Fe2O3(012) is only 2.32 eV.The adsorption energy of HMX on TiO2is 3.45 eV,which is close to that of ɑ-Fe2O3(001).Here three nitro groups of HMX are tightly adsorbed on the surface Ti and break the Ti-O bonds leading to surface deformation.The adsorption energy of HMX on ɑ-Al2O3is 3.28 eV along with the formation of two Al-O bonds and three hydrogen bonds.Among these structures,it is noteworthy that the N-O bond of HMX is stretched the longest on Cr2O3(001)and two Ti-O bonds of TiO2are broken.
Moreover, the adsorption of HMX on NiO, Bi2O3(120), γ-Al2O3,MgO and SnO2is relatively weak and their adsorption energy are 2.99 eV, 2.65 eV, 3.00 eV, 2.54 eV, 2.45 eV, respectively, which is attributed to the less bonding between HMX and metal oxides.For example,four Ni-O bonds are formed with an average bond length of 2.09 Å on the surface of NiO.There are no bonds formed on the surface of Bi2O3(120) and γ-Al2O3, but surface deformation is obvious.The interaction of HMX with MgO and SnO2is not even as strong as that of NiO.HMX has weak adsorption energy on the surface of Bi2O3(002), PbO(010), PbO(002), ZnO,γ-Fe2O3and CuO,which are 2.28 eV,1.94 eV,1.71 eV, 2.08 eV, 2.18 eV and 1.75 eV,respectively.On these surfaces,usually only one or two nitro groups interact with the surface metal atoms and no bond is formed.

Fig.1.Adsorption configurations of HMX adsorbed on metal oxide.
The most stable adsorption configuration of HMX on metal oxides is that all three nitro groups interact with the surface metal atoms, and part of H forms hydrogen bonds with surface O.Apparently, the interaction of nitro on HMX with the metal atoms of the metal oxide and the formation of hydrogen bonds promote the strong adsorption energy.The N-O bond of HMX is obviously elongated comparing other bonds,while the C-N bond,N-N bond,and C-H bond are basically unchanged.
3.2.Adsorption process analysis
The difference of density of states (DOS) before and after adsorption was analyzed.The crystal face of metal oxides with high adsorption energy is preferentially considered.The total DOS and the partial DOS of the metal oxides(TiO2,CuO,Co3O4,ɑ-Fe2O3(001),NiO, Cr2O3(001), ZnO, ɑ-Al2O3, Bi2O3(120), PbO(010)) were calculated and shown in Fig.2.
Generally,the contribution of s,p orbitals primarily comes from O and the contribution of d orbitals primarily comes from metal atoms in the DOS of metal oxides.HMX mainly hybridizes with metal-d orbitals and O-p orbitals for the DOS of HMX adsorbed on metal oxides.And the interaction is mostly in the range of -7.5-0 eV and -20-(-15) eV limited by the characteristics of metal oxides.There is basically no change except for TiO2comparing the DOS of metal oxides before and after adsorption.This may be due to the broken Ti-O bonds of TiO2during the adsorption, the dissociated O atoms weakens the peak of O-s, p orbital and Ti-d orbital.For non-transition metal oxides (NTMO)with weak d orbitals, only O-p orbitals interacts with HMX resulting in weaker adsorption capacity than transition metal oxides(TMO).The DOS of γ-Al2O3and γ-Fe2O3is similar with ɑ-Al2O3and ɑ-Fe2O3and not displayed.And the DOS of CdO,MgO,SnO2was omitted owing to its relative weak adsorption.
The charge transfer of HMX on metal oxides is shown in Table 2 and Fig.3.HMX loses the most electrons on TiO2and SnO2,followed by a few electrons on Fe2O3, ZnO and CuO.The acquirement ofelectrons on TiO2maybe owing to the broken Ti-O bond.Amount of other metal oxides tend to lose electrons after adsorbing HMX.
