Diffusion Characteristics and Removal of Cyclohexane in Polyolefin Elastomer Melt
Qi Jibing; Yang Tong; Liu Yandong; Yuan Zhiguo; Zhang Qiaoling; Liu Youzhi; Yi Jianjun
(1. Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering, North University of China, Taiyuan 030051, China; 2. Petrochemical Research Institute, PetroChina Co., Ltd., Beijing 102206, China)
Abstract: The diffusion coefficient of volatiles in polymer solutions is a crucial parameter to describe the mass transfer efficiency and ability of volatiles. In this research, polyolefin elastomer (POE) was used as a polymer, and cyclohexane was used as a volatile. A gravimetric analysis was applied to measure the diffusion coefficient of cyclohexane in POE. The devolatilization rate of the POE-cyclohexane system under different conditions was measured. The effects of temperature, film sample thickness, and initial concentration of volatiles on the devolatilization rate were discussed. Based on the devolatilization rate data, the average diffusion coefficient of cyclohexane in POE was obtained by fitting with a mathematical model. The experimental results indicate that the devolatilization rate increased with increasing temperature and initial concentration of volatiles, but it decreased with increasing sample thickness. As the thickness increased, the overall diffusion resistance increased. As the temperature increased, the molecular movement increased, resulting in the increase of average diffusion coefficient. The relationship between the diffusion coefficient of the POE-cyclohexane system and temperature follows the Arrhenius law. The diffusion activation energy E=6201.73 J/mol, and the pre-exponential factor of the diffusion coefficient D0=2.64×10-10 m2/s. This work can provide basic data for exploring the devolatilization of POE polymers and serves as a useful reference for enhancing the effect of devolatilization.
Key words: diffusion coefficient; gravimetric analysis; polyolefin elastomer (POE); cyclohexane; devolatilization rate
1 Introduction
In polymer synthesis, there are a small number of unexpected components in the products, such as unpolymerized monomers, solvents, and even byproducts. These components are also known as volatiles, which directly affect the performance of the product, limit the application of the polymers, and even do harm to the environment and users’ health[1-7]. Therefore, the removal or recovery of the residual volatile components from the polymer is crucial[6]. The removal efficiency depends on the diffusion capacity of volatile molecules in the polymer system. The research on diffusion coefficient is of theoretical significance and practical value to strengthen diffusion capacity, reduce energy consumption, and guide the design and practical operation of diffusion processes. With the development and highend application of polymer materials, more stringent technical indicators of polymer devolatilization have been put forward. Therefore, it is particularly important to study the diffusion of small molecules in polymers, as it has broad theoretical and practical significance[8-9]. The diffusion coefficient of small molecules in polymers is the most direct physical quantity to measure their diffusion properties[10-11]. The diffusion coefficient is a basic physical parameter describing transport phenomena[12-15], characterizing the degree of diffusion and mass transfer capacity[16], and it has important theoretical properties and broad application prospects in the development of polymer materials[17].
The diffusion coefficient of volatiles in polymer solutions is an important parameter to describe the mass transfer efficiency and ability of volatiles[16]. At present, the diffusion coefficient is mainly obtained by experimental measurement and estimated from empirical formulas[18]. Various methods can be used to determine the diffusion coefficient of small molecules in polymer systems, mainly including gravimetric analysis, inverse gas chromatography, nuclear magnetic resonance, dissolution infiltration, and laser holography[1,18-21]. Gravimetric analysis is a common method to determine the mutual diffusion coefficient of solvent molecules in polymer systems[22]. Inverse gas chromatography is mainly used to determine the self-diffusion coefficient of a solvent at an infinite dilution solvent concentration[11,23-24], and preparing the chromatographic column is troublesome[20]. Nuclear magnetic resonance is used to determine the self-diffusion coefficient, but it is only suitable for the determination of solvent molecules that can generate NMR. Furthermore, it has the problems of high price and high equipment maintenance costs[10,19]. Dissolution infiltration was one of the first main methods to study gas diffusion in polymer membranes[17]. Laser holography is a modern method to measure the self-diffusion coefficient of small molecules in polymer systems[1], and it has high precision and sensitivity. Each determination method has its own advantages in terms of measurement time, accuracy, and applicable concentration range[19].
