Combination of Nitrogen-Rich Skeleton and Coordination Group:Synthesis of a High-Energy Primary Explosive Based on 1H-Tetrazole-5-Carbohydrazide
Tingwei Wng , Zuji Lu , Shu Bu , Bolong Kung , Lu Zhng , Zhenxin Yi ,Kun Wng , Shungun Zhu , Jinguo Zhng ,*
a State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China
b School of Chemical Engineering, Nanjing University of Science and Technology, 200 Xiaolingwei Street, Xuanwu, Nanjing 210094, China
c Department of Chemistry, Laboratory of Structure and Functional Regulation of Hybrid Materials, Anhui University, Ministry of Education, Hefei 230601,China
Keywords: 1H-tetrazole-5-carbohydrazide Primary explosive Decomposition mechanism Coordination polymers Laser
ABSTRACT The high energy coordination compounds Cu(TZCA)2(ClO4)2 (ECCs-1) was prepared by 1H-tetrazole-5-carbohydrazide (TZCA) with a high energy skeleton and a strong coordination ability group.At the same time, the reaction activity of the ligand was explored, and the single crystal structure of it and intermediate were obtained.The structures of all substances were characterized by IR and EA.And the structure and composition of ECCs-1 are confirmed by ESP, AC, SEM and ICP-OES.Physical and chemical properties tests show that ECCs-1 has an acceptable thermal stability (Td = 177°C) and extremely sensitive mechanical stimulation(IS=1 J,FS=5 N).The comprehensive performance test results show that ECCs-1 has excellent initiation ability.In addition, the decomposition mechanism of ECCs-1 is explored from two aspects of experiment and theoretical calculation.
1.Introduction
The essence of energy materials (EMs) determines their work conditions: only relying on tiny stimuli, chemical reactions can occur, and a large amount of energy can be released [1-4].Of course, the process is short, intense, autonomous and without the participation of external factors.These stimuli can be mechanical(such as impact, friction), or light, heat, electricity, and so on [1,5].With the deepening of research,more and more new concepts and new types of materials are used in energetic materials,such as cocrystals [6-10], PBX (polymer bonded explosive) [11,12], metallic hydrogen[13,14],metastable interstitial composites(MICs)[15,16],and so on.In particularly, due to the concept of 'nitrogen-rich',energetic materials, including explosives and pyrotechnics, have developed to a higher level [17-20].These nitrogen elements can not only come from heterocyclic skeletons, such as pyrazole,triazole, tetrazole, but also come from substituent groups, such as-NH2, -NO2, -N3[21-24].
Thanks to the contribution of'rich nitrogen',the development of primary explosive has also made great achievements, including metal salts, metal-free organics and so on [25-27].Among them,energetic coordination compounds (ECCs), especially those containing energetic anions such as -ClO4, -NO3and -ClO3, have entered the field of vision of researchers with their unique advantages [28-30].The uniqueness of ECCs is reflected in the adaptability of sensitivity and energy,which is mainly contributed by different ligands.The high energy of ECCs is reflected not only in the use of ligands, but also in the use of inorganic salts, such as Cu(ClO4)2, Cu(NO3)2and so on [31,32].

Fig.1.The combination of nitrogen-rich skeleton and coordination group.

Fig.2.The route of synthesizing TZCA and ECCs-1.
Initially,the ligands which used in the preparation of ECCs were some very simple small molecules, such as NH2NH2and CHZ (carbohydrazide) [33,34].Limited choices have prevented the development of ECCs, so heterocyclic molecules have attracted more attention.We have done a lot of research in this area, such as the preparation of high energy complexes by using triazole, urazine and furazan as the ligands (see Fig.1).Nowadays, tetrazole and its derivatives are more widely used, and scholars represented by Klap¨otke have done a lot of researches[35-43].However,the single coordination site tetrazole skeleton participating in the formation of coordination bonds only can form ECCs with negative oxygen balance like Cu(L)4(ClO4)2types(L:ligands)[41,42,44].The binding force of the single-dentate structure ligand is small, which makes the decomposition temperature of the complex low and the oxygen balance constant small.Therefore, it is necessary to design a new ligand,which not only meets the requirements of tetrazole fuel,but also meets the needs of multi-dentate coordination.