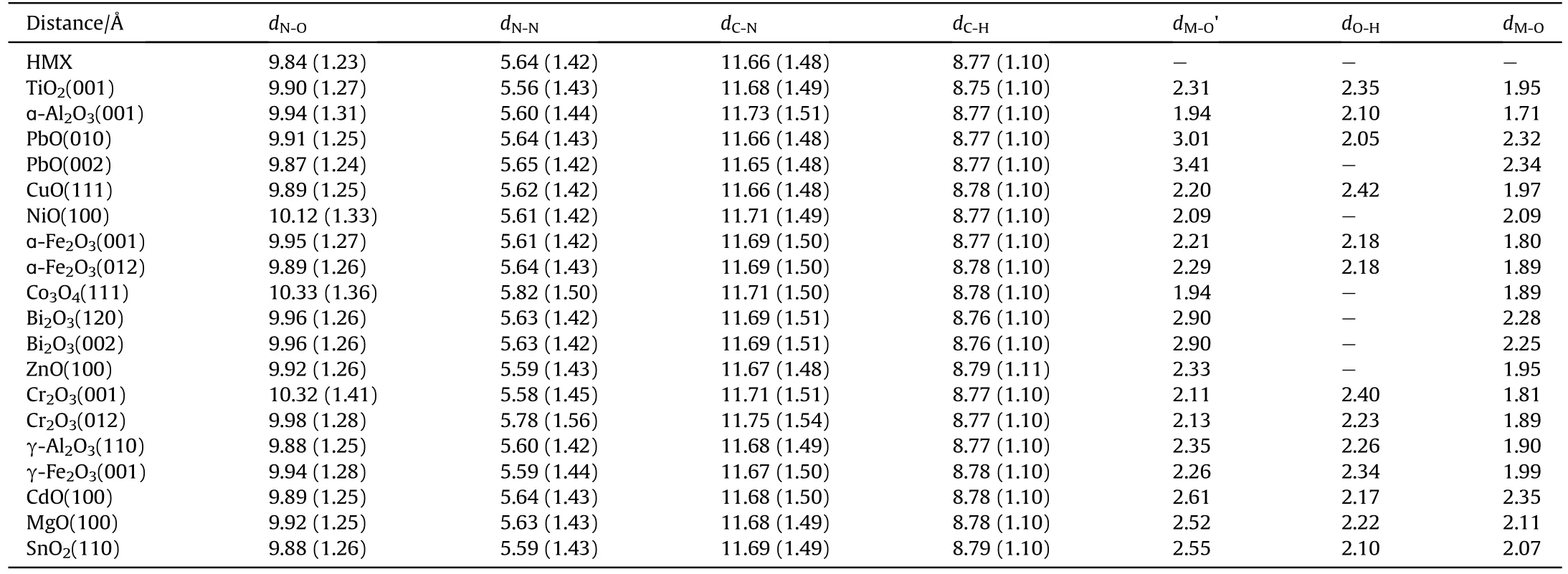
Table 1The bond length of HMX adsorbed on metal oxide before and after adsorption.
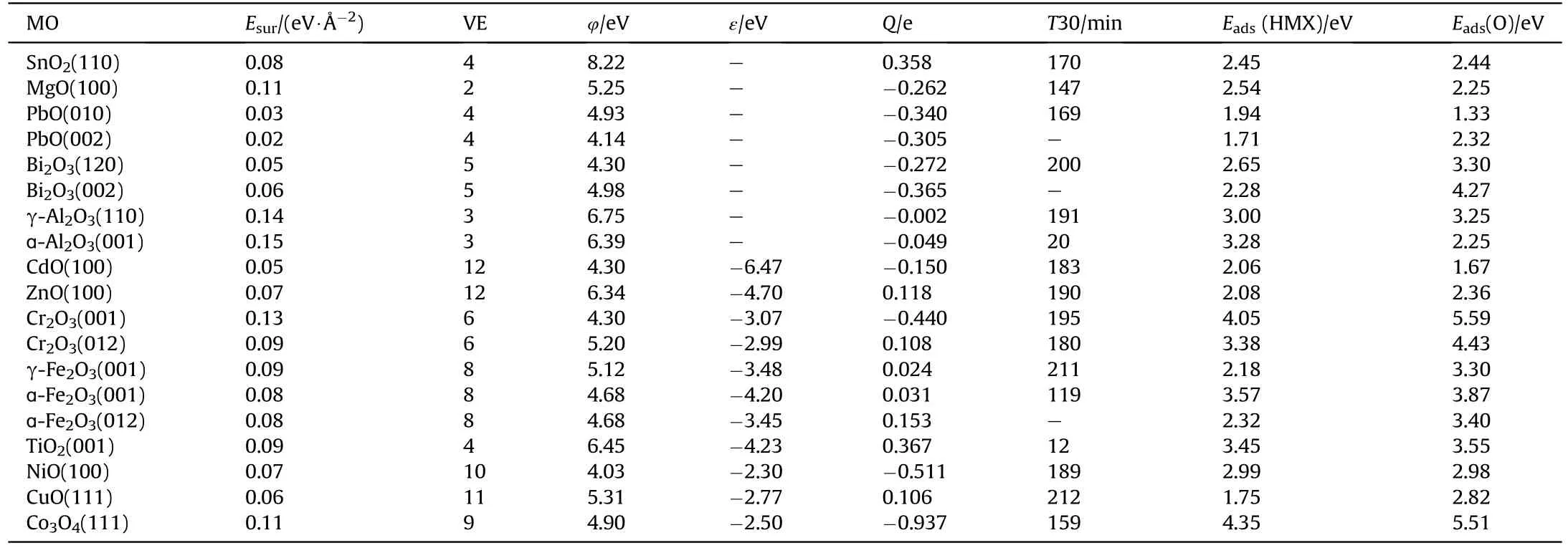
Table 2Structural properties of metal oxide and adsorption energy of HMX.
Previous study thought that the nitro of HMX gaining more electrons would promote the dissociation of N-NO2bond [34].Here HMX on Co3O4gain the most electrons and the N-NO2bond is stretched by 0.08 Å.There is no elongation of N-NO2bond on other metal oxide,only N-O bond was significantly elongated.This weak charge transfer is not enough to induce N-NO2decomposition.
3.3.Association between adsorption energy and activation energy
The adsorption energy of HMX on metal oxides was correlated with the experimentally obtained activation energies.Here, the slab model of metal oxides chooses the crystal surface with similar surface energy but higher adsorption energy.Activation energy in the same paper were selected due to the existence of sample size and instrument error during the experiment.
Liu et al.[35]studied the catalytic effect of several metal oxides(Al2O3, TiO2, Fe2O3, Co3O4, PbO, Bi2O3, NiO, CuO) on HMX, and measured the decomposition temperature and apparent activation energy by TG and DSC.The adsorption energy of HMX on these metal oxides is fitted with the activation energy from Liu et al.[35],as shown in Fig.S1, theR2value is 0.8.As the adsorption energy increases,the activation energy gradually decreases.But these four points is unable to represent the overall situation.