Gravimetric analysis is a method in which the mass change caused by the absorption or desorption of volatile molecules by the polymer is input into the integrated diffusion equation to obtain the diffusion coefficient[22,25]. Common gravimetric analysis methods include the quartz spring method, the microbalance method, the leaching weighing method, and the piezoelectric crystal method[17,25-26]. The significant advantages of gravimetric analysis are that its experimental equipment is relatively simple[25], its cost is low, and its applicable temperature and pressure ranges are wide[19]. In the existing diffusion coefficient measurement methods, different measurement methods are applicable to different material systems. For high-viscosity polymer systems, such as polyolefin elastomer (POE), the measurement of each parameter is not easy due to the high viscosity of the polymer system. Inverse gas chromatography is not suitable for high-viscosity materials, and if inverse gas chromatography is used, they may block the injection tube and chromatographic column, whereas gravimetric analysis is suitable for a wide range and has looser sample requirements. Therefore, for high-viscosity systems, gravimetric analysis has shown unique advantages.
As a high-viscosity polymer, POE has a narrow relative molecular mass distribution. In the molecular structure, ethylene and higher α-olefin copolymers play the role of physical cross-linking through the crystallization of polyethylene chain segments, which makes POE have the dual characteristics of plastic and rubber, uniquely combining the flexibility and toughness of synthetic rubber and the processability of plastics[27]. Additionally, POE polymers possess many excellent properties, such as low density, good resistance to aging and ultraviolet radiation, excellent elasticity, low costs[28], and excellent mechanical and rheological properties[29-32]. Therefore, POE is widely used in many fields. With the deepening of research and the progress of industrialization, the application of POE will become more and more extensive[33-37]. Nevertheless, POE production technology remains absent in China. To change the present situation that China relies on imported POE products, some domestic enterprises have devoted themselves to POE research. However, there is little research available on the diffusion coefficient of volatiles in POE systems with high viscosity.
In this work, the POE-cyclohexane system was taken as an example, and gravimetric analysis was applied to efficiently measure the diffusion coefficient of cyclohexane in POE. Firstly, the devolatilization rate of the POE-cyclohexane system under different conditions was measured, and the effects of temperature, film sample thickness, and initial concentration of volatiles on the devolatilization rate were discussed. Then, using the devolatilization rate data obtained experimentally, the average diffusion coefficient of cyclohexane in POE was obtained by fitting with a mathematical model. The effects of temperature, film sample thickness, and initial concentration of volatiles on the average diffusion coefficients were investigated.
2 Experimental
2.1 Materials
The following chemical reagents were used in the experiment: polyolefin elastomer (POE) and cyclohexane (99.5%). All chemicals were used without any further purification.
2.2 Preparation of POE melt
In the study, the solvent cyclohexane should be fully dissolved into the POE under the conditions of high temperature and high pressure. This dissolution process is a key step in preparing the qualified POE melt samples.
According to the required mass fraction, POE commodity material and cyclohexane solvent were added to a customized melting kettle. Then, nitrogen was pumped in to ensure that the entire melting kettle was under high pressure. When everything was ready, the heating device and stirring device were turned on, so that cyclohexane was dissolved into the POE under high temperature and high pressure. The temperature was 50–180 °C, and the pressure was 0.5–1 MPa. The whole dissolution process took about 5 hours (Figure 1). After the cyclohexane was fully dissolved into the POE, the melting kettle was cooled down, and the pressure was released. The POE melt was successfully prepared, and it could be used for the volatilization experiment.
Figure 1 Schematic diagram of the POE dissolution experiment.
2.3 Preparation of sample
In the experiment, a certain amount of POE melt prepared in the melting kettle was taken out for tableting, which was pressed into a circular slice with a diameter of 80–90 mm as the experimental sample. The thickness of the circular slice depended on the experimental conditions, which was generally 0.2–1 mm.
2.4 Experimental setup of devolatilization experiment
Gravimetric analysis was used to continuously measure the mass of the POE melt samples containing volatile components at different times. Thus, the mass of volatile matter released from the POE melt at the corresponding time was obtained, and the corresponding devolatilization rate was calculated. The mass change caused by the desorption of volatile molecules by the polymer was input into the integrated diffusion equation to obtain the diffusion coefficient.