In ours work, the ligand TZCA was prepared by one-step method.The coordination sites and properties were analyzed,and the coordination model was predicted.The structures of all intermediate compounds were characterized by IR, EA (elemental analysis) and single XRD (single crystal X-ray diffractometry).The structure and composition of ECCs-1 was determined by IR, ICPOES, EA and SEM.In addition, the stability and detonation performance analysis are carried out, and the results show that ECCs-1 has an acceptable thermal stability and detonation performance.
2.Experimental
2.1.Caution!
In this work,the target product we prepared is very dangerous.Violent explosions occurred during some tests, such as DSC, TGA and HN tests.We strongly recommend that operators should make safety protection (masks, gloves, explosion-proof panels) when synthesizing and using these substances.All operations must be carried out in professional and protective equipment.Physical treatment of these target products,such as grinding,crushing,etc.,is not recommended.
2.2.Synthesis of 1H-tetrazole-5-carbohydrazide (TZCA 2)
The raw material (compound 1, 100 mmol) was suspended in 200 mL anhydrous methanol and slowly added 120 mmol hydrazine hydrate (80%) under intense stirring.The reflux reaction was carried out at 85°C for 8 h.After the reaction is over, put it in the refrigerator(3-5°C)for 5-6 h,and a large number of white target products can be obtained.Yield: 95%.IR (KBr, ν/cm-1): 3336(m),3253(m), 1670(m), 1591(m), 1522(s), 1389(s), 1288(s), 1092(s),985(s), 937(s), 644(s).MS (ESI),m/z: 127.9 [C2H3N6O-].Elemental analysis(%)for C2H4N6O(Mr=128.04 g/mol):calcd.C 18.74,H 3.12,N 65.60; found C 18.82, H 3.17, N 65.53.13C NMR (125 MHZ,DMSO‑d6):δ 161.2,157.1.
2.3.Synthesis of Cu(TZCA)2(ClO4)2 (ECCs-1)
The ligand (10 mmol) was dissolved in 20 mL water, and then Cu(ClO4)2solution(15 mL,1 M)was added.At this time,there will be a lot of green precipitation immediately.The reaction was continued for 10-20 min at room temperature.Filtered, washed with ethanol and dried to obtain the blue-green target compound.Yield: >98% (based on TZCA).IR (KBr, ν/cm-1):1628(s), 1345(s),1096(s), 931(m).Elemental analysis (%) for C4H8N12O10Cl2Cu(Mr=518.53 g/mol):calcd.C 9.26,H 1.54,N 32.40;found C 9.31,H 1.58, N 32.36.
3.Results and discussion
3.1.Preparation of ligands
Based on the mechanism of hydrolysis reaction,1H-tetrazole-5-carbohydrazide (TZCA) can be prepared by the reaction of Ethyl tetrazole-5-carboxylate with hydrazine hydrate.TZCA has good solubility and can be easily dissolved in water.However, when we cultivated the ECCs crystal, we found a very special phenomenon:when HClO4was added to the complex powder, although the powder could be dissolved and the solution became clear,it would immediately produce new precipitation, which was a very rapid process.And the new precipitate is difficult to dissolve in water,acid,DMSO and other solvents.Fortunately,we got a single crystal to prove that the new material is N'-(1H-tetrazole-5-carbonyl)-1Htetrazole-5-carbohydrazide.The reaction mechanism is very simple:the N-bridging reaction about acylhydrazide(R-CONHNH2)is occurred under acidic, which removes the one molecule of[NH2NH2] and forms the new structure (R-CONHNHCO-R).
In order to explore the reaction activity of TZCA, we changed many conditions, such as acid, solvent and temperature.Table S5 shows the reaction conditions including reaction temperature,solvent, acids and feed molar ratios.Because of the high reaction activity, short reaction time and poor solubility of TZCA, it can be proved that the products are the same by simple drying and infrared characterization.The experimental results show that this reaction can occur at different temperatures with different solvents and acids (see Fig.3).
3.2.Synthesis of ECCs-1
TZCA has strong coordination ability and can form green powder when reacting with Cu(ClO4)2.This process is instantaneous and can be carried out without the addition of other catalysts or external conditions.The reaction can be stopped after 5-10 min,and the experiment can also be carried out under room temperature condition.Because of this, the yield is very high.In addition,TZCA can also produce the same phenomenon as other perchlorate reactions, such as Zn(ClO4)2, Ni(ClO4)2and Co(ClO4)2(as shown in Fig.4).
