Deflagration characteristics of freely propagating flames in magnesium hydride dust clouds
Qiwei Zhng , Yngfn Cheng ,,*, Beiei Zhng , Dnyi Li ,c, Zhowu Shen
a School of Chemical Engineering, Anhui University of Science and Technology, Huainan 232001, Anhui, PR China
b School of Civil Engineering and Architecture, Anhui University of Science and Technology, Huainan 232001, Anhui, PR China
c Anhui Engineering Laboratory of Explosive Materials and Technology, Anhui University of Science and Technology, Huainan 232001, Anhui, PR China
d School of Engineering Science, University of Science and Technology of China, Hefei 230027, Anhui, PR China
Keywords: Magnesium hydride dust Flame combustion mechanism Particle size Dust explosion Two-color pyrometer
ABSTRACT The flame propagation processes of MgH2 dust clouds with four different particle sizes were recorded by a high-speed camera.The dynamic flame temperature distributions of MgH2 dust clouds were reconstructed by the two-color pyrometer technique, and the chemical composition of solid combustion residues were analyzed.The experimental results showed that the average flame propagation velocities of 23 μm, 40 μm, 60 μm and 103 μm MgH2 dust clouds in the stable propagation stage were 3.7 m/s,2.8 m/s, 2.1 m/s and 0.9 m/s, respectively.The dust clouds with smaller particle sizes had faster flame propagation velocity and stronger oscillation intensity, and their flame temperature distributions were more even and the temperature gradients were smaller.The flame structures of MgH2 dust clouds were significantly affected by the particle sinking velocity, and the combustion processes were accompanied by micro-explosion of particles.The falling velocities of 23 μm and 40 μm MgH2 particles were 2.24 cm/s and 6.71 cm/s,respectively.While the falling velocities of 60 μm and 103 μm MgH2 particles were as high as 15.07 cm/s and 44.42 cm/s,respectively,leading to a more rapid downward development and irregular shape of the flame.Furthermore, the dehydrogenation reaction had a significant effect on the combustion performance of MgH2 dust.The combustion of H2 enhanced the ignition and combustion characteristics of MgH2 dust, resulting in a much higher explosion power than the pure Mg dust.The micro-structure characteristics and combustion residues composition analysis of MgH2 dust indicated that the combustion control mechanism of MgH2 dust flame was mainly the heterogeneous reaction,which was affected by the dehydrogenation reaction.
1.Introduction
As a new type of weapon, thermobaric bombs have received extensive attention in recent years.The unique damage effect with pervasive high heat and shock wave produced by its explosion is incomparable to traditional ammunition, and it is especially suitable for killing the enemy in closed spaces such as caves, underground fortifications and buildings [1].Thermobaric bombs have the characteristics of both high explosives and fuel air explosives.Powders such as aluminum, titanium, magnesium, zirconium,silicon and boron are added to thermobaric bombs, which further enhances their thermal and pressure effects with a significant energy multiplication function [2-4].Thermobaric bombs are originated from early fuel air explosives, after more than 20 years of development, they have formed a series of products including not only the short-range thermobaric bombs for individual soldiers,but also the artillery-launched thermobaric grenades and thermobaric rockets, as well as the fighter-launched thermobaric bombs and thermobaric air-to-ground missiles.After continuous development and improvement, thermobaric bombs have been gradually expanding to the directions of miniaturization multi-carrier launching.In order to improve the energy density of thermobaric bombs, countries in the world have begun to look for novel combustion materials [5].Hydrogen storage alloys served as additives are widely used in energetic materials such as explosives,pyrotechnics and propellants because of their high energy density,combustion calorific value and energy release efficiency [6-9],among which MgH2is one of the most representative materials[10].Xue et al.[11]found that RDX with 10%MgH2powders had the optimal shock wave parameters,and compared with the reference sample, its peak overpressure, specific impulse and shock wave energy were increased by 5.7%, 7.0% and 8.4%, respectively.Cheng et al.[12,13] demonstrated that MgH2powders could significantly increase the shock wave overpressure,specific impulse,shock wave energy and bubble energy of emulsion explosive in the underwater explosion, and improve their anti-dynamic pressure ability in the delay blasting operation.Liu et al.[14]explored the effects of MgH2powders on the thermal decomposition, combustion rate and explosion heat of ammonium perchlorate-based composite solid propellant, and found that MgH2powders could reduce its peak temperature of thermal decomposition and increase its combustion rate.Chen et al.[15] studied the properties of novel hydrogen storage alloys dust clouds,and the result showed that MgH2in the dust mixture could effectively reduce its minimum ignition energy and increase its peak explosion pressure.Hydrogen storage alloy MgH2has the dual characteristics of metal fuel and hydrogen,so it also has a good application prospect in thermobaric bombs [16].

Abbreviations mp Particle mass, kg dp Particle diameter, m g Gravitational acceleration, m/s2 t Time, s up u Particle velocity, m/s2 Fluid velocity, m/s2 ut Particle settling velocity, m/s v Fluid kinematic viscosity, m2/s ρp Particle density, kg/m3 or g/cm3 ρg Air density, kg/m3 or g/cm3 ζd Drag coefficient Re Reynolds number g gas p Particle
Studying the dust explosion characteristics of hydrogen storage alloy MgH2is helpful to reveal the damage mechanism of thermobaric bombs containing MgH2powders.The flame structures and propagation characteristics of the dust clouds are extremely important parameters to characterize its detonation characteristics,and researchers have done a lot of works on them [17].Gao et al.[18] studied the effects of particle size distributions on the flame propagation characteristics of octadecanol dust clouds in an open space, and the experimental results showed that the flame front structures were changed with the varying particle size distributions, and the flame propagated in the dust cloud with a smaller particle size had a regular shape,which was similar to the premixed gas explosion.Chang et al.[19]observed the flame development of nano and micron aluminum dust clouds,and found that the flame propagation of nano dust clouds had higher temperature gradients in the preheating zone and lower minimum explosive concentrations as compared to micron aluminum dust clouds.Huang et al.[20]studied the combustion characteristics of aluminum dust with different particle size distributions in the laminar air flow, and found that with the particle size decreased from micron to nano scale,the flame propagating velocity increased and the combustion mechanism changed from a diffusion-controlled mode to a kinetically-controlled mode.Yu et al.[21] observed the flame propagation behaviors and micro-structures in micron and nano titanium dust explosions, and discovered that the flame of nanotitanium dust cloud propagated rapidly and had an obvious micro-explosion phenomenon.Jing et al.[22,23] studied the explosion mechanism of micron-sized flake aluminum powder, and found that the transient reaction rate of flake aluminum powder/air was mainly determined by oxygen diffusion rate and chemical kinetic rate.
