Facile preparation of Fe/N-based biomass porous carbon composite towards enhancing the thermal decomposition of DAP-4
Er-hi An , Xio-xi Li , Cun-jun Yu , Ying-xin Tn , Zi-jun Fn , Qing-xi Li ,Peng Deng ,*, Xiong Co ,***
a School of Environment and Safety Engineering, North University of China, Taiyuan, 030051, PR China
b College of Safety and Emergency Management Engineering, Taiyuan University of Science and Technology, Taiyuan, 030024, PR China
Keywords: Biomass materials Porous carbon DAP-4 Thermal decomposition Facile method
ABSTRACT Fe/N-based biomass porous carbon composite(Fe/N-pCarbon)was prepared by a facile high-temperature carbonization method from biomass, and the effect of Fe/N-pCarbon on the thermal decomposition of energetic molecular perovskite-based material DAP-4 was studied.Biomass porous carbonaceous materials was considered as the micro/nano support layers for in situ deposition of Fe/N precursors.Fe/NpCarbon was prepared simply by the high-temperature carbonization method.It was found that it showed the inherent catalysis properties for thermal decomposition of DAP-4.The heat release of DAP-4/Fe/N-pCarbon by DSC curves tested had increased slightly, compared from DAP-4/Fe/N-pCarbon-0.The decomposition temperature peak of DAP-4 at the presence of Fe/N-pCarbon had reduced by 79°C from 384.4°C (pure DAP-4) to 305.4°C (DAP-4/Fe/N-pCarbon-3).The apparent activation energy of DAP-4 thermal decomposition also had decreased by 29.1 J/mol.The possible catalytic decomposition mechanism of DAP-4 with Fe/N-pCarbon was proposed.
1.Introduction
The ammonium perchlorate (AP) based molecular perovskite energetic material DAP-4 has excellent strong oxidation, heat resistance,and detonation characteristics,which makes it possible to become a substitute for AP in solid propellants [1-5].It is necessary to burn catalysts in propellant to enhance energy release.In recent years, metal-doped carbon substrates such as graphene and graphene oxide to prepare composite catalysts have become a hot topic [6-9].Yang et al.used balsa wood as a carbon matrix to support Ti3C2, showing the superior catalytic effect on AP [10].However, graphene or other high-cost materials are not the best choice for carbon-based materials,more and more scholars begin to choose porous biomass materials.
Biomass materials,as important green renewable resources,had attracted much attention in industrial and agricultural production[11-14].They usually are organic polymers that are composed of carbon, hydrogen and oxygen elements.These elements are not only green and biodegradable, but are also used to achieve more value by exploiting their potential properties [15,16].For example,Zhu et al.reported that biomass konjac glucomannan was used to prepare highly conductive carbon aerogel with superior mechanical property via high-temperature carbonization strategy [17].Yu et al.described that biomass polymer materials were considered to fabricate the organic supporting layers,which was used to fabricate bulk synthetic composites with the nacre-like hierarchical structure [18].In our previous work [19], biomass poly glucomannan materials were used to design and fabricate lamellar ammonium perchlorate-based composites, which have excellent performance in the thermal decomposition characterization [20,21].These studies indicated that the biomass material was useful for potential application due to its unique properties.
Inspired by multifunctional applications of the biomass materials[22,23],We notice that steamed bread with low cost is a good biomass material because of its inherent porous conditions.In this report, we reported herein that biomass glucose-based polymer materials were used as the micro/nano support layers for in situ deposition of Fe/N precursors.Fe/N-pCarbon composites were prepared facilely by the high-temperature carbonization method.The micro morphology and element states of Fe/N-pCarbon were characterized.The effect of Fe/N-pCarbon as catalysts on the thermal catalytic decomposition of molecular perovskite-based energetic material DAP-4 was studied.It was found that Fe/N-pCarbon composites, which were originated from biomass polymer materials, showed a good catalysis thermal decomposition property on DAP-4.This work offered a new idea for potential application in the field of energetic materials.