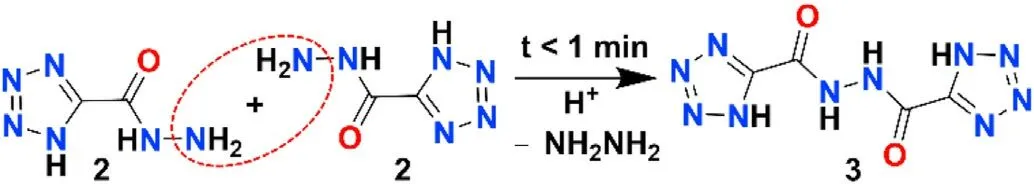
Fig.3.Reaction mechanism of TZCA.

Fig.4.(a)The experimental phenomena of TZCA and Cu(ClO4)2,(b)Reaction results of TZCA with four metals.
In order to explore the morphological structure and composition of ECCs-1,we used SEM-EDS mapping method to characterize it.Because the grinding operation cannot be performed (danger),we can only observe very few solid particles even at the minimum magnification.It can be seen from Fig.5 that ECCs-1 is an amorphous particle with smooth surface.We emphatically analyzed the five elements C, N, H, O, Cl and Cu of ECCs-1.According to the analysis of the EDS test results, their proportion is 10.3, 31.7, 1.4,30.5,13.7 and 12.4, which are similar to the theoretical results.
It is because of the particularity of TZCA that we cannot get the single crystal of ECCs-1 smoothly.In addition to using EA and SEMEDS, we also use the ICP-OES (inductively coupled plasma-optical emission spectroscopy) testing method to obtain the ECCs-1 chemical formula.Dissolve 10 mmol TZCA in 500 mL deionized water (5 parts in total).Then 5 mL, 6 mL, 7 mL, 8 mL and 9 mL of Cu(ClO4)2solution(1 mol/L)were added,and deionized water was added to the total volume of 1000 mL after 4 h.Then the reaction solution was placed for 1 h, and the supernatant was taken to determine the solubility of Cu2+.(M0: The number of moles ofTZCA.M1: The total number of moles of Cu2+.CfandM2: The concentration and molar number of unreacted Cu2+which were determined.M1-M2:the number of moles of Cu2+participating in the reaction.M2=Cf/63.546).From the test results shown in Table 1,it can be seen that TZCA reacts with Cu2+in a ratio of 2:1,so it is inferred that the molecular formula of ECCs-1 is Cu(TZCA)2(-ClO4)2(see Table 2).

Table 1ICP-OES test results of TZCA for Cu2+ ions.
3.3.Coordination site analysis and crystal structure prediction
Fortunately, we got 2 and 3's single crystals and used them for structural verification (shown in Fig.6).Because the crystal structures of them are not our key research objects, we do not need to focus on their structures.Unfortunately, we did not get the crystal of ECCs-1,but we were still very concerned about it.Therefore,we use AC (atomic charge) and ESP (electrostatic surface potential)analysis to explore the coordination sites, coordination elements and coordination ability of the ligand.On this basis, we try to simulate the stacking structure of ECCs-1.The structure and parameters of the ligand are constructed by gaussian 6.0, and the atomic charge analysis is carried out.The extreme points were fitted and analyzed by VMD.
From the point view of AC analysis, all N and O elements are negative (shown in Fig.S4), but this does not mean that each can participate in the formation of coordination bonds.Among the acylhydrazide, there is no doubt that O1 has the strongest coordination ability.Although N5 has more abundant electrons than N6,it does not have the coordination ability because of the conjugation effect and steric hindrance.In the tetrazole skeleton, N1 and N2 have greater potential because their values are smaller.