Despite the explosion parameters, flame temperature is also a very important parameter for evaluating the thermal damage effect of dust explosions.In recent years,with the continuous maturity of photoelectric technology, non-contact optical temperature measurement methods have been developed rapidly,among which the two-color pyrometer technique is the most popular method for it is no need to determine the optical properties(such as transmittance and emissivity)of the flame before the temperature measurement[24,25].In our previous works, we also carried out a lot of studies on the two-color pyrometer technique[26-28],which provided an experimental technical support for measuring the flame temperature distribution of MgH2dust cloud.
In the study, flame dynamic evolution processes and temperature distribution in the micron scale MgH2dust cloud were studied using a modified Hartmann tube.The flame structures and propagation processes were recorded with a high-speed camera,and the transient two-dimensional temperature distribution of MgH2dust explosion could be reconstructed with the high-speed images and a Python code.Furthermore, the combustion residues of MgH2dust were analyzed by the scanning electron microscopy and x-ray diffraction method, and the combustion mechanism of MgH2dust was also discussed on basis of the deflagration characteristics and combustion residues analysis.
2.Experiments
2.1.Experimental materials
In the experiments,MgH2(hydrogen storage capacity of 7.6 wt%,Alfa Aesar, USA) dust was sieved into four groups of samples with different particle size.The particle size distribution and microstructure of the four different samples were measured by a laser particle size analyzer (Mastersizer 2000, Malvern, UK) and a scanning electron microscope (SEM, VEGA3 SBH, TESCAN), as shown in Fig.1.The mean diameters (D50) of MgH2powders were 23 μm,40 μm,60 μm and 103 μm,respectively.Fig.1 shows that the particle surfaces of MgH2powders were smooth and spherical,and those withD50of 23 μm had a slight agglomeration phenomenon.
2.2.Experimental apparatus
A modified 1.2 L Hartmann tube was used to create an open space for studying the dust explosions, as shown in Fig.2.It consisted of a combustion tube, a gas supplying system, a dispersion system,an ignition system,a high-speed photography system and a time controller system,Before the ignition,the movable part in the center of Hartmann tube was fixed, and the inlet solenoid valve,ignition electrode, retractable electromagnet and camera system were synchronously controlled by a programmable logic controller.In the experiments, 0.5 MPa compressed air was used to disperse the MgH2particles.After 0.12 s delay of dust dispersion, the suspended particles were ignited with a 15 kV high voltage transformer.At the same time, a high-speed camera (Memrecam HX-3,NAC Japan)equipped with a macro lens(AF Micro-Nikkor 200 mm f/4D IF-ED, NAC Japan) was triggered to record the propagation process of the dust flame.At the moment of ignition, the movable tube was moved down to provide an open space for the combustible dust cloud, so that the flame could propagate in the open space without the restriction of the tube wall,facilitating the direct observation of flame structure and propagation behaviors.

Fig.1.Particle size distribution and micro-structure of MgH2 powders: (a) 23 μm; (b) 40 μm; (c) 60 μm; (d) 103 μm.
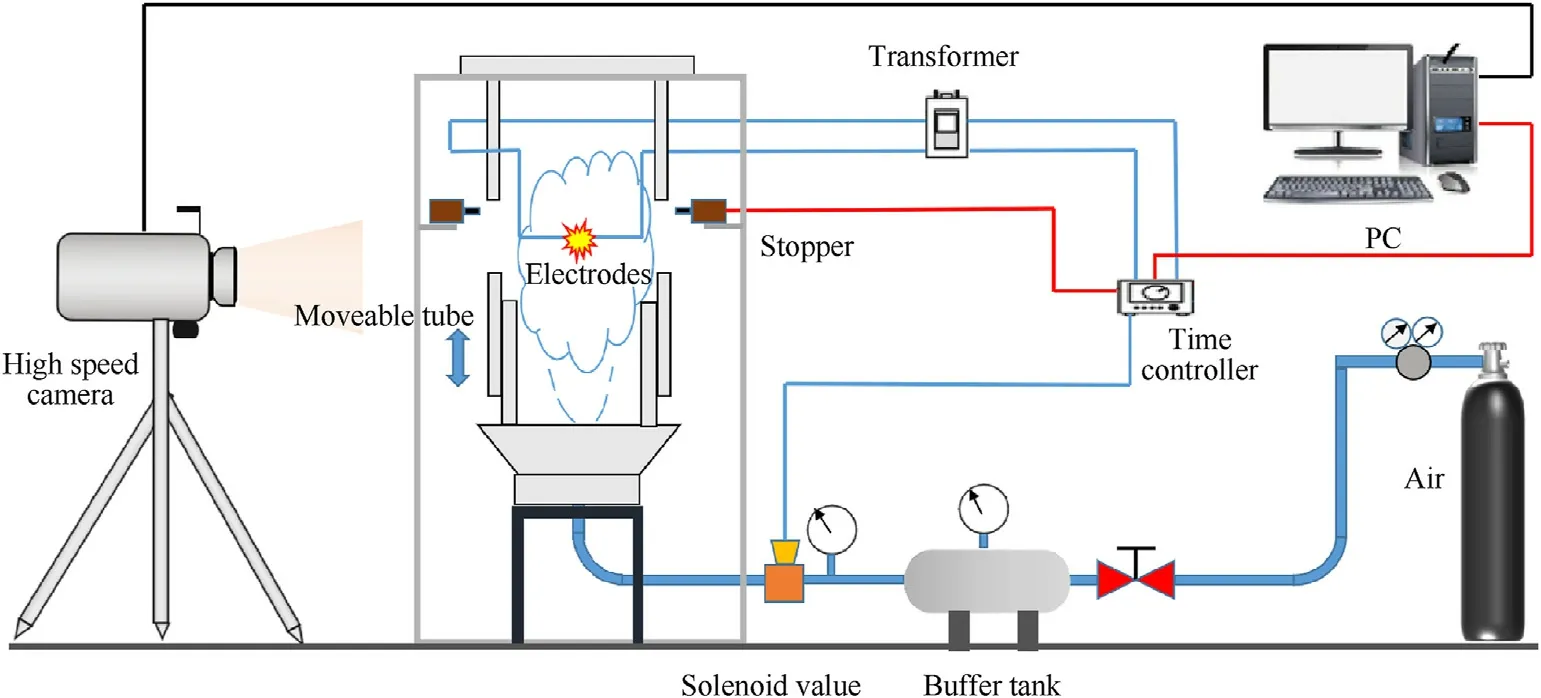
Fig.2.Schematic of the experimental apparatus.
2.3.Calibration of temperature measurement system
As a non-contact combustion diagnostic technique, two-color pyrometer technique has been applied in measuring particle temperature of metal dust [27,28].The two-color pyrometer system was calibrated before measuring the temperature field of MgH2.In the experiment, the tungsten filament lamp was selected for temperature calibration(tungsten is the metal with the highest melting point in the world,and its melting point is as high as 3695 K)[26].By calculating the corresponding relationship between theR/Gratios of the camera sensor and the temperature of the tungsten filament lamp, the variation trend of correction coefficient with temperature was acquired through the calibration (as shown in Fig.3) [29].