2.Experimental section
2.1.Chemicals and materials
Perchloric acid (HClO4, 70%), Triethylenediamine (dabco,C6H12N2), and K4Fe(CN)6·3H2O were purchased from Shanghai Aladdin Biochemical.Technology Co., Ltd.Ammonium perchlorate(AP) was provided from Jiangyang Chem.Eng Co., Ltd (China,Shanxi).DAP-4 was prepared in our own laboratory.The biomass materials (Chinese steamed bun) were obtained from market.
Fe/N-pCarbon was prepared as shown in Fig.1.To begin, the steamed buns was cut into 2×2×1 cm3pieces and place them in an oven at 60°C for 8 h to eliminate moisture.Immediately after,the K4Fe(CN)6·3H2O(Fe/N preperous)with a fixed mass percentage(25%, 50%, 75%) of the piece was weighed to dissolve in 5 mL of water, gently soak it to cover the full piece, and then put it in an oven to dry.Finally,it is placed in a tube furnace(20-1100°C,2 h)for high temperature treatment to get the sample.Depended on the contents of Fe/N preperous(25%,50%,75%),the sample was named by Fe/N-pCarbon-1, Fe/N-pCarbon-2, and Fe/N-pCarbon-3, respectively.Fe/N-pCarbon-0 was also prepared by carbonization of biomass materials without Fe/N prosperous added.DAP-4/Fe/NpCarbon was pretreated before test.DAP-4 and Fe/N-pCarbon with the weight ratio of 95:5 was physically mixed in a mortar for 30 min.
2.2.Material characterization
Scanning electron microscopy (SEM) and coupling energy dispersion X-ray spectrometer (EDX) mapping images were obtained by a Hitachi 4800 apparatus with the electric voltage of 15 kV.Use the Brunauer-Emmett-Teller (BET) method on the Nova Station A specific meter analyzer (N2, 77 K) to analyze the adsorption surface areas of the samples.X-ray photoelectron spectroscopy (XPS) was conducted using an ESCALAB 250 photoelectron spectrometer (Thermo Fisher Scientific Co., Ltd.).
DAP-4 was mixed with Fe/N-pCarbon.The DSC curve of mixture was recorded by differential scanning calorimetry (DSC) in a DSC131 thermal analyzer (SETARAM, Caluire, France).The heating range was 30-500°C with high purity nitrogen.The heating rates were selected as 5,10,15, and 20°C/min.
3.Results and discussion
Fe/N-pCarbon composites was prepared from the carbonization of biomass amylum materials and showed in Fig.1(d).The micro morphology of Fe/N-pCarbon was shown in Fig.2.The hierarchical macro and nano porous structures of Fe/N-pCarbon-0 and Fe/NpCarbon-1 were seen in Figs.2(a) and 2(c), indicated that micro/nano framework structure had no affected, before and after Fe/N precursors were added.Fe/N precursors were introduced, and in situ deposited on the surface of biomass layers.They were carbonized further at the higher temperature to form Fe/N-doping carbon composite.As shown in Fig.2(b),Fe/N-pCarbon-0 prepared by no Fe/N element added suggested that the lower content of N element could be seem.When the Fe/N precursors were added,Fe/N-pCarbon-1, -2, and -3 showed novel EDX element mappings in Fig.2(d).It was found that the doping contents of Fe and N elements had been increased with the content of precursors increased,as shown in Table 1.