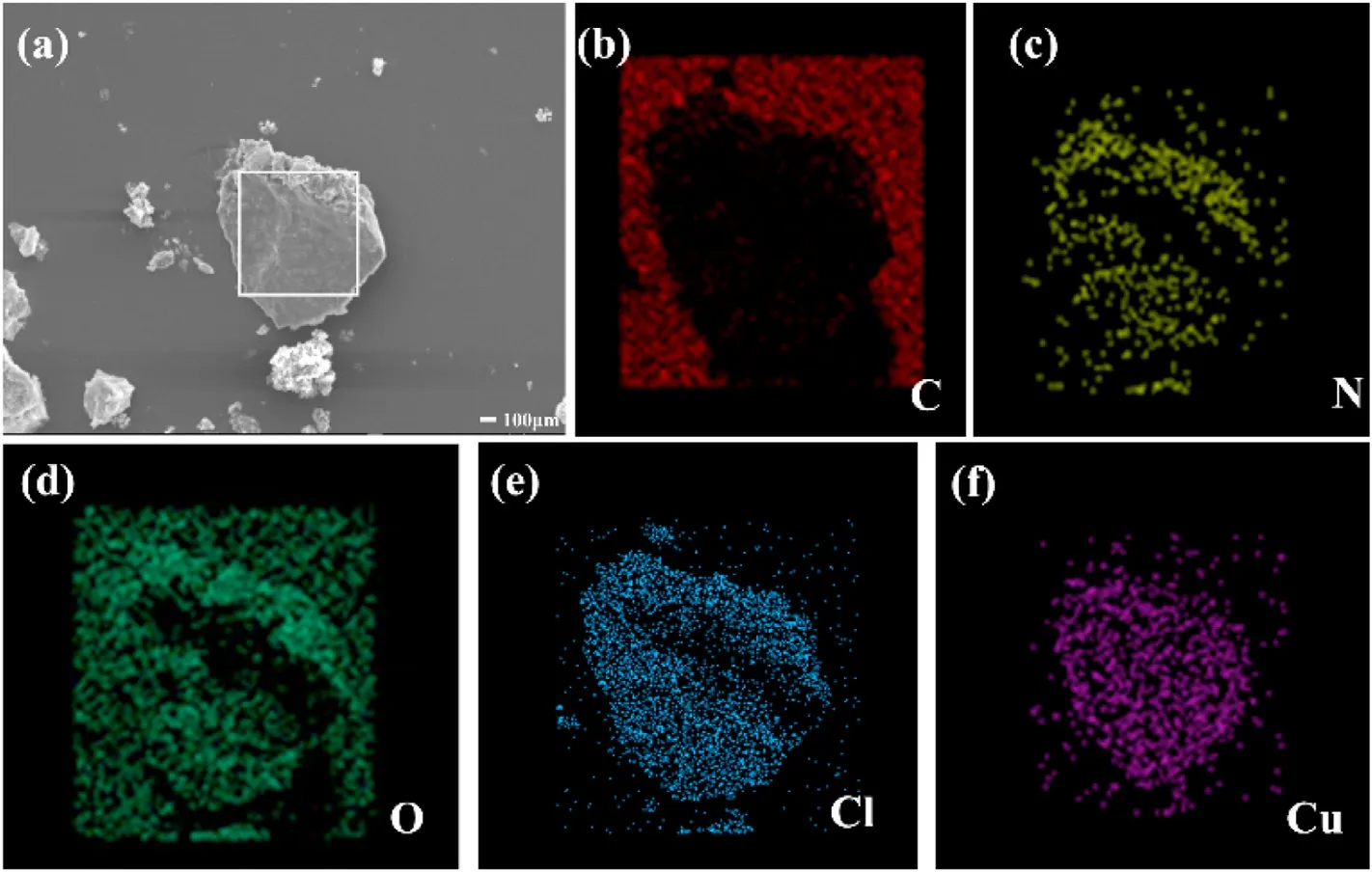
Fig.5.SEM images and EDS spectra of ECCs-1.

Table 2Energetic properties and physical of selected compounds.
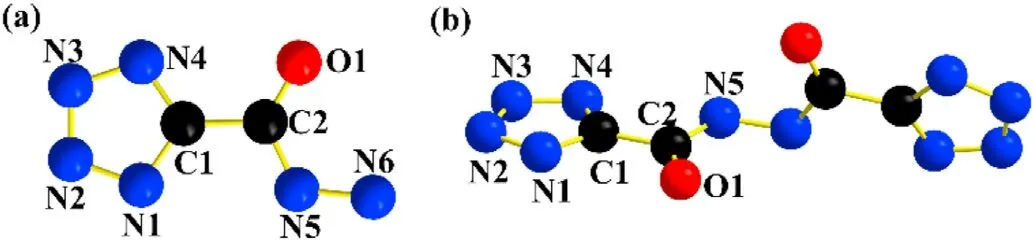
Fig.6.Crystal structure of (a) TZCA and (b) compound 3.