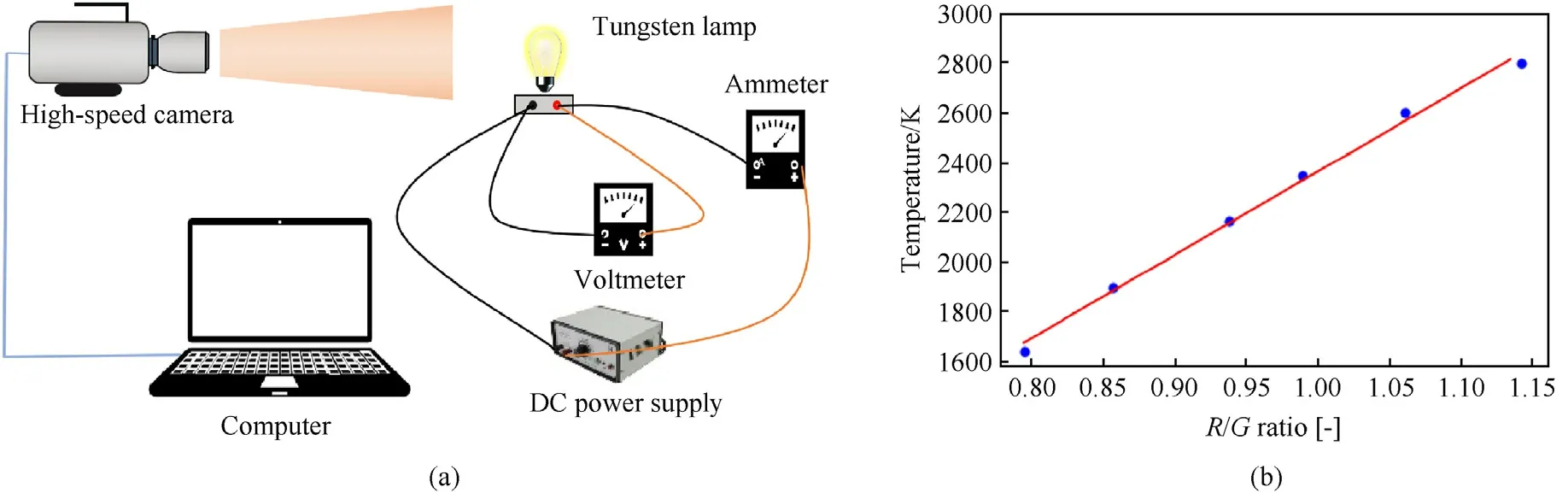
Fig.3.Temperature calibration with tungsten filament lamp: (a) schematic diagram of experimental set-up; (b) temperature-(R/G) ratio fitting curve.
3.Results and discussions
3.1.Kinetics of MgH2 dehydrogenation and oxidation
The thermal decomposition and dehydrogenation characteristics of MgH2samples were studied by the thermogravimetrydifferential scanning calorimetry under the argon atmosphere,and the thermogravimetry (TG) and differential scanning calorimetry(DSC)curves of MgH2with different particle sizes are shown in Fig.4.Since the thermal expansion coefficient of MgH2is much larger than that of MgO [17], MgH2expanded after being heated,which would break the MgO film layer outside, and the internal MgH2would be decomposed into H2and Mg.The TG curves and DSC curves had a descending peak and an endothermic peak at 455-475°C, respectively, indicating that the dehydrogenation reaction began in this temperature range.As the particle size decreased, the mass loss was greater, so the hydrogen content of the small sized MgH2particles was higher.By comparing the TG and DSC curves of MgH2with different particle sizes under the argon atmosphere,it could be found that the smaller particle size of MgH2had the lower initial decomposition temperature,maximum heat absorption and energy required for hydrogen release.
Fig.5 shows the TG and DSC curves of MgH2powders in air atmosphere.MgH2powders with different particle sizes had two rapid weight gain stages in the range of 370-450°C and 600-650°C, respectively.The first rapid weight gain stage (dehydrogenation and oxidation stage of MgH2powders), in which the TG curve rose rapidly around 370°C with the increase of temperature, corresponding to the hydrogen release from the particle interior, the diffusion combustion of the particle surface, and the oxidation reaction of the decomposition product Mg particles,and comparing Figs.4(a)and 5(a),it could be seen that the weight gain of Mg particles oxidation was much greater than the weight loss of hydrogen release, that was why the weight of sample increased.The second rapid weight gain stage (high-temperature oxidation stage of Mg particles),as in Fig.4(a),there was no dehydrogenation reaction of MgH2powders at 600-650°C,so the weight gain of the sample was mainly caused by the complete oxidation of the remaining Mg particles(boiling combustion),as in Fig.5(a),the TG curve started to show a second rise at 600-650°C.In addition, as shown in Fig.5(b), the first exothermic peak of the DSC curve of MgH2powders in air atmosphere appeared at 370-450°C,but the endothermic peak of the DSC of MgH2powders in argon atmosphere did not appear at this stage in Fig.4(b),which was because the endothermic reaction of MgH2powders dehydrogenation overlapped with the oxidation reaction,and the heat release of the oxidation process was much greater than the heat absorption of the decomposition hydrogenation process, so the overall performance presented an exothermic reaction.The DSC curve of MgH2powders in air atmosphere had a second exothermic peak at 600-650°C,which was caused by the complete oxidation(boiling combustion)of the remaining Mg particles.