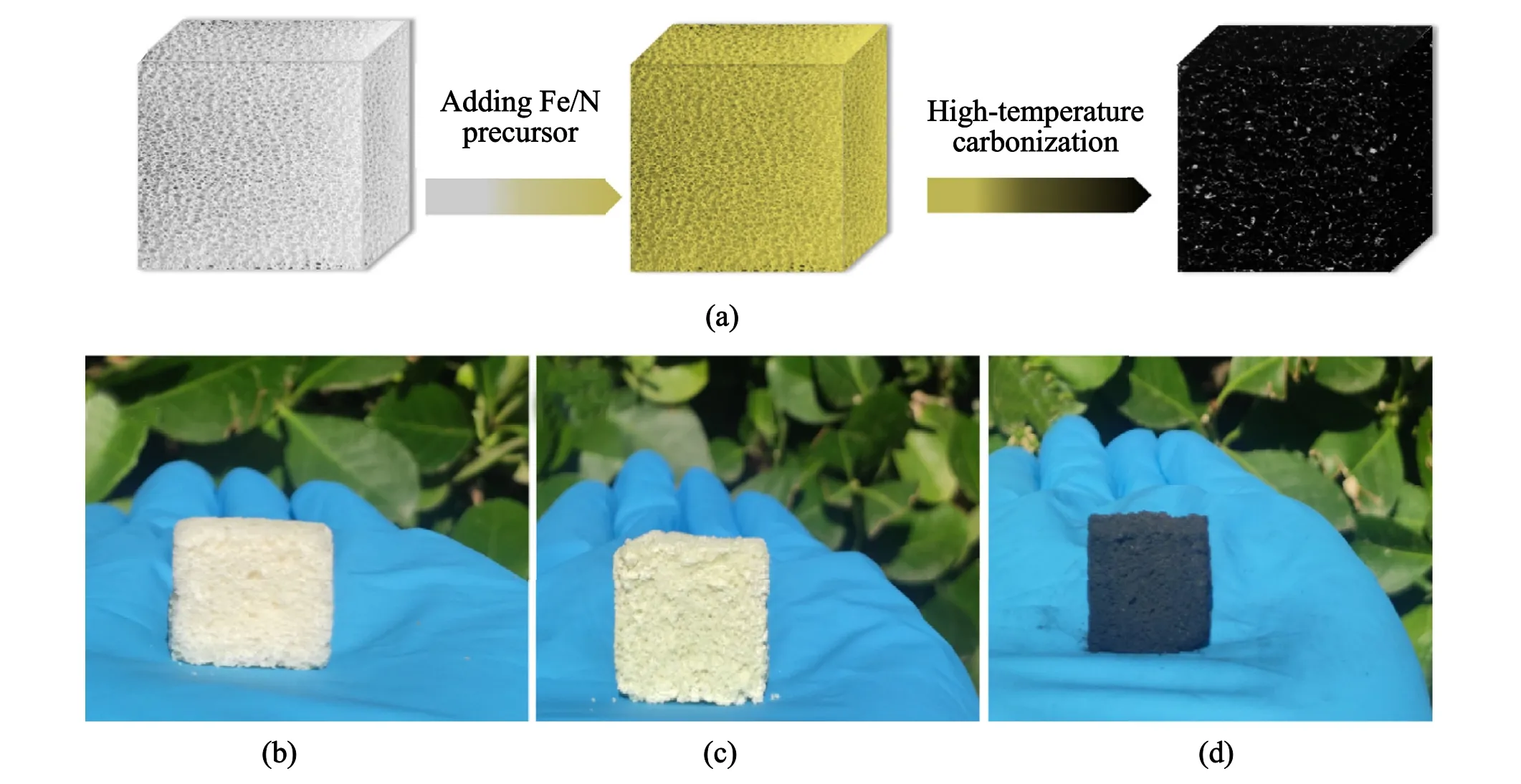
Fig.1.(a) Preparation scheme of Fe/N-pCarbon; (b) Biomass polymer material; (c) Carbonized precursor with Fe/N modified; (d) Fe/N-pCarbon.

Fig.2.(a) SEM and EDX mapping images of (a), (b) Fe/N-pCarbon-0, and (c), (d) Fe/N-pCarbon-1.