Extreme point and ESP can express the coordination ability of ligands more intuitively(shown in Fig.7(a)).O1 and N6 constitute the strongest extreme point together (-43.25), which is the potential position of the metal.Therefore, the hydrazide group can form the coordination bond,and it is a double-dentate coordination model.In the tetrazole skeleton,the strongest value of the extreme point is N2 (-38.17).However, because there is one coordination element, only single-dentate coordination model can be formed.We choose the most commonly used copper for the construction of coordination bonds.Because its chemical valence is[+2],according to the judgment of coordination chemistry,it will construct ECCs in six-coordination mode(that is,it contains six coordination bonds).Considering thatwill also be involved in the formation of coordination bonds, there are four remaining.Among the two coordination sites, the hydrazide group has the priority because its extreme point is the smallest and only two ligands are needed to construct ECCs.If the complex is constructed with N2 as the coordination site, four ligands are needed, which is obviously very complicated.Therefore, the double-dentate 0D complex constructed by hydrazide can meet the optimal conditions, and this method is the most common.Therefore, we use gaussian 6.0 to construct the coordination mode shown in Fig.7(b).Other detailed parameters about ECCs-1 are shown in the support information.According to our research content, 4-amino-1,2,5-oxadiazole-3-carbohydrazide (AOCA), 1H-pyrazole-4-carbohydrazide (PZCA)and TZCA are all containing hydrazide groups which participating in coordination, and their differences are only due to different skeleton rings.Of course, we are very lucky to get compounds Cu(AOCA)2(ClO4)2and Cu(PZCA)2(ClO4)2by reacting ligands with Cu(ClO4)2, respectively.The structures of Cu(AOCA)2(ClO4)2and Cu(PZCA)2(ClO4)2are consistent, so we simulated the structure of ECCs-1 according to them.
3.4.Physicochemical stability
For Cu(II)-based ECCs, we are most concerned about thermal stability and mechanical sensitivity.The thermal behaviors of TZCA and ECCs-1 were measured by TG-DSC and DTA (shown in Figs.8 and S6; heating rate:5°C/min, atmosphere: N2).
The initial melting temperature of TZCA is 188°C (shown in Fig.S6), and its melting process is accompanied by serious sublimation(the weight loss rate is 43%).When the temperature reaches 242°C,an obvious exothermic peak appears and ends at 282°C(The weight loss rate is 19%).The physicochemical properties of ligands do not seem to affect the that of ECCs-1, because the initial decomposition temperature of ECCs-1 is 177°C, which is much lower than the melting temperature and decomposition temperature of TZCA.Due to the role of coordination bonds and the use of high-energy skeletons, ECCs-1 has violent thermal behavior(shown in Fig.8(a)).Even though the testing sample was only 0.02 mg, there was a violent explosion.Fortunately, we used steel containers to keep the instrument from being damaged.However,during the DTA test, due to the limitations of the conditions, no more suitable container was used and the glass tube was blown up(shown in Fig.8(b), only 0.2 mg for the test).
In addition, the BAM standard method is used to evaluate the mechanical sensitivity of ECCs-1.The results show that it is extremely sensitive (IS = 1 J, FS = 5 N) and more than any other primary explosive we have prepared in previous.Even if TZCA is very insensitive, combining it with Cu(ClO4)2through a simple“coordination bond”makes the whole substance very dangerous.It is even more dangerous than LA,which probably limits the value of its practical application.

Fig.7.ESP analysis of (a) TZCA (a) and the calculated molecular structure of (b) ECCs-1.