Since the value ofT30 (time required for the decomposition depth of HMX to reach 30%)in the experiment is identical with TG results and has a correlation of 93% with the activation energy(Fig.S2),T30 is used to replace activation energy and fitted with adsorption energy, as illustrated in Fig.4.TheR2value of 0.28 demonstrates poor linear correlation.In general, the catalytic activity of ɑ-Al2O3,TiO2,Fe2O3,Co3O4with strong adsorption energy is better than that of PbO, Bi2O3and CuO with weak adsorption.A rough qualitative relationship can be established between adsorption and activation energy.

Fig.2.Density of states of HMX adsorbed on meatal oxide and pure metal oxide.
Some metal oxides are of strong adsorption energies but low catalytic efficiency for decomposition of HMX.For example, Co3O4and NiO stretched the N-O bond of HMX by 0.13 Å and 0.10 Å,respectively, but poor catalytic effect.Analysis of adsorption configuration manifests that the O atoms of HMX were tightly bonded to the surface Co and Ni,and the formed bond length were comparable to those of metal oxides.It was speculated that O would occupy the surface metal site when the elongated N-O bond of HMX was broken, thereby reducing the surface activity.Hence the adsorption of O radicals on the surface of metal oxides is emphatically considered, the adsorption configuration and energy can be seen in Fig.S3.The strong adsorption energy of O radicals on Co3O4indicates that the dissociated O prefers to firmly adsorb on the surface and occupy the active site.