The experimental setup is shown in Figure 2, which mainly included two parts: a weighing system and a temperature control system. The weighing system contained a petri dish, a support frame, an analytical balance, a computer, and a balance holder. A Sartorius Practum-type high-precision analytical balance was used to measure the mass change of the POE samples in real time.

Figure 2 Diagram of the experimental setup for devolatilization
The range of the analytical balance was 0–220 g, the accuracy was 0.0001 g, and the error did not exceed 1 mg, which met the experimental requirements. The computer collected the mass data of each sample in real time every 10 s. The temperature control system consisted of a drying oven and a PY-SM5 LCD high-precision temperature control component. The temperature control drying oven combined with the PY-SM5 LCD high-precision temperature control component to maintain a constant temperature in the experimental environment. The temperature accuracy of the temperature control component was ±0.1 °C. The temperature measuring probe of the temperature control component was placed above the sample. When the ambient temperature exceeded the set temperature, the heating control device responded accordingly. Therefore, the system temperature could be considered constant during the experiment.
There was a hole at the bottom of the drying oven, and the support frame was passed through the hole. The top of the support frame was extended into the drying oven, and the bottom was placed on the analytical balance, so as to realize a connection between the weighing system and the temperature control system. The POE sample was placed in a petri dish, which was put on the top of the support frame. The drying oven was preheated in advance. Each experiment was repeated at least twice under the same conditions.
At a certain temperature, the volatile matter would be separated from the sample, and the sample lost mass weight. The mass of the sample was continuously monitored, and the above-mentioned mass loss was measured and recorded. The data were read and recorded every 10 s until the POE sample had a constant weight. The devolatilization rate was calculated by recording the continuous changes in the mass of the film samples of the POE and volatile component mixture. When the mass reached a stable level, it can be considered that the volatiles had been completely removed, and the experiment was finished.
In this study, a gravimetric analysis device was used to record the weight change of the sample in the process of absorption or desorption of volatiles, and the mass change curve during the devolatilization process was obtained. This method is often used to study the diffusion problem in polymer films. In a solution consisting of volatiles and polymer, the polymer can be considered as a stagnated component because of its high viscosity and poor liquidity[38]. Meanwhile, it is considered that the volatiles have one-dimensional diffusion along the thickness direction of the film, so it can be described by the onedimensional model of Fick’s diffusion law[6].
2.5 Calculation of the average diffusion coefficient of POE
In order to calculate the average diffusion coefficient, it is necessary to convert the relationship of the sample mass with time into the relationship of the devolatilization rate with time. It is assumed that from moments 0 tot, the mass of the sample decreases fromm0tomt. At the end time (tend) of the experiment, the reduction of volatile matter ism0–mend. Therefore, the devolatilization rate is defined as shown in Equation 1, whereyt,expis the devolatilization rate of the mixture at momenttin the experiment:

wherem0,mt, andmendare the initial mass, the mass of the sample at momentt, and the mass of the sample at the end timeof the experiment(tend), respectively.
According to Equation 1, the relative change curve of the sample mass can be processed to obtain the change curve of the devolatilized rate.
The average diffusion coefficient of volatiles and polymer can be obtained by establishing the one-dimensional model according to Fick’s diffusion law and calculating[6]:

with the following boundary and initial conditions:

whereCis the mass concentration (g/g) of the volatile solvent in the polymer,DABis the average diffusion coefficient of the volatile component and polymer,L(m) is the thickness of the sample,t(s) is the time of diffusion, andx(m) is the vertical coordinate in the sample. Equation 2 was resolved to the integral formula Equation 6, and an average diffusion coefficient (DAB) of the volatiles in the diffusion process can be calculated by this equation in the literature[39-40]:

wheremeq,m0, andmtare the equilibrium mass and initial mass, and the mass of the sample at momentt, respectively.
Based on Equation 6 and combined with the theoretical analytical formula, MATLAB was used to fit the devolatilization rate obtained from the experiment to obtain the average diffusion coefficient.
3 Results and Discussion
3.1 Influence of the thickness of film sample
In the experiment, the POE-cyclohexane system withT=323.15 K,Φ=85 mm, andC0=31.8% was used as an example. Figure 3(a) is the curve of the volatile matter removal rate of film samples with different thicknesses over time, whereyis the devolatilization rate,Tis the temperature,δis the thickness of the sample,Φis the diameter of the sample, andC0is the initial concentration of the volatiles.