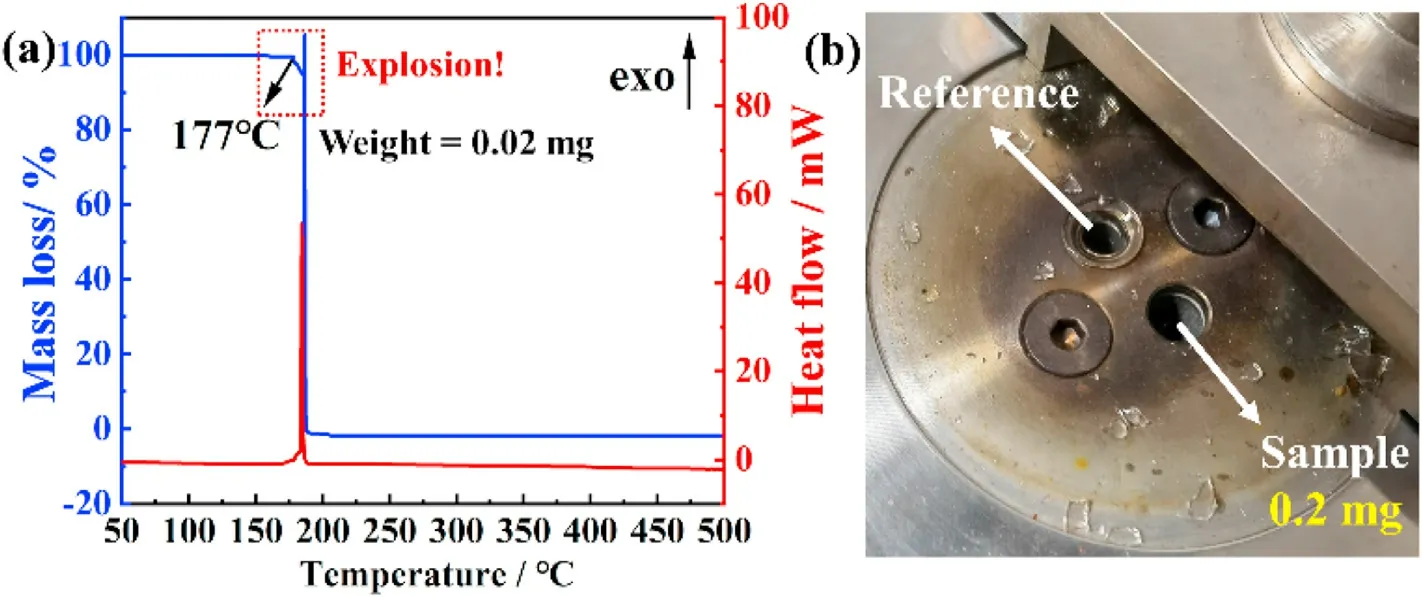
Fig.8.TG-DSC of (a) ECCs-1 and (b) DTA testing result.
3.5.Detonation performance
The ΔcU(constant pressure reaction heat) of ECCs-1 was determined to be 8594 J/g by oxygen calorimeter, and the labeled molar enthalpy of formation was calculated to be 1496 kJ/mol by Hess's law.Compared with our previous work, ECCs-1 has higher detonation velocity (D) and detonation pressure (P) because of its high nitrogen content, oxygen balance constant and density.Judging from the point view of primary explosives,these values are higher than LA (D= 5920 m/s) and [Cu(AzMT)6](ClO4)2(D=7222 m/s).And judging from the explosive point of view,it is close to RDX(D=8878 m/s).Although the value obtained by EXPLO 5(D=8351 m/s,P=33.1 GPa)is larger than that calculated by K-J equations (D= 7945 m/s,P= 29.1 GPa), it does not affect our judgment.
3.6.Performance evaluation
In fact, more scholars prefer the intuitive methods to judge the performance of primary explosives.These methods usually include thermal stimulation (such as hot needle and hot plate tests), laser initiation and lead plate damage tests [17,18,45,41].
Because of the potential danger of ECCs-1, we only used the amount of 1 mg to carry out hot needle (HN) experiments.In the process of testing,the sample explodes directly,resulting in a shrill roar.This rapid process is captured by high-speed cameras, as shown in Fig.9, where there is only a tiny spherical Mars.Unlike flames produced by deflagration or combustion, the explosion has only one spot because more energy is expressed in detonation waves.This fully shows that ECCs-1 has a violent explosion phenomenon.During the test,we could hear a violent explosion.

Fig.9.HN test result of ECCs-1.
Secondly,in order to ensure safety,we carried out an improved hot plate test: put the sample on the filter paper, then ignite the filter paper,and further ignite ECCs-1.By observing the combustion of the sample, we can preliminarily judge its detonation performance.In addition, we used high-speed cameras to capture the explosion process.In addition,we used high-speed cameras to capture the explosion process(see Fig.10):only after 2.08 ms,ECCs-1 exploded violently and produced dazzling light.The explosion produced a violent detonation wave, causing flames to spray around (see Fig.2).