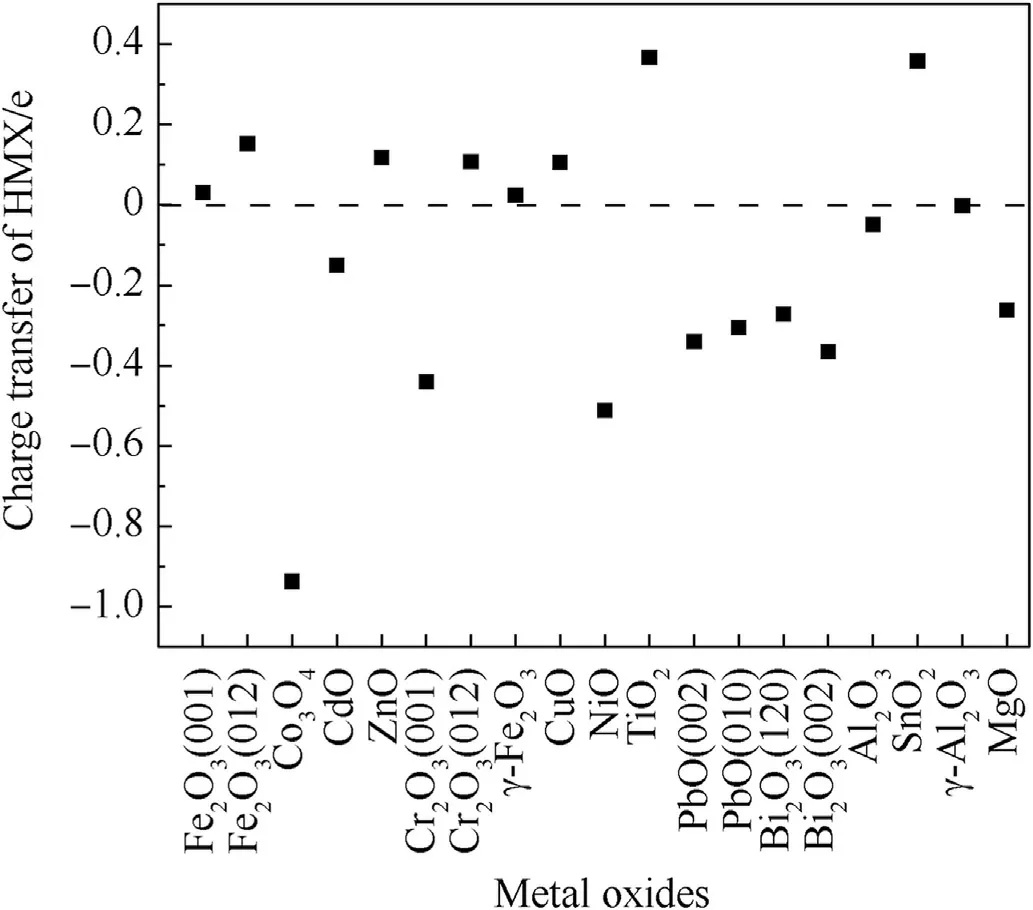
Fig.3.Charge transfer of HMX on metal oxide.

Fig.4.Adsorption energy of HMX is correlated with T30.
On the one hand, it is hoped that HMX has a strong adsorption energy on the surface of metal oxides, which promotes the longstretching of HMX bonds and easy decomposition.On the other hand,the O atom on the nitro group of HMX tends to dissociate and occupy the active center,thus reducing the activity.To maintain the activity of the metal oxide surface, the adsorption capacity of the dissociated O radical can not be too strong.Therefore, the correlation betweenT30 and the adsorption energies of HMX and O radical on metal oxides (ɑ-Al2O3, ɑ-Fe2O3, Co3O4, NiO, PbO, Bi2O3and CuO) is presented in Fig.5, based on the research of Liu et al.[35] The TiO2was not taken into account due to its broken Ti-O bond,the dissociated O may slow down the occupation of O radical.Obviously, an ideal combustion catalyst ought to have strong adsorption of HMX and weak adsorption of O free radicals located at the bottom right corner of Fig.5.

Fig.5.Relationship between T30 and adsorption energy of HMX and O.