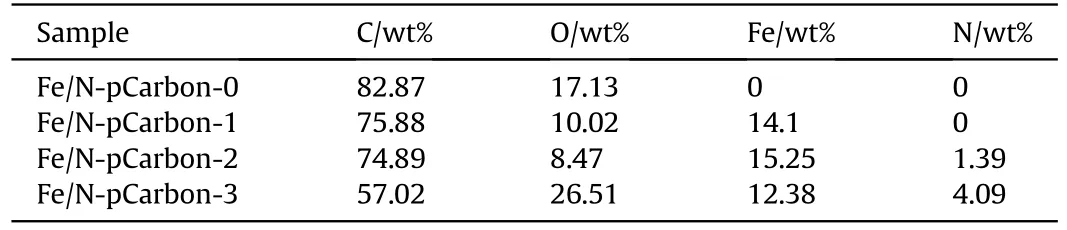
Table 1The element contents from EDX data.
Table 1 showed that the main elements of Fe/N-pCarbon are C and O, which are originated from the biomass materials.With the Fe/N precursors introduced, addition elements (Fe and N) were found in Fig.2(d).And, the contents of them had been increased.For Fe/N-pCarbon-1,-2,and-3,the contents of Fe element had been increased in proper order, and O element also had a similar trend,although C content had been reduced.This indicated that Fe/N precursors at surface of biomass supporting layer were carbonized in high-temperature environment to form iron-based compound.They deposited on the surface of carbon materials.And, little N elements were absorbed by the carbonized sample.
The isotherm adsorption curves of Fe/N-pCarbon-0 and Fe/NpCarbon-3 were tested on the prepared samples with an automatic specific surface area meter,as shown in Fig.3.Figs.3(a)and 3(b) curves under low relative pressure (0-0.3), the adsorption reaction is mainly single molecule adsorption.The high adsorption potential (micropore filling) at low relative pressure confirms that both materials contain a large number of micropores.
According to the data of adsorption curve and BET equation,its specific surface area was analyzed.
In the equation,V, volume of adsorbed gas;Vm, volume of gas monolayer;P, equilibrium pressure of adsorbed gas;P0, saturated vapor pressure of adsorbed gas at the same temperature;C, constant (related to adsorption heat).
The analysis results of BET are shown in Table 2.The C values of Fe/N-pCarbon-0 and Fe/N-pCarbon-3 are 468.61 and 149.61,respectively.This also proves that both materials are strong adsorbents.The specific surface area of Fe/N-pCarbon-0 is very huge,up to 1190 m2/g.However, the specific surface area of Fe/NpCarbon-0 is 175 m2/g.The filling of Fe/N reduces the specific surface area of the material.This is because Fe/N occupies a certain number of pores when filling the pores of Carbon.
XPS analysis of Fe/N-pCarbon was showed in Fig.4 to characterized the chemical states of addition elements.The contents of Fe/N precursors are more,the XPS peaks had become more obvious,as Fe/N-pCarbon-3 sample shown in Fig.4(a).For Fe2p XPS spectrum in Fig.4(b),the peak of Fe/N-pCarbon-3 could be deconvoluted into four peaks,which revealed that the form of Fe2+and Fe3+existed in composites [24].And, N1s XPS peak of Fe/N-pCarbon had a shift towards lower binding energy in Fig.4(c),indicated more elements were absorbed to form more N-based structure.The above characterization results reveled the Fe/N-pCarbon composites with hierarchical porous frameworks and high hetero-element distributions were prepared.

Fig.3.Isothermal adsorption curve of (a) Fe/N-pCarbon-0; (b) Fe/N-pCarbon-3.

Table 2Fitting results of B.E.T specific surface area of two samples.

Fig.4.(a) XPS survey; (b) Fe 2p, and (c) N1s XPS of Fe/N-pCarbon.