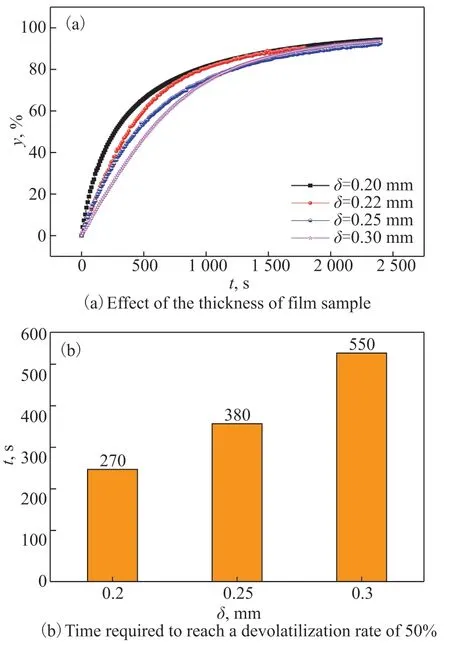
Figure 3 Effect of the thickness of the sample on the devolatilization rate
In the present work, the thickness of the polymer film sample is the main factor affecting devolatilization, so it needs to be determined first mainly because of the following reasons:
(1) When the thickness of the thin film sample is large, the resistance of the volatiles at the bottom of the sample will increase in the process of diffusion to the gas–solid interface.
(2) With the increase of sample thickness, the content of the volatiles in the sample will also increase, resulting in the partial pressure of the gas phase at the gas–solid interface above the sample deviating from the assumption that the partial pressure of the gas–phase space is 0.
(3) Considering the error of actual operation, in the process of pressing the film sample, it is inevitable that there will be volatilization of volatile matter, which will cause the actual initial concentration of the film sample to be lower than the initial value. When the thickness of the film sample is large, the loss of volatiles is relatively small, and the experimental measurement error will also be reduced. However, if the thickness of the sample is too thick, the assumption of a one-dimensional diffusion model will be deviated. Furthermore, a large thickness is not conducive to devolatilization.
It can be seen from Figure 3(a) that, in the early stage of the experiment, the removal rate of volatiles was faster, and the devolatilization rate of the sample with a larger thickness was lower than that of the sample with a smaller thickness. The sample withδ=0.20 mm reached 50% at about 270 s, and the sample withδ= 0.22 mm reached 50% at about 380 s. It took 550 s for the sample withδ=0.30 mm to reach 50%, as shown in the Figure 3(b). And the thickness increased the diffusion resistance during the whole devolatilization process. In the devolatilization process, the greater the thickness of the sample, the greater the devolatilization resistance, and the smaller the devolatilization rate over the same time. The smaller the thickness of the sample, the smaller the devolatilization resistance, and the greater the devolatilization rate over the same time. With the thickness decreases, the devolatilization path of volatiles is shortened, the overall devolatilization resistance is reduced, and the overall devolatilization efficiency is improved. Therefore, in the process of devolatilization, the thickness of the sample should be as thin as possible, which can reduce the devolatilization resistance and increase the devolatilization rate. The experimental results provide technical support for the subsequent design and operation of the devolatilization apparatus.
It is observed from Figure 4 that the average diffusion coefficient increased with the increase of the thickness of the thin-film sample. The increase of the thickness increased the diffusion resistance during the entire devolatilization process. In the devolatilization process, the greater the thickness of the sample, the greater the diffusion resistance, and the greater the average diffusion coefficient. As the thickness increased, the diffusion path of the volatiles was lengthened, the overall diffusion resistance was increased, and the overall diffusion coefficient was improved. When the thickness of the sample increased by 50%, the average diffusion coefficient increased by 26.9%.
So the maiden departed and climbed up the mountain, but before she reached the top the giant heard her footsteps, and rushed out breathing fire and flame, having a sword in one hand and a club in the other

Figure 4 Effect of the thickness of the sample on the diffusion coefficient of the volatiles.
3.2 Influence of temperature
In this part, a film sample of the POE-cyclohexane system withδ=0.25 mm andC0=32% was selected to study the influence of temperature on the devolatilization rate. Figure 5 is the curve of the devolatilization rate of the film samples versus time at different temperatures.