Then, we also carried out the laser ignition test (test dosage is 1 mg; test method and equipment are shown in the supporting information).The results show that only the energy of 40 mJ is enough to detonate ECCs-1 completely.As shown in Fig.11, The moment of the explosion glowed brightly and filled the whole window.From the beginning of the explosion to the end, it only took 4.17 ms (the minimum measuring time of the instrument is 4.17 ms).
Finally,the actual lead plate damage initiation performance test still shows that ECCs-1 has a strong explosive performance.When it is used as a primary explosive, it can completely detonate RDX(shown in Fig.12(c)).The hole in the punctured lead plate reached 23 mm.In addition,the RDX was replaced with equal quality ECCs-1, and the test was carried out under the same conditions.The results show that ECCs-1 can not only detonate itself,but its power is comparable to that of RDX, because the hole of lead plate reaches 21 mm (shown in Fig.12(b)).However, TAT cannot detonate RDX under the same conditions (shown in Fig.12(d) and 12(e)).
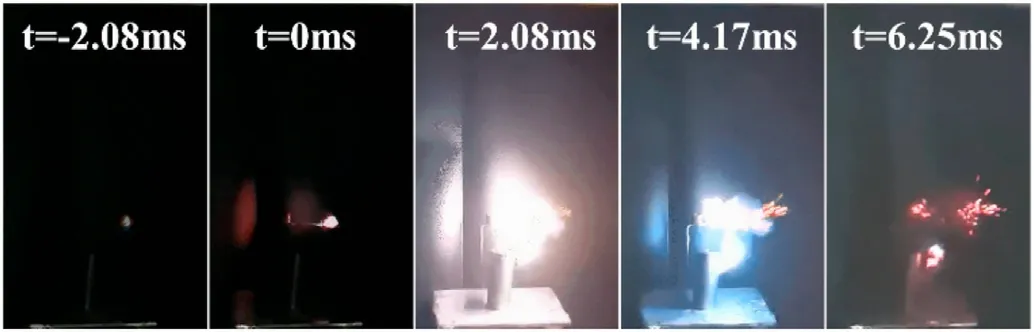
Fig.10.The improved hot plate test result of ECCs-1.

Fig.11.Laser test result of ECCs-1.
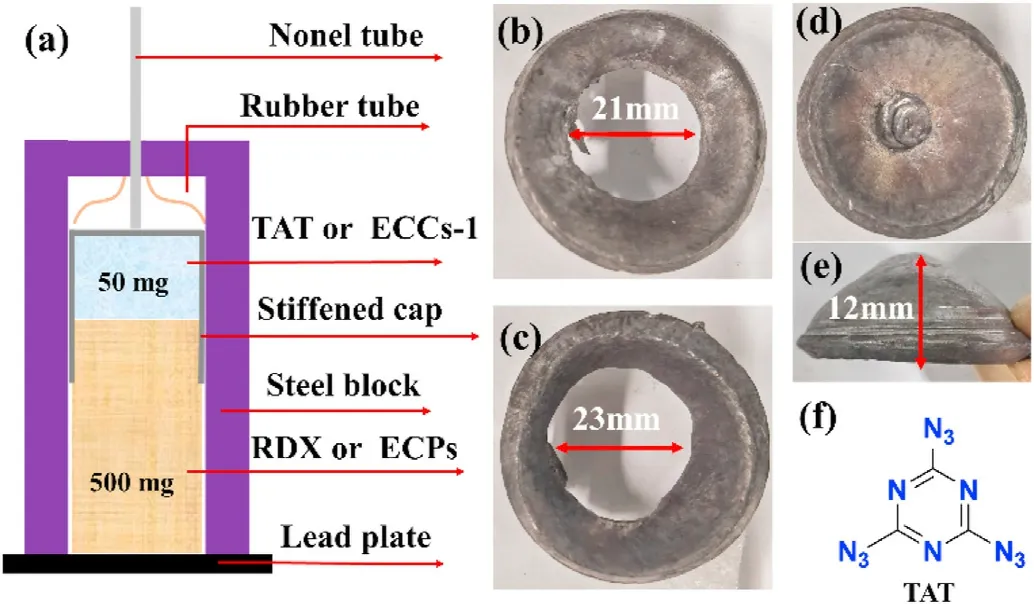
Fig.12.(a)Schematic diagram;(b)550 mg ECCs-1;(c)50 mg ECCs-1 and 500 mg RDX;(d) and (e) 50 mg TAT and 500 mg RDX; (f) the molecular formula of TAT.