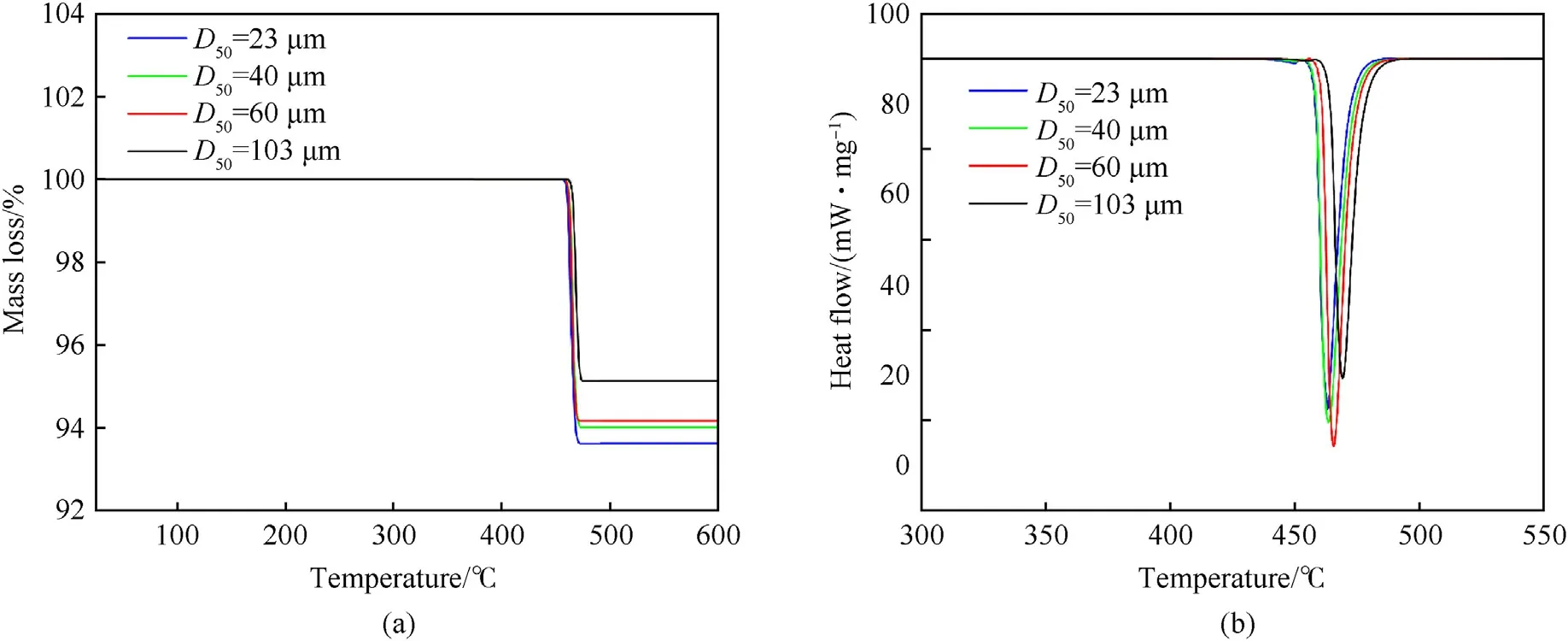
Fig.4.Thermal analysis for pure MgH2 at the heating rate of 10°C/min in argon: (a) TG curves; (b) DSC curves.
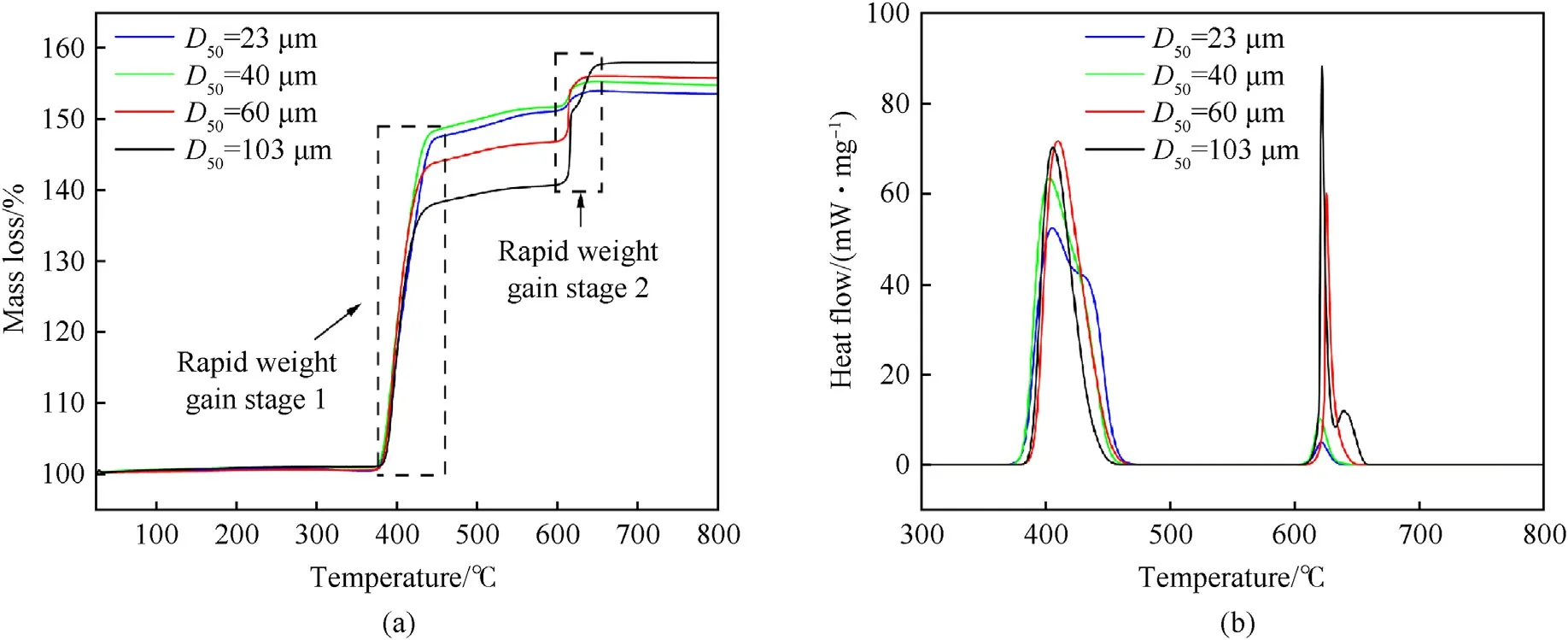
Fig.5.TG and DSC results for pure MgH2 at the heating rate of 10°C/min in air: (a) TG; (b) DSC.
Fig.5 shows that in the first weight gain stage (370-450°C),compared with the larger particles with the particle sizes of 60 μm and 103 μm, the particles of 23 μm and 40 μm had lower initial oxidation temperatures,higher weight gain and lower heat release.The reasons for that may concluded as follows:smaller particle size resulted in lower apparent activation energy of MgH2,leading to a lower initial oxidation temperature, and smaller particles had larger contact area with air to be oxidized more completely and weight gained more obviously, furthermore, due to the higher hydrogen content of the smaller MgH2powders, as the dehydrogenation reaction needed to absorb heat, so the heat released by the first rapid weight gain stage of smaller MgH2powders were lower.In the second rapid weight gain stage (600-650°C), during which a high-temperature oxidation(boiling combustion)reaction occurred, and the Mg powders were completely reacted, so the smaller MgH2powders had less weight gain and heat release due to the relative lower Mg content.
3.2.Flame propagation behaviors
The flame propagation processes in the MgH2dust clouds(830 g/m3concentration) with four different particle size distributions are shown in Fig.6.The initial shape of the flames was approximately spherical,and the flame fronts were smooth.As the combustion progressed, the flame fronts gradually became irregular, and bright cluster flames appeared in the lower part of the combustion zones, which was attributed to the uneven dispersion of dust particles caused by the gravity effect and turbulence[28].It was notable that dust cloud with the smaller particle size had a brighter flame and a faster propagation velocity.According to the diffusion oxidation mechanism of Mg particles, particle size is the main factor affecting the oxidation rate of Mg particles [27,30].Smaller particle had a longer suspension time and a larger specific surface area, which led to the faster heat rate and more violent combustion reaction [31].Furthermore, for both hydrogen release rate and hydrogen storage capacity of MgH2powders with small particle size were higher than those with large particle size,and the combustible gas H2released around the particles would reduce the influence of the 'gap effect' between particles [32,33], thus accelerated the combustion reaction of particles.