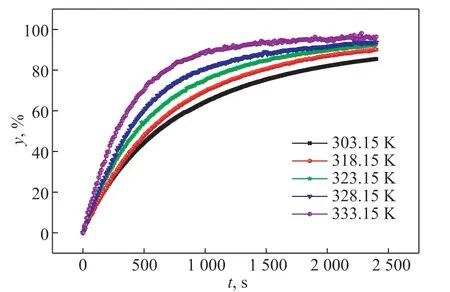
Figure 5 Effect of temperature on the devolatilization rate
As shown in Figure 5, with the increase of temperature, the devolatilization rate of the thin-film samples increased. The variation trend of the devolatilization rate with time at different temperatures was consistent, and the devolatilization rate curve was smooth. With the increase of temperature, the molecular movement speed increases, resulting in the increase of the devolatilization rate of the sample. At the same temperature, the devolatilization rate increased gradually. In the initial stage of the devolatilization, the escape rate of volatiles was faster, and the devolatilization rate increased greatly. As time going on, the total content of volatiles in the samples decreased, and the increase of the removal rate of volatiles tended to be slow.

Figure 6 Effect of temperature on the diffusion coefficient of the volatiles.
The increase of temperature is helpful for devolatilization. Therefore, in order to increase the devolatilization rate and improve the degree of devolatilization, in designing a devolatilizer, the heating and thermal insulation components should be considered.
As shown in Figure 6, the average diffusion coefficient of volatiles in the thin-film samples increased with the increase of temperature. Higher temperature will make the polymer molecular chain move more violently, and increase the rate of molecular diffusion. As a result, the average diffusion coefficient of volatiles in the sample will increase.
As illustrated in Figure 7, at different temperatures, the logarithm of the diffusion coefficients had a linear relationship with the reciprocal of the temperature, which can be expressed by Equation 7:
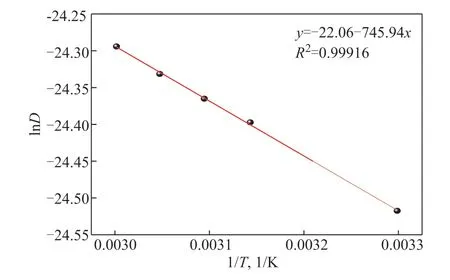
Figure 7 Logarithm of diffusion coefficients under different 1/T of POE-cyclohexane

For Equation 7,R2=0.99916, and it can be seen that the fitting is good.
According to the Arrhenius law, Equation 7 can be expressed as follows:

whereEis the diffusion activation energy (J/mol)—which is the potential barrier when atoms overcome the constraints and transition, and is related to the structure of polymers and small molecules—andD0is the preexponential factor of the diffusion coefficient. According to the Arrhenius law, the diffusion activation energy of each mixed system can be obtained. In Figure 7, the slope of the lnD-1/Tcurve is the value ofE/R, whereRis the ideal gas constant (J/(mol·K)).
It can be concluded that the law of the diffusion coefficient of POE-cyclohexane system being affected by temperature was in accordance with the Arrhenius law. The diffusion activation energy (E) was 6201.73 J/mol, and the pre-exponential factor of the diffusion coefficient (D0) was 2.64×10-10m2/s.
3.3 Influence of the initial concentration of volatiles
In the process of polymer devolatilization, the concentration of volatiles in the polymer also affects the devolatilization rate. Figure 8 shows the variation of the devolatilization rate of polymer samples with different initial concentrations of volatiles over time. In the POEcyclohexane system, the volatile was cyclohexane with initial mass fractions of 21.5%, 27.2%, 31.8%, and 35.2%. The experimental temperature was 323.15 K, the system was under atmospheric pressure, and the thickness of the sample (δ)was 0.22 mm.
Figure 8 shows that, in the same time, the greater the initial concentration of volatiles, the greater the corresponding devolatilization rate. The higher initial volatiles mass fraction improved the mass transfer driving force (i.e., concentration gradient), which promoted the removal of volatiles.