Since the adsorption energy of other metal oxides (Cr2O3, γ-Al2O3,γ-Fe2O3,ZnO,CdO,MgO,SnO2)have also been calculated,the T30 values of these metal oxides can be roughly inferred from the volcanic plot.The adsorption energy of HMX and O radicals on these metal oxides was marked with triangle and pentagram in Fig.5 and the estimated T30 values was listed in Table 2.To validate the accuracy of the inferred T30 values,the catalytic efficiency(Ka)of various metal oxides (Cr2O3, γ-Al2O3, γ-Fe2O3, ZnO) on HMX researched by Pivkina was compared with our estimated values[36].As researched by Tang et al., different crystal planes of ZnO have different catalytic effects on AP [37], the (012) surface and(001) surface of Cr2O3also exhibit different catalytic effect.The obtained T30 values are in order of TiO2<ɑ-Al2O3<ɑ-Fe2O3<Cr2O3(012)<NiO<Cr2O3(001)<ZnO<γ-Al2O3<γ-Fe2O3and the value of NiO,ZnO and Cr2O3are close together,which basically agree well with the experimental Ka values TiO2>ɑ-Al2O3>Cr2O3>γ-Fe2O3>γ-Al2O3(nano-sized)and ɑ-Al2O3>NiO>Cr2O3>ZnO(micron-sized), indicating rationality of our speculation.The difference of γ-Fe2O3and γ-Al2O3is mainly attributed to their specific surface area in the experiment, which are 161 m2/g, 36.4 m2/g,respectively [36].In general, this method of evaluating catalytic capability is feasible.
Thus, the catalytic effect of metal oxides on HMX can be qualitatively estimated according to the adsorption energy of HMX and O radical.The order of predicted catalytic activity is ɑ-Al2O3>ɑ-Fe2O3>MgO>Co3O4>PbO>SnO2>CdO>Cr2O3>NiO>ZnO>γ-Al2O3>-Bi2O3>γ-Fe2O3>CuO based on Fig.5.However,this investigation is concentrated on those ineffective combustion catalysts which tend to dissociate O,the applicability for those deep dissociated catalysts along with gas product and free radicals should be further discussed.For example, HMX would dissociates NO2on some combustion catalyst,the adsorption of NO2on active site is not as strong as that of O radical.
3.4.Effect of structural properties
Previous studies have revealed that structural properties such as surface energy,valence electron,d-band center and work function are related to adsorption energy [38-40].These important properties are listed in Table 2.The main factors affecting HMX adsorption and decomposition are analyzed based on these structural properties, which facilitates the screening of catalysts and saves computational resources.The fitting results of these structural properties and adsorption energies are displayed in Fig.6.Valence electron and work function shows poor correlation with adsorption energy as shown in Fig.S4.
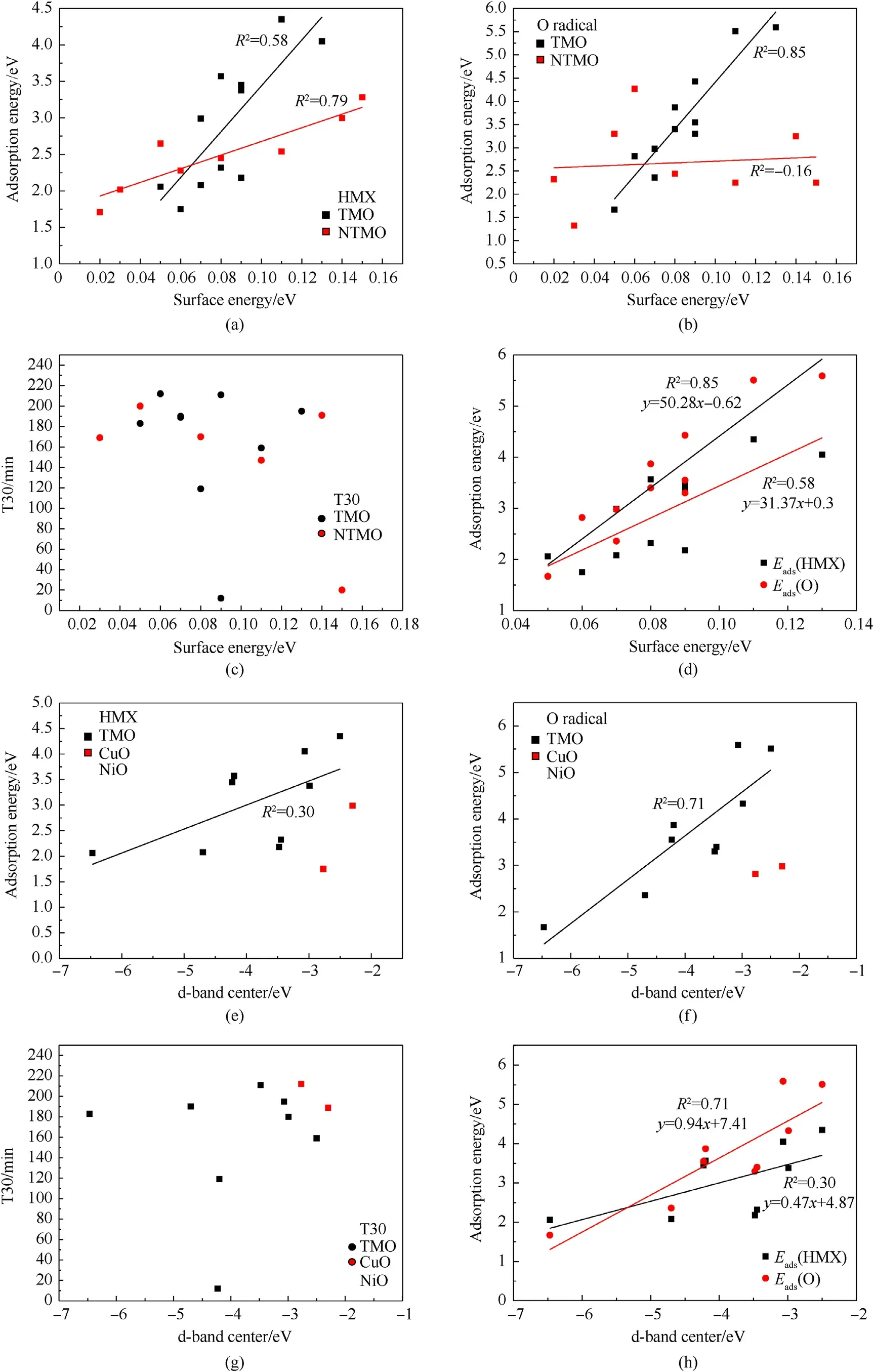