3.7.Study on decomposition mechanism
We used solid state UV-VIS-IR spectroscopy to measure any possible correlation between laser ignitability and the absorption at the corresponding wavelength, and the test results are shown in Fig.13.[42,46-48]TZCA has a strong absorption in the wavelength range of ultraviolet (300-400 nm).With the increase of wavelength, the absorbance decreases sharply.There is almost no absorption of TZCA in the visible (400-760 nm) and infrared region(760-1300 nm).Due to the perfect configuration of the complex,ECCs-1 has a completely different absorption behavior.Among the 300-400 nm, the absorption of ECCs-1 is the strongest and the absorbance decreases slowly with the increase of wavelength.In 400-760 nm, the absorbance increases with the increase of wavelength,which is completely different from TZCA.In the range of 760-1300 nm,although the absorbance changes,but the overall decrease is not much.In the whole test range, ECCs-1 showed strong absorptive capacity.At 915 nm (the output wavelength of the instrument), the absorbance of ECCs-1 is 0.45, while that of TZCA is only 0.01.This shows that ECCs-1 has excellent d-d transitions ability at this laser wavelength.
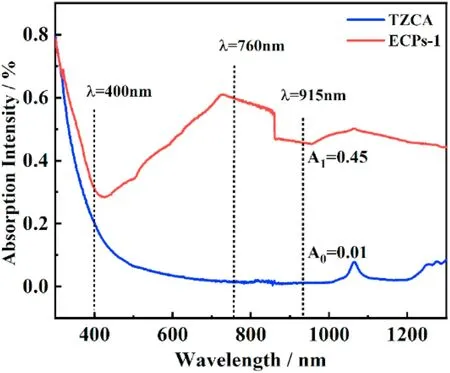
Fig.13.The UV-VIS-IR spectra of ECCs-1.

Table 4The typical structural parameters of ECCs-1.

Fig.14.The decomposition mechanism of ECCs-1.
Once again,we carried out the decomposition mechanism study of ECCs-1.The typical structural parameters are shown in Table 4,with the discussions of WBI(Wiberg bond index)and MBO(Mayer bond order) [45].The specific research methods and testing methods are shown in the supporting information.
As shown in Fig.14, although the WBI and MBI values of these six coordination bonds are very weak, they are not easy to break according to the predicted results.On the contrary,the Cl-O bond(O13-Cl19 and O12-Cl18) with strong WBI and MBO values is broken, which may be due to the strong absorption of ECCs-1.Therefore, [ClO3] and [O] radicals are all produced in this way (at 0.21 ps).The highly active[O]radical attacks the-NH2at end of the nearest TZCA, resulting in the production of [NH] radical (at 0.27 ps).The continuous production of free radicals causes the ligand to break the coordination bond with [Cu], and further produce a violent explosive reaction.
4.Conclusions
In this work, we used TZCA as the ligand to prepare the coordination polymers ECCs-1 in very high yield.Although ECCs-1 has an acceptable thermal stability (Td= 177°C) and detonation performance (DK-J= 7945 m/s,PK-J= 29.1 GPa), it is very sensitive to mechanical stimulation (IS = 1 J, FS = 5 N).The results of HN test,laser ignition test and lead plate initiation test all show that ECCs-1 has excellent initiation ability and explosive power.The results of mechanism research show that ECCs-1 has good light absorption ability and reaches 0.45 at 915 nm.In addition, the theoretical calculation shows that the initiation mechanism of ECCs-1 is free radical mechanism.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are thankful to the projects of National Natural Science Foundation of China(Grant Nos.22175025 and 21905023)for their generous financial support.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.02.014.
- Defence Technology的其它文章
- The interaction between a shaped charge jet and a single moving plate
- Machine learning for predicting the outcome of terminal ballistics events
- Fabrication and characterization of multi-scale coated boron powders with improved combustion performance: A brief review
- Experimental research on the launching system of auxiliary charge with filter cartridge structure
- Dependence of impact regime boundaries on the initial temperatures of projectiles and targets
- Experimental and numerical study of hypervelocity impact damage on composite overwrapped pressure vessels