Fig.7 is the flame propagation process of 23 μm Mg dust cloud with concentration of 830 g/m3.Mg and MgH2dust clouds had different flame phenomena.Comparing Figs.6(a) and 7, it can be seen that the flame propagation velocity of Mg dust cloud was significantly lower than that of MgH2dust cloud,that was because MgH2dust cloud would release H2during its burning process,forming the gas-solid two-phase combustion of H2and dust mixture.The heat released by H2combustion would accelerate the combustion of MgH2powders(a'positive feedback'effect[32]),and the combustion product water vapor would continue to react with Mg to produce new H2, this reciprocating cycle of feedback effect would accelerate the total combustion reaction [34].In addition,unlike the combustion of Mg dust cloud,a large number of floating flocculus could be observed in the MgH2dust cloud flame.In the combustion process of MgH2dust cloud, the combustion product MgO powders would diffuse outward under the action of H2and water vapor, and they gradually cool ed and eventually formed the flocculent structure with water in the propagation process.
Fig.8(a)and 8(b)show the flame front propagation distance and velocity as a function of time in the MgH2dust clouds with four different particle sizes, respectively.In order to reduce the influence of gravity and buoyancy, the horizontal displacement of the flame was used to calculate the flame propagation velocity.As shown in Fig.8,in the 23 μm,40 μm,60 μm MgH2dust clouds with the concentration of 830 g/m3,there was a significant acceleration of flame propagation in the initial stage, while that in the 103 μm MgH2dust cloud with the same concentration was relatively stable.The average flame propagation velocities of 23 μm, 40 μm, 60 μm and 103 μm MgH2dust clouds in the stable propagation stage were 3.7 m/s, 2.8 m/s, 2.1 m/s and 0.9 m/s, respectively.The dust cloud with a smaller particle size had the faster flame propagation velocity.This was because smaller particle had larger specific surface area, shorter preheating time, and faster particle decomposition/gasification rate, so it was much easier to release H2.The combustion of H2had a large improvement in the flame velocity and initial acceleration[35].The flame propagation velocities were not constant but fluctuated.The flame velocity fluctuation phenomenon could be attributed to the velocity slip between gases and particles.During the flame propagation, MgH2powders would release H2and Mg vapors when heated.Initially,the velocity of gas was higher than that of the particle because of strong expansion;thus the particle number density ahead of the flame front would decrease due to the higher inertia of particles.The reduced particle number density would decrease the rate of surface reactions and consequently the heat release by both gas-phase and particlephase reactions.This decrease in reaction intensity would lower both gas and the particle velocities.However, gas responses were much faster than those by the particles.The consequence was that the particles were moving much faster than gas,and this essentially increased the particle number density ahead of the flame front.As a result,the reactions become intensified because of a higher particle number density.This led to more heat release and enhanced the gas and particle velocities, but the gas velocity increased more than particle velocity.This alternating change of gas and particle velocities resulted in a periodic change of local particle number density (that was, the local fuel equivalence ratio), and consequently it caused flame-speed oscillation [36].Furthermore, the oscillation frequency of the flame was irregular due to the effect of turbulence and buoyancy [37], and the oscillation phenomenon became stronger with the decreasing particle size.The thermal decomposition rate of smaller particles was relatively faster, and the change of its gas phase velocity was greater than that of large particles,so the oscillation amplitude enhanced for the velocity slip between the smaller particles and gas was larger.

Fig.6.Flame propagation behaviors of MgH2 dust clouds with different particle size: (a) 23 μm MgH2 dust cloud; (b) 40 μm MgH2 dust cloud; (c) 60 μm MgH2 dust cloud; (d)103 μm MgH2 dust cloud.
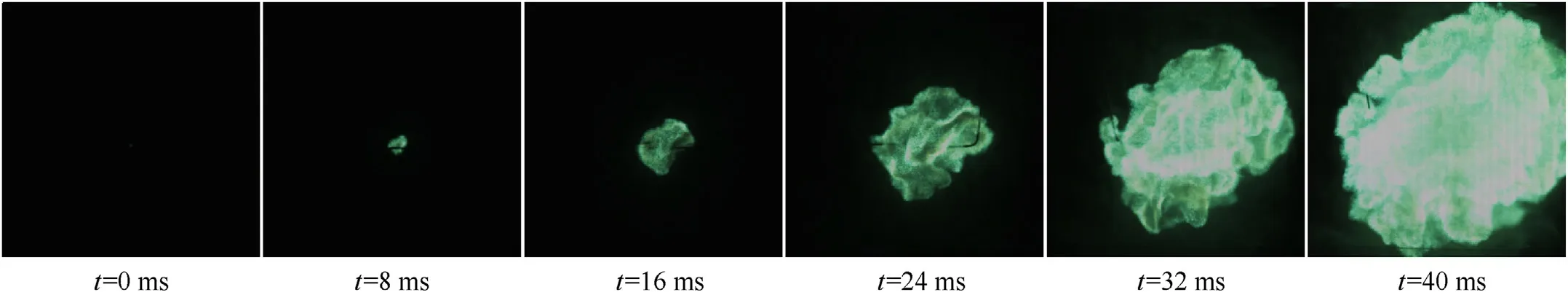
Fig.7.Flame propagation of 23 μm Mg dust cloud with concentration of 830 g/m3.
3.3.Flame micro-structures
The local magnification view of the combustion processes of 103 μm MgH2dust cloud are shown in Fig.9.After the ignition of MgH2powders, the smaller particles in the flame front were decomposed or gasified to form a local premixing flame.The surrounding large particles absorbed heat through thermal radiation and thermal convection, then participated in the combustion reaction to gradually form a propagating cluster flame.The light blue flame appeared during the combustion of the MgH2dust cloud,which was caused by the combustion of H2released from MgH2powders.As the combustion intensified,the decomposition rate of MgH2powders accelerated to release more H2, and the light blue flames became more visible.Different from the Mg dust cloud,there were many micro-diffuse flames around the burning particles in the combustion processes of MgH2dust clouds.At the initial stage of particle combustion, the particles had a bright spherical structure and continued to form a micro-diffusion flame(the effect of H2combustion), which rapidly expanded and ignited the unburned dust particles in the preheating zone [38].