Fig.6.The correlation of structural properties with adsorption energy and activation energy.
Figs.6(a) and 6(b) present the positive correlation between surface energy and adsorption energy.Metal oxides are divided into TMO and NTMO for fitting.There is a linear relationship between adsorption energy of HMX and the surface energies of TMO and NTMO.TheR2values are 0.59,0.80,respectively.As the surface energy increases, the adsorption energy increases as well.The adsorption energy of O correlates well with the surface energy of TMO, while the correlation with NTMO is poor.The R2values are 0.85, -0.16, respectively.Fig.6(c) displays non linear relationship between surface energy and T30.Since there is a linear relationship between surface energy and adsorption energy on TMO as shown in Fig.6(d), the adsorption energy of HMX and O on TMO can be predicted according to this linear relationship.
The d-band center as an important structural feature of TMO is correlated with adsorption energy in Figs.6(e)and 6(f).Majority of the points are positively correlated with the adsorption energy,and the remaining outliers(CuO,NiO)are analyzed in conjunction with the DOS in Fig.2.The electron distribution of CuO and NiO in dorbital mainly concentrates on the peak near-2.50 eV,comparing with other TMOs.Hence the calculated d-band centers are -2.77 eV, -2.30 eV, respectively, which are naturally close to the location of peak.Norskov J.K.revealed the linear relationship between d-band center and adsorption energy of O on metal.The electrons of metal is uniformly distributed in energy band [41].However, the electrons of metal oxides (CuO, NiO) usually concentrates on small region,this situation is different from metal.The HMX interacted with TMO at a wide range of-7-0 eV,not only at one point in the electron concentration.Such d-band centers of TMO with excessively concentrated electrons is not suitable to describe the absorption energy at this time.The relationship between T30 and d-band center are displayed in Figs.6(g) and 6(h)presents the linear relationship between adsorption energy and dband center.
In summary, the surface energy and d-band center are linearly related to the adsorption energy of HMX and O,and the slope of O is larger than HMX.The fitting effect of surface energy is better than that of d-band center and these structural properties are more relevant to the adsorption energy of O.Therefore, using these simple properties can preliminarily predict adsorption energy,then the T30 values of metal oxides can be obtained by taking adsorption energy into volcano plot.This also shows the importance of surface energy and d-band center for catalytic activity from the side.