Energetic molecular perovskite-based material DAP-4 was shown in Figs.5(a) and 5(b).The cubic shape of DAP-4 with 4-10 μm was seem, which are originated from the crystal growth process of molecular perovskite cell.And, XRD pattern of DAP-4 was showed in Fig.5(b).The main strong XRD peaks was found at 21.8°,and 25.2°,which are reflected from the crystal face(222),and (400).Fig.5(c) showed the DSC curves of DAP-4 with and without Fe/N-pCarbon.For DAP-4,the thermal decomposition peak was located at 384.4°C.And pure carbon-based materials (Fe/NpCarbon)without Fe/N modified was introduced to had no obvious effect on the DAP-4 thermal decomposition.But,for Fe/N-pCarbon-1,-2,and-3,a great decreasing tendency(at least 26°C)of thermal decomposition peak of DAP-4 with 5wt%Fe/N-pCarbon added was shown in Fig.5(c).Maximum reduction temperature was 79°C from 384.4°C (DAP-4) to 305.4°C (DAP-4/Fe/N-pCarbon-3), which indicated Fe/N-pCarbon composites showed the inherent catalysis properties for thermal decomposition of DAP-4.The tested heat release of samples was shown in Fig.5(d).DAP-4/Fe/N-pCarbon had a higher heat release than DAP-4.But,pure carbon-based material Fe/N-pCarbon-0 reduced the total heat release.Therefore, it was found that pure carbon-based materials can’t effectively catalyze the thermal decomposition of DAP-4, and it will also reduce total heat release of the system.But,in this work,hetero-elements were introduced into carbon-based composite catalysts,which are useful for enhanced the thermal decomposition properties of DAP-4.
The apparent activation energy of thermal decomposition of DAP-4 was investigated in Fig.6.The DSC curves of DAP-4 with and without Fe/N-pCarbon at different heating rates were shown in Figs.6(a)-6(e), which showed a main increasing tendency of the thermal decomposition temperature peak of samples with the heating rate added.Depended on the relationship between peak temperature and heating rate, the apparent activation energy of samples can be calculated by the Kissinger model [25].The activation energy for pure DAP-4 is calculated to be 229.4 kJ/mol,which are consistent with the previous study [26].Pure carbon-based material Fe/N-pCarbon-0 reduced the activation energy.However,when Fe/N-pCarbon-1 was added,DAP-4 increased to 296.7 kJ/mol,and the pre-exponential factor(lgA)also increased to 24 min-1.But the activation energy and the pre-exponential factor showed a decreasing trend with the increase of Fe/N content in the porous biomass carbon(Table 3).Especially for DAP-4/Fe/N-pCarbon-3,the activation energy of its thermal decomposition had been decreased to 220.3 kJ/mol,which is reduced by 29.1 kJ/mol from pure DAP-4.
The catalytic thermal composition mechanism of DAP-4 with the present of Fe/N-pCarbon was proposed in Fig.7.As shown in Fig.2(c),Fe/N-based compounds were seen on the surface of Fe/NpCarbon-1 microstructure, compared from the smooth surface of Fe/N-pCarbon-0 in Fig.2(a).It was found that pure amorphous carbon-based materials had weak catalytic thermal decomposition properties, resulted from lower electron transfer capability of disordered carbon structure [27].Hetero-elements, such as Fe and N,were introduced into carbon-based catalysts Fe/N-pCarbon-1,-2,and-3 herein.They generate Fe/N-based compounds,such as FexOy,FexNy, FexCyNz, and etc, these compounds at the nanoscale are beneficial to modify the structure of pure amorphous carbon-based material, to enhance the electron transfer capability [28].As reported in previous literature,the thermal decomposition process of DAP-4 can be catalyzed at a lower decomposition temperature,when high thermal stability is from the cage-like framework was destroyed.With the present of Fe/N-pCarbon composites, Fe/Nbased compounds have a strong heat and mass transfer capabilities,leading in a reduced thermal decomposition threshold.They could support an accelerated proton transfer from H2dabco2+andtoquickly to promote form HClO4,as well as super-oxide radical anion -O2· further [29].More reducing agents were oxidized continually.As shown in Fig.5(d), more heat was released from DAP-4/Fe/N-pCarbon.The decomposition reaction products were CO2, CO, H2O,NO, NO2, N2, HCl, and soon.