As illustrated in Fig.10,the phenomenons of particle explosions and ejections of smaller luminescent substances often occurred during the combustion of MgH2powders,which was named microexplosion [28].The direct cause of the micro-explosion was the rapid increase of internal pressure due to the gas expansion inside of the particles, and the particles would be broke into small fragments as the internal stress exceeded the maximum bearing capacity of the liquid particle surface.The probability and intensity of micro-explosion depended largely on the speeds of generation and expansion of gas bubbles within the particles [39].There were three potential sources of gas inside MgH2particles: first, a large amount of H2was stored inside of the particles;second,due to the high temperature inside of the particles, the vapor of Mg or its oxides might be produced;third,the surrounding gas would diffuse and dissolve into the molten MgH2powders.The occurrence of micro-explosions inhibited particle agglomeration to a certain extent, which was conducive to improving the rate of hydrogen release and the combustion of MgH2powders [40,41].
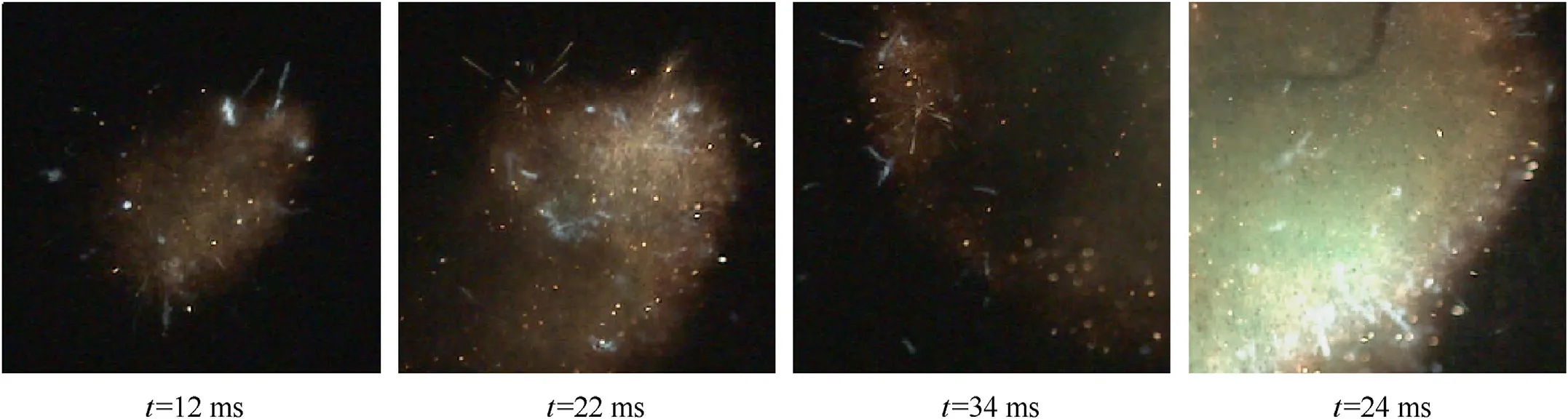
Fig.9.Flame micro-structure of 103 μm MgH2 dust cloud.

Fig.10.Micro-explosion phenomenon occurred in MgH2 dust flame propagation.
The micro-explosion process of a single MgH2particle is shown in Fig.11.The MgH2particle surface burned and formed a bright tail-like light.As the temperature increased,the thermal expansion of the liquid or vapor Mg inside the particles increased the internal pressure of the particles.When the stress reached a certain threshold, the particles began to expand and rupture, ejecting Mg vapor to the surroundings and eventually leading to microexplosion phenomenon [42].
3.4.Flame temperature distribution
In the experiments, the combustion processes of MgH2dust clouds were recorded by a high speed camera, and the corresponding temperature distributions were calculated and rebuilt by the two-color pyrometer technique, as shown in Fig.12.The temperature of each propagation direction in the early stage of the flame was almost the same,and the temperature of the internal and external flames were also very close.With the development of the flame propagation,the temperature of each stage increased,but the temperature of the flame front was relatively lower than that of inside, which was closely related to the dehydrogenation reaction of MgH2powders and the gasification of Mg.Fig.4(a)demonstrates that the dehydrogenation temperature of MgH2powder was low and its dehydrogenation rate was fast, and a large amount of H2would be quickly released after heating.H2diffused and burned rapidly at the flame front, and the decomposed product Mg particles inside the flame vaporized and underwent a combustion reaction at the same time.For the combustion temperature of Mg powders was higher than that of H2[27],and the decomposition of MgH2powders at the flame front would absorb part of the heat generated by H2combustion, so that the temperature at the dust flame front was lower than that inside.Furthermore,by comparing the temperature distributions of MgH2dust clouds with different particle sizes, it could be seen that local high temperature areas would appear at the bottom part of the dust cloud,and the overall flame temperature distributions in the later stage of the dust clouds with small particle sizes were more uniform and the temperature gradient were smaller than those in the dust clouds with large particle size.That was because the gravity effect could not be neglected with the increasing particle size [28].In this regard, we performed analysis from the gravitational sedimentation perspective on the spatial distribution characteristics of dust flame temperature.
Firstly, assuming that these particles were spheres with a diameter ofdp, they were sedimenting under the forces including gravity, buoyancy, and airflow drag force [43].According to the Newton's second law,
wherempis the particle mass;upis the particle velocity;ugis the flow velocity of air,ρpis the density of particles;ρgis the density of air;dpis the diameter of particles;ζdis the drag coefficient;gis the gravitational acceleration.
The drag force increases as the air velocity increases.When a certain velocityutis reached, the sum of gravity, buoyancy and airflow drag force acting on the particles is zero, and the particles fall at a uniform velocity ofut,which is the final settling velocity of the particles.Based on Eq.(1), when dup/dt= 0, the final falling velocity of the particles can be calculated.

Fig.11.Micro-explosion of a single MgH2 particle.