3.5.The experiment detection of thermal behavior
The catalytic activity of metal oxides was obtained with the help of above method.However, the activity of some metal oxides predicted by us has not been experimentally verified.And roughly estimation of the activity according to the surface energy is not implemented.Therefore, DSC technology was used to study the thermal decomposition of HMX/MgO, HMX/SnO2, HMX/ZrO2, and HMX/MnO2mixtures.
Nanosized MgO, SnO2, ZrO2and MnO2with particle sizes of 30 nm, 30 nm, 20 nm, 50 nm, were provided by Zhongmai Metal Materials Limited Company.HMX were provided by institute of Xi'an Modern Chemistry Research Institute.These metal oxides were fully mixed with HMX in the mass ratio of 1:4.Then the mixtures were used as samples for DSC tests.DSC were determined with a 200F3 differential scanning calorimeter(Netzsch,Germany)and the conditions were: nitrogen gas purity, 99.999%; N2flowing rate, 40 mL/min; sample mass, about 0.5 mg; heating rate(β): 5.0,10.0, 15.0 and 20.0°C/min-1.Apparent activation energy (Ea) of decomposition process was evaluated by Ozawa method with DSC data from different heating rates.DSC heat flow curve was shown in Fig.S5.The peak temperature(Tp)of decomposition,Eavalues,and coefficient(r) for these four mixtures were obtained and listed in Table 3.
TheEavalue of MgO is lower than SnO2,which is consistent with the lower T30 value of MgO in Fig.5.The stable crystal face of ZrO2and MnO2was calculated and listed in Table 4.Then the adsorption energy of HMX on ZrO2was estimated referring to the linearrelationship between surface energy and adsorption energy in Fig.6(c).The predicted adsorption energy of ZrO2and MnO2was listed in Table 4 and marked with pentagram in Fig.5.Since the R2value is only 0.58,the adsorption energy of HMX and O on ZrO2and MnO2was calculated,as listed in Table 4.The calculated adsorption energy of HMX on ZrO2and MnO2are 2.09 eV,1.74 eV,respectively.And the adsorption energies of O are 1.49 eV, 0.2 eV, respectively.Configurations were listed in Fig.S6.Obviously,the predicted value has a large error.The estimated T30 values based on the predicted adsorption energy are also listed in Table 4.The low surface energy of MnO2(002) surface indicate an extremely weak adsorption.Apparently, the T30 value of ZrO2is smaller than that of MnO2,which is consistent with the apparent activation energy measured by us.
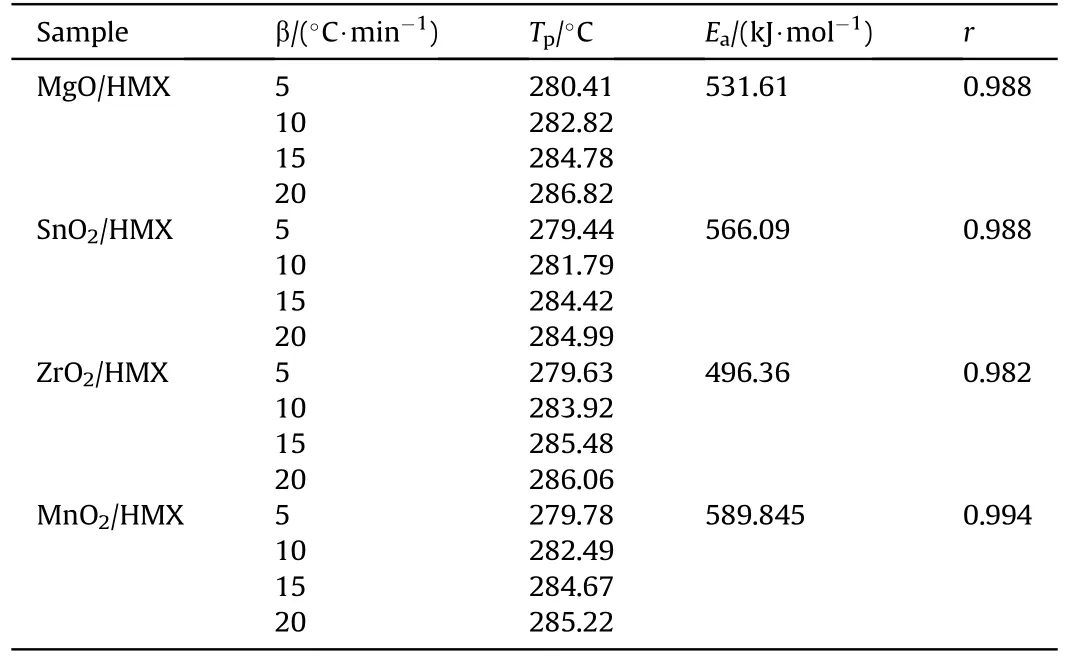
Table 3The apparent activation energies of samples.