Fig.5.(a) SEM image and (b) XRD patterns of DAP-4, (c) DSC curves and (d) decomposition heat release of DAP-4 and DAP-4/Fe/N-pCarbon.

Fig.6.DSC curves of (a) DAP-4, (b) DAP-4/Fe/N-pCarbon-0, (c) DAP-4/Fe/N-pCarbon-1, (d) DAP-4/Fe/N-pCarbon-2, (e) DAP-4/Fe/N-pCarbon-3 at different heating rates, and (f)fitting lines display the linear fitting result of the tested data with the dependence of ln (β/Tp2) on 1/Tp for above samples.
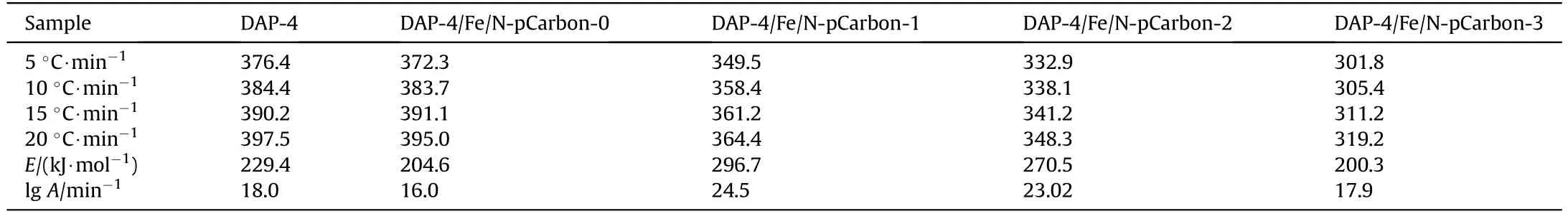
Table 3The kinetic parameters for decomposition of DAP-4 and mixtures.
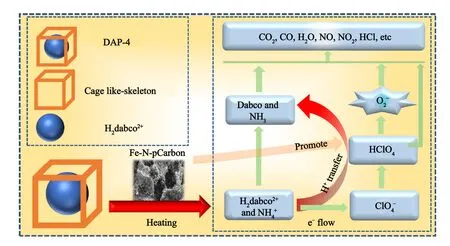
Fig.7.Schematic illustration of thermal decomposition process of DAP-4 with Fe/N-pCarbon catalysts.
4.Conclusions
In summary, Fe/N-pCarbon was prepared by a facile hightemperature carbonization method, and its effect on the thermal decomposition of DAP-4 was studied.It was found that a lowercontent Fe/N-based compounds at the nanoscale deposited on the surface of Fe/N precursors.They showed the good inherent catalysis properties for thermal decomposition of DAP-4.With 5wt% Fe/N-pCarbon-3 added, the decomposition temperature peak of DAP-4 had reduced by 79°C from 384.4°C of DAP-4 to 305.4°C of DAP-4/Fe/N-pCarbon-3.The apparent activation energy of DAP-4/Fe/N-pCarbon-3 thermal decomposition also had decreased by 29.1 J/mol from 229.4 J/mol of DAP-4 to 200.3 J/mol of DAP-4/Fe/NpCarbon-3.In addition, the catalytic decomposition mechanism of DAP-4 with Fe/N-pCarbon was provided.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the National Natural Science Foundation of China(Grant No.21975227) and the Found of National defence Science and Technology Key Laboratory(Grant No.6142602210306).
- Defence Technology的其它文章
- The interaction between a shaped charge jet and a single moving plate
- Machine learning for predicting the outcome of terminal ballistics events
- Fabrication and characterization of multi-scale coated boron powders with improved combustion performance: A brief review
- Experimental research on the launching system of auxiliary charge with filter cartridge structure
- Dependence of impact regime boundaries on the initial temperatures of projectiles and targets
- Experimental and numerical study of hypervelocity impact damage on composite overwrapped pressure vessels