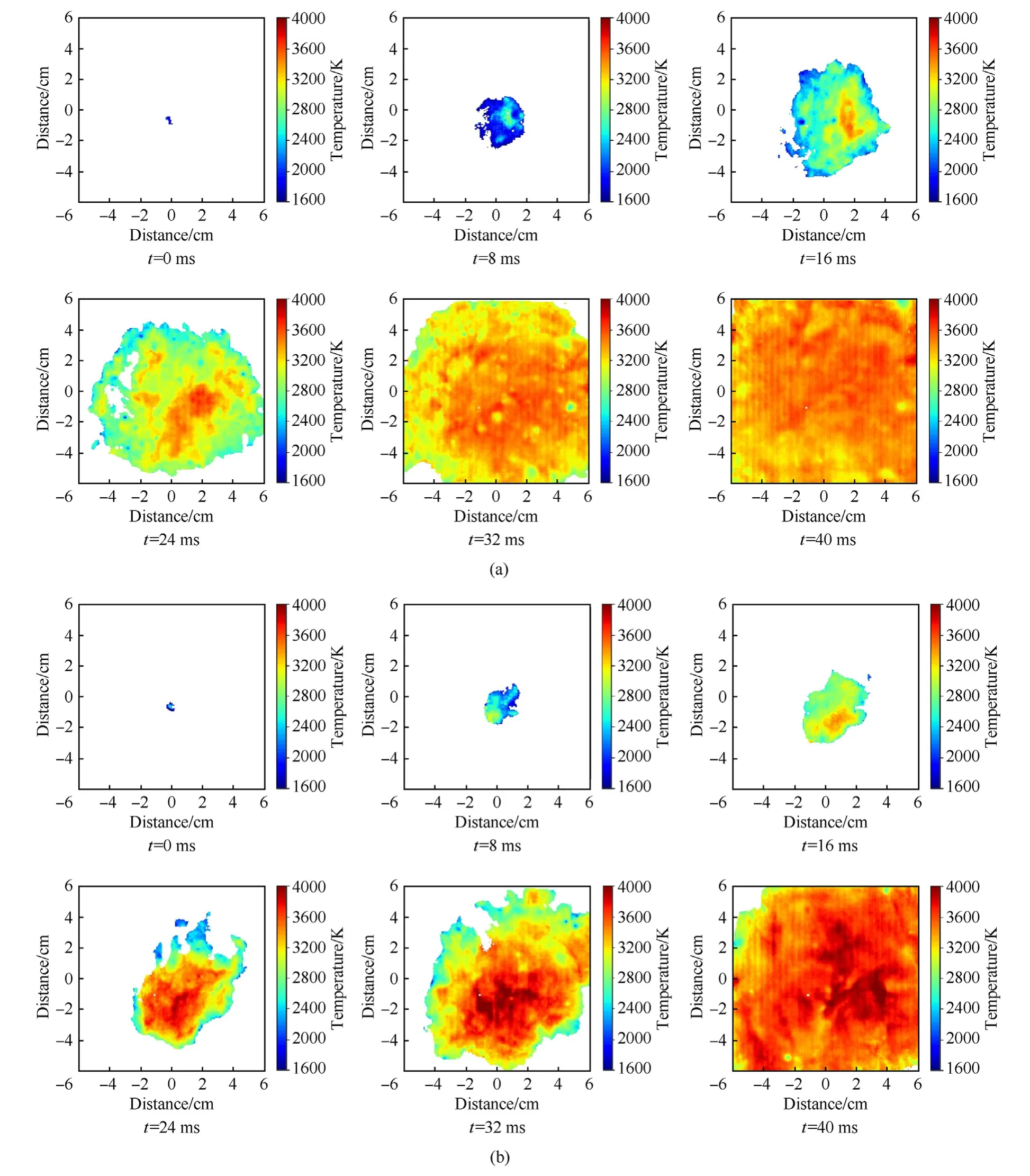
Fig.12.Flame temperature distribution in micron MgH2 dust clouds:(a)23 μm MgH2 dust cloud;(b) 40 μm MgH2 dust cloud;(c)60 μm MgH2 dust cloud;(d)103 μm MgH2 dust cloud.
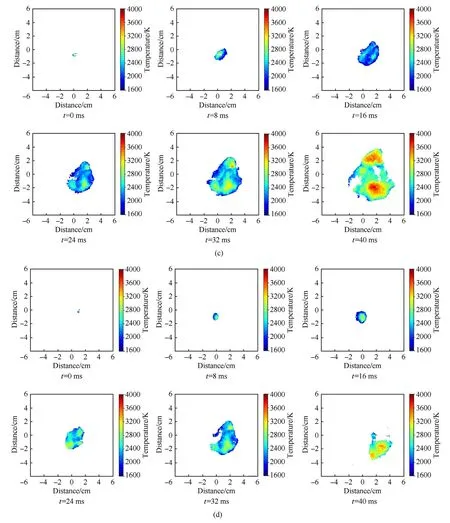
Fig.12.(continued).
The drag coefficient ζdchanges with Reynolds numberRe, and, when Reynolds numberRe≤1, the drag coefficient ζd=24/Re,so the particle sedimentation formula can be expressed as
where ν is the kinematic viscosity of air, ν = 15.7 × 10-6m2/s;ρg= 1.20 kg/m3.
The falling velocity of the particles were approximated calculated according to the density of MgH2(1450 kg/m3),and the results are listed in Table 1.Eq.(3)shows that the falling velocity of the particles was proportional to the square of the particle size.Smaller particle had lower falling velocity of particles in the air,which was more conducive to the suspension and diffusion movement of particles as well as the heat transfer between particles.As shown in Table 1,the falling velocities of 23 μm and 40 μm MgH2particles were 2.24 cm/s and 6.71 cm/s, respectively, which was much smaller than those of 60 μm and 103 μm MgH2particles.Therefore, the flame shape were more spherical and the temperature distribution were more uniform in the 23 μm and 40 μm MgH2dust clouds.While the falling velocities of 60 μm and 103 μm MgH2particles were as high as 15.07 cm/s and 44.42 cm/s, respectively,leading to a more rapid downward development and irregular shape of the flame, which also explained why the cluster flame usually appeared at the bottom of the MgH2dust cloud with large particle sizes.

Table 1Falling velocity of MgH2 particles.
3.5.Analysis of MgH2 combustion residues
Chemical composition analysis of solid combustion residues could invert the development process of MgH2dust explosion[44].Fig.13 shows the micro-structures of solid residues with a scanning electron microscopy(SEM).As a reference,Fig.13(a)demonstrates the SEM picture of an unreacted MgH2particle.Fig.13(b) shows that the solid combustion residues of MgH2particle became loose,porous and larger than the unburned ones.Floating flocculus formed during the MgH2combustion covered the surface of the solid combustion product,as shown in Fig.13(c).With the increase of temperature and the deepening of dehydrogenation degree,the pores on the surface of the combustion residues became larger and penetrated deep into the particles or even produced cracks under the action of micro-explosion, as shown in Fig.13(d).To further understand the combustion reaction of the MgH2dust cloud,X-ray diffraction experiments were performed on its combustion residues, and the results are shown in Fig.14.Fig.14 shows that only MgO and Mg were contained in the solid residues after MgH2dust cloud combustion,indicating that MgH2particles were completely decomposed in the combustion reaction, and MgO was the oxidation product of the combustion reaction of MgH2particles, while Mg was the decomposition product of MgH2that did not participate in the combustion.