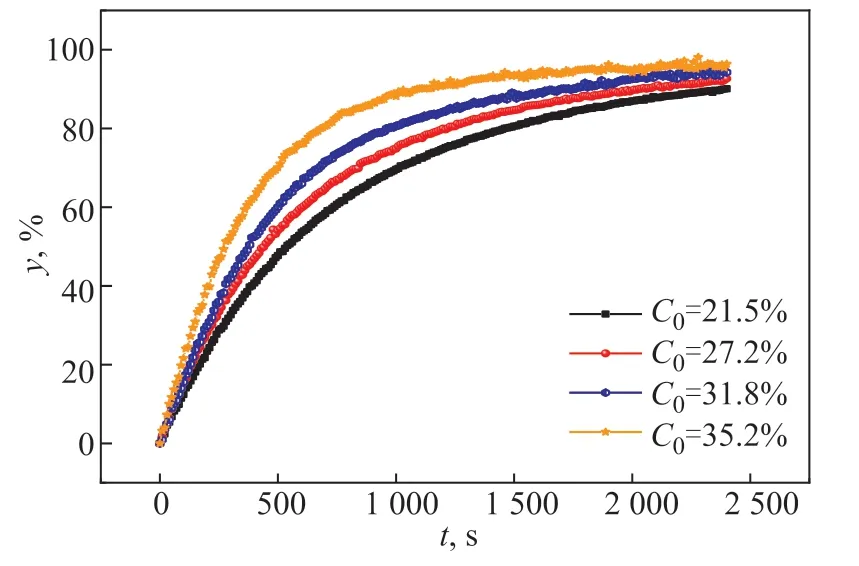
Figure 8 Effect of the initial concentration of the volatiles on the devolatilization rate
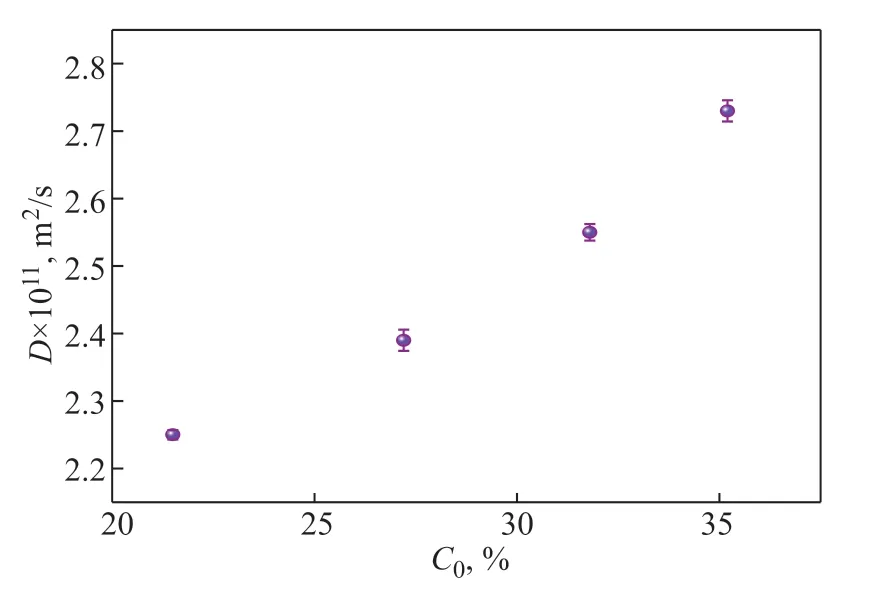
Figure 9 Effect of the initial concentration of the volatiles on the diffusion coefficient.
As shown in Figure 9, the average diffusion coefficient increased with the increase of the initial concentration of volatiles. The initial concentration of volatiles increased as the content of volatiles in the sample increased. With the increase of initial volatiles mass fraction, the concentration gradient grew, favoring the faster mass transfer process, leading to the increase of the corresponding devolatilization rate and the average diffusion coefficient. When the initial concentration of volatiles increased by 63.7%, the average diffusion coefficient increased by 21.3%.
3.4 Influence of natural placing time
A film sample withΦ=90 mm,δ=0.8 mm, andC0=63.8% was selected and placed naturally at room temperature (299.15 K) to study the effect of placing time on the volatilization of volatiles in the film samples. Figure 10 shows the effect of natural placing time on the devolatilization rate.