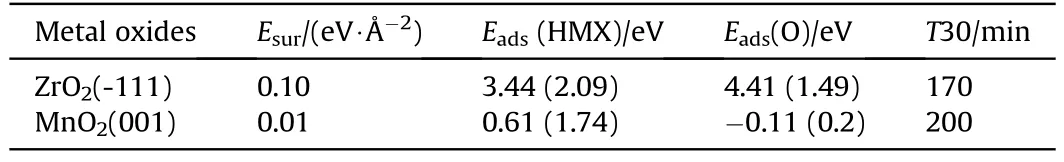
Table 4The predicted adsorption energy and T30 values of HMX.
Finally,theEavalues of these four mixtures was compared with their T30 values.The order of T30 values (MgO<ZrO2≈SnO2<MnO2) is roughly consistent with tendency ofEa(ZrO2<MgO<SnO2<MnO2).The relatively small T30 value of ZrO2maybe due to the error in estimating adsorption energy,as the fittingR2value of adsorption energy is only 0.59.Besides,the smaller particle size of ZrO2maybe decrease its activation energy.According to the general trend, it is feasible to replace the experiment with simple adsorption calculation.
4.Conclusions
The adsorption configuration of HMX on numerous metal oxides(Al2O3,TiO2,Fe2O3,Co3O4,PbO,Bi2O3,NiO,CuO,Cr2O3,γ-Al2O3,γ-Fe2O3, ZnO, CdO, MgO, SnO2) was calculated to establish the correlation between adsorption energy and activation energy.
(1) NiO and Co3O4with strong adsorption energy performs relatively weak catalytic effect among these metal oxides.TiO2exhibits excellent catalytic performance owing to broken Ti-O bonds.It is concluded that the O atoms are easily dissociated from HMX and tightly adsorbed on the metal atoms, resulting the reduced active site and weak catalytic performance.
(2) The connection betweenT30 values and adsorption energy of HMX and O was established and descripted as volcano plot.It is found that metal oxides which possess strong adsorption energy of HMX and weak adsorption energy of O have low apparent activation energy on decomposing HMX.Moreover,T30 values of other metal oxides predicted by this volcano plot are consistent with experiment.
(3) The adsorption energy of HMX and O are positively correlated with surface energy and d-band center of TMO.Hence the adsorption energy of HMX on ZrO2and MnO2was predicted by this linear relationship, then theT30 values were estimated based on the volcano plot.Finally,DSC technology was used to validate the predicted T30 values by analyzing the thermal decomposition behavior of HMX/MgO, HMX/SnO2,HMX/ZrO2,and HMX/MnO2mixtures.The obtainedEavalues are roughly consistent with our predictions.
It is hoped to predict the adsorption energy through simple structural properties, and then predict the activation energy according to the volcanic plot, so as to save computational and experimental resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Key Science and Technology Innovation Team of Shaanxi Province (No.2022TD-33), National Natural Science Foundation of China (Grant Nos.21373161,21504067).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.03.002.
- Defence Technology的其它文章
- The interaction between a shaped charge jet and a single moving plate
- Machine learning for predicting the outcome of terminal ballistics events
- Fabrication and characterization of multi-scale coated boron powders with improved combustion performance: A brief review
- Experimental research on the launching system of auxiliary charge with filter cartridge structure
- Dependence of impact regime boundaries on the initial temperatures of projectiles and targets
- Experimental and numerical study of hypervelocity impact damage on composite overwrapped pressure vessels