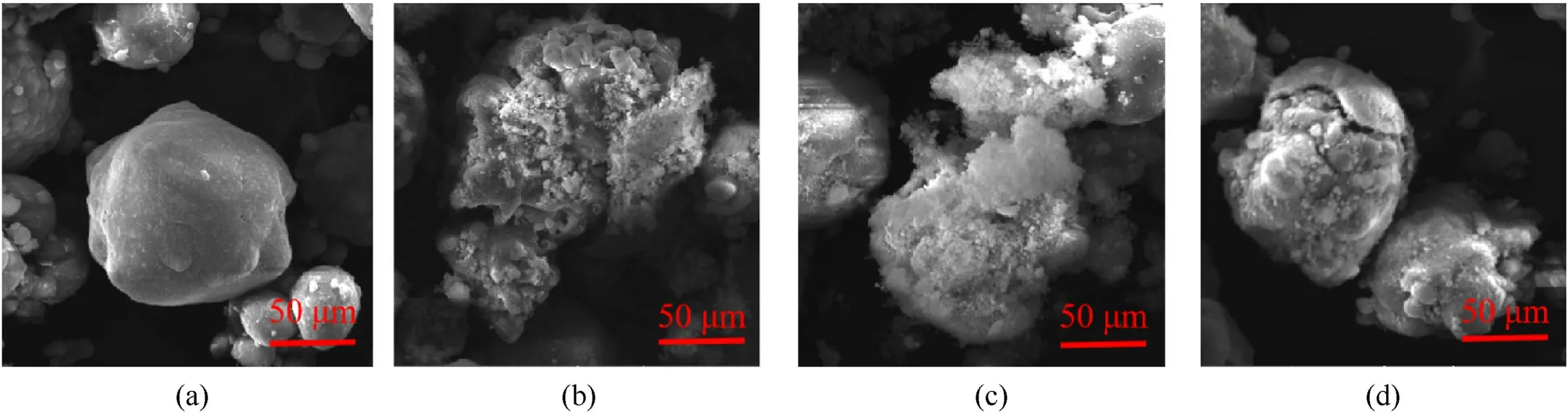
Fig.13.Micro-structures of MgH2 combustion residues:(a)Pure MgH2 particle surface;(b)Holes on residue particle surface;(c)MgO floccules on residue particle surface;(d)Crack on residue particle surface.

Fig.14.XRD patterns of MgH2 combustion residues.
Combining the experimental results of the flame propagation behaviors, flame micro-structure characteristics and combustion residues composition analysis, the dehydrogenation and combustion processes of MgH2powders in air can be obtained,as shown in Fig.15.After the MgH2particles were heated, heterogeneous surface reaction occurred between particle surface and oxygen to form a protective MgO layer.Since H2would be released from MgH2particle, and the thermal expansion coefficient of MgH2particle was significantly larger than that of MgO [17], so holes and cracks would be formed on the surface of the MgH2particles.Meanwhile,the transformation of MgH2to Mg was accompanied by volume shrinkage, which led to further expansion of pores and cracks formed on the surfaces of MgH2particles [45].H2has the characteristics of low ignition point and wide explosion limit,and formed a gas-solid two-phase combustion reaction with MgH2particles,which enhanced the explosion intensity.In addition, the water vapor of H2combustion product could promote the hydrolysis reaction of Mg to released H2and large amounts of heat, thereby accelerating the propagation of MgH2dust flame.At the same time,oxygen could enter the interior of MgH2particles through the cracks, and the MgO layer gradually expanded to the inside of the particles, while some Mg vapor and H2could escape through the cracks of the MgO layer, so that the degree of MgH2dehydrogenation deepened gradually from the surface to the interior.MgH2particles were not completely melted in the initial heating stage,at this moment the interaction of MgH2particle with oxygen mainly occurred on its surface.After the ignition of MgH2dust cloud, the surface of the dust particle was completely melted and its vapor was generated, the released H2and metal vapor were mixed with oxygen and then vapor phase diffusion combustion occurred.The flocculent MgO generated by vapor phase combustion of particles diffused into the surrounding space, and part of them diffused inward and deposited on the surface of the solid residues.Therefore,the combustion control mechanism of MgH2particles was mainly heterogeneous surface reaction.Furthermore, the specific surface area,hydrogen release rate and hydrogen storage capacity of MgH2powders with small particle size were higher than those with large particle size, thus the dust clouds with smaller particle sizes had faster flame propagation velocity and more violent combustion reaction, and their flame temperature distributions were more uniform and the temperature gradients were smaller.

Fig.15.Schematic of the dehydrogenation and passivation process of MgH2.
4.Conclusions
The experimental results of thermal analysis experiments showed that, under argon atmosphere, the dehydrogenation of MgH2particles occurred at 455-475°C.MgH2particles with smaller particle size had higher H2content, but the initial decomposition temperature, maximum heat absorption and the energy required for H2release were lower.Under air atmosphere, MgH2particles had two rapid weight gain stages in the range of 370-450°C and 600-650°C, respectively.In the first rapid weight gain stage,the initial oxidation temperature and heat release of the smaller particles were both lower but the weight gain was greater.In the second rapid weight gain stage,MgH2powders with smaller particle sizes had less weight gain and heat release due to less Mg content.
Flame propagated in the MgH2dust clouds all presented an approximately spherical shape at the early stage, the average propagation velocity,initial acceleration,and oscillation amplitude of the dust flame were related to the particle size distributions of MgH2powders.The smaller particle sizes resulted in the greater flame propagation speed and initial acceleration.In addition, the velocity oscillation became more obvious as the particle sizes decreased,owing to that the thermal decomposition rate of smaller particles was relatively faster, and their gas phase velocities changed more significantly than larger particles, which made the velocity slip between particles and gas larger, and the oscillation amplitude increased.Furthermore, different from Mg dust clouds,MgH2dust clouds had many combustion particles with microdiffusion flame structures during the combustion processes.Micro-explosion occurred during the combustion of MgH2dust clouds to inhibit particle agglomeration in a certain extent, which was conducive to improving the combustion of MgH2powders.
The flame temperature of MgH2dust cloud in the early stage was almost the same in all directions.With the development of the dust flame, the temperature of each stage increased, but the temperature of the flame front was lower than that inside.Local high temperature appeared at the bottom of the MgH2dust cloud due to the dust falling,and in the later stage of the dust clouds with small particle size, the overall flame temperature distribution was more uniform and the temperature gradient was smaller than those with large particle size.In addition, combining with the experimental results of flame propagation behaviors, flame micro-structure characteristics and combustion residues composition analysis, it could be concluded that the combustion control mechanism of MgH2particles was mainly heterogeneous surface reaction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grant Nos.12272001, 11972046), the Outstanding Youth Project of Natural Science Foundation of Anhui Province (Grant No.2108085Y02), and the Major Project of Anhui University Natural Science Foundation (Grant No.KJ2020ZD30),and the authors would like to thank these foundations for the financial supports.
- Defence Technology的其它文章
- The interaction between a shaped charge jet and a single moving plate
- Machine learning for predicting the outcome of terminal ballistics events
- Fabrication and characterization of multi-scale coated boron powders with improved combustion performance: A brief review
- Experimental research on the launching system of auxiliary charge with filter cartridge structure
- Dependence of impact regime boundaries on the initial temperatures of projectiles and targets
- Experimental and numerical study of hypervelocity impact damage on composite overwrapped pressure vessels