Figure 10 shows that the devolatilization rate gradually increased over time. In the 0–600 min timeframe, the devolatilization rate increased rapidly. With time going on, the devolatilization rate increased relatively slowly. At 1200 min, the devolatilization rate reached the maximum value and tended to be stable at 99.81%. During the process of devolatilization, with time going on, the content of volatiles in the sample gradually decreased. Before the devolatilization rate reached the maximum value, the content of volatiles in the sample decreased rapidly. At 1200 min, the content of volatiles in the sample decreased to almost zero. Therefore, the devolatilization rate reached its maximum value at 1200 min.
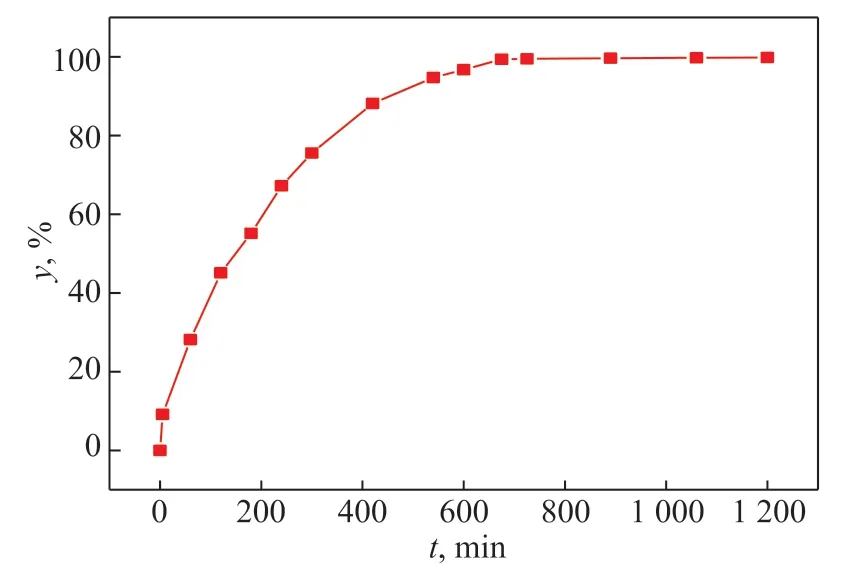
Figure 10 Effect of natural placing time on the devolatilization rate
4 Conclusion
In this work, the average diffusion coefficient of volatiles in a high-viscosity POE polymer system was studied by gravimetric analysis, and POE-cyclohexane was used as the research system. A device for gravimetric analysis was designed and constructed to measure the removal of volatiles from the polymer melt. The temperature control accuracy was 0.1 °C, and the mass measurement accuracy was 0.0001 g. The device was proved to be feasible by several tests on the removal rate of cyclohexane in the POE polymer melt. The experimental results indicate that the devolatilization rate increased with the increase of both temperature and initial concentration of volatiles, but it decreased with the increase of sample thickness. With the progress of devolatilization, the concentration of cyclohexane in the polymer decreased, which made devolatilization more difficult. In order to improve the devolatilization effect, it is necessary to increase the temperature, decrease the size of the polymer melt, and increase its dispersion. It is noteworthy that the relationship between the diffusion coefficient of the POE-cyclohexane system and temperature follows the Arrhenius law. The diffusion activation energy (E) was 6201.73 J/mol, and the pre-exponential factor of the diffusion coefficient (D0) was 2.64×10-10m2/s. This work can provide basic data for exploring the devolatilization of POE polymers and provide a useful reference for enhancing the effect of devolatilization.
Acknowledgments:The authors wish to express their thanks for the financial support from the Polyolefin Elastomer Technology Development project (2020B-2619).
- 中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Microporous Metal Organic Frameworks for Light Hydrocarbon Adsorption
- Selection of Extraction Solvents for Bitumen from Indonesian Oil Sands through Solubility Parameters
- Synthesis of Hierarchical Porous Fe2O3/Al2O3 Materials and Study on Catalytic Viscosity Reduction of Heavy Oil
- Activated Carbon from Rice Husk with One-Step KOH Mechanical Mixing Activation as Adsorbent for Treating Phenolic Wastewater
- Effect of Particle Shape on Catalyst Deactivation during 2-Butene and Isobutane Alkylation of Liquid Phase in Fixed-Bed Reactor Using Particle-Resolved CFD Simulation
- Experimental and Numerical Investigation on Erosion Corrosion of the Air Cooler Tube Bundle in a Residue Hydrotreating Unit

