Synthesis and Evaluation of Microporous Metal Organic Frameworks for Light Hydrocarbon Adsorption
Wen Guilin; Li Ying; Li Jianzhe; Li Qingrun; Zhang Hongxing; Xiao Anshan; Tao Bin
(State Key Laboratory of Safety and Control for Chemicals, SINOPEC Research Institute of Safety Engineering Co., Ltd., Qingdao 266101, China)
Abstract: Five microporous MOFs were synthesized and their static adsorption properties for light hydrocarbons were experimentally investigated at 298 K and 150 kPa. Among the five MOFs, HKUST-1 and Ni(bdc)(ted)0.5 exhibited much higher uptakes of ethane and propane than PCN-250, UiO-66, and ZIF-8. Breakthrough experiments were carried out at 298 K and atmospheric pressure on HKUST-1 and two commercially used adsorbents. HKUST-1 exhibited a much lower dynamic than static adsorption capacity. Moreover, HKUST-1 and the two traditional adsorbents could effectively separate binary (ethane/propane) and ternary (ethane/propane/toluene) mixtures.
Key words: light hydrocarbons; metal–organic frameworks; adsorption; breakthrough curve; adsorption selectivity
1 Introduction
The increasing focus on environmental protection leads to stricter emission standards for air pollutants. The recovery of volatile organic compounds (VOCs) in the refining and oil storage industry is a significant task, not only for its beneficial effect on environmental protection, but also because it can create economic value[1-2]. Among the recovery methods of VOCs[3-5], the adsorption technique represents an effective and energysaving approach[6]. Common adsorbents include activated carbon, silica gel, and molecular sieves, which have a wide range of applications in the field of gas adsorption. Currently, the emissions contain a large amount of light hydrocarbons, whose small sizes hinder their adsorption by existing adsorbent materials. The search for more effective adsorbents has led to the emergence of improved materials. Metal–organic frameworks (MOFs)[7]are a class of organic–inorganic hybrid materials with intramolecular pores formed by the self-assembly of organic ligands and inorganic metal ions or clusters through coordination bonds; these materials are considered as promising adsorbents. The huge specific surface area of MOFs results in excellent adsorption performance, and the good designability of MOF materials enables the development of highly selective adsorption materials for specific types of gases[8-11]. Bao et al. synthesized a magnesium-based MOF (Mg-MOF-74) and examined its adsorption capacity for C2/C3hydrocarbons at 1 bar and 298 K[12]. The Mg-MOF-74 material had a higher affinity for C3than C2, mainly due to the significant dipole moment of propylene and propane; the adsorption capacities for C2H6and C3H8reached 6.6 and 7.2 mmol/g, respectively. Liang et al. reported that Ni(bdc)(ted)0.5(bdc = 1,4-benzenedicarboxylic; ted = triethylenediamine) exhibited a high C2H6adsorption capacity of 6.93 mmol/g at 100 kPa and 273 K[13]. In addition, Pires et al. reported that MOFs with highly aromatic ligands preferentially adsorbed C2H6over C2H4[14]. Wang et al. explored the effect of the functional groups of UMCM-151 on the adsorption of light hydrocarbons[2]. The results showed that the -F, -Cl, -NH2, -CH3, and -OCH3functional groups significantly increased the adsorption capacity, and electronic effects resulted in an improved affinity of the porous MOFs for light hydrocarbons. Shi et al. conducted gas adsorption experiments and revealed that the light hydrocarbon uptake of PCN-224 followed the trend C3> C2> C1at any temperature[15].
An appropriate pore size is one of the key criteria for the selection of MOF adsorbents[16]. The gas molecules cannot be adsorbed when their size exceeds that of the MOF pores, whereas the adsorbed molecules will easily leak out when their size is much smaller than the pore diameter. Due to the smaller sizes of light hydrocarbons, microporous MOFs should be selected for their effective adsorption. In this work, we synthesized five microporous MOFs (HKUST-1, Ni(bdc)(ted)0.5, PCN-250, UiO-66, and ZIF-8) and investigated their performance in the adsorption of two typical light hydrocarbons, C2H6and C3H8.
2 Experimental
2.1 Synthesis of MOFs
Five microporous MOFs (HKUST-1, Ni(bdc)(ted)0.5, PCN-250, UiO-66, and ZIF-8) were synthesized following typical procedures reported in the literature, with some modifications.
Preparation of HKUST-1:According to the synthesis method reported in the literature[17], we dissolved copper hydroxide (Cu(OH)2, 0.065 mol) and 1,3,5-benzenetricarboxylic acid (0.05 mol) in ethanol aqueous solution (ethanol 80 mL/H2O 60 mL) under stirring. After 24 h of reaction with medium-speed stirring, the reaction system turned into a uniform turbid liquid with a peacock blue color. The corresponding powders were obtained by centrifugation and then washed with water and ethanol in sequence. Finally, the product was dried in a vacuum oven at 350 K for 24 h.
Preparation of Ni(bdc)(ted)0.5:The Ni(bdc)(ted)0.5MOF was synthesized following the procedures reported by Guerrero-Medina[18]. First, terephthalic acid (H2bdc, 1 mmol) and ted (0.5 mmol) were dissolved inN,Ndimethylformamide (DMF, 30 mL) in sequence. Then, nickel chloride hexahydrate (NiCl2·6H2O, 1 mmol) was added to the mixture with homogeneous stirring. After that, the resulting green homogeneous solution was put into a Teflon-lined vessel and placed in oven heated at 390 K for 48 h. Green crystals were recoveredvialowspeed centrifugation and washed several times with DMF and ethanol, followed by drying in vacuum at 350 K for 24 h.
Preparation of PCN-250:The PCN-250 MOF was synthesized by a typical procedure[1]. First, Fe3(μ3-O)(CH3COO)6was prepared by adding ferric nitrate nonahydrate (Fe(NO3)3·9H2O, 0.02 mol) and sodium acetate (CH3COONa, 0.31 mol) in H2O (50 mL) with stirring at room temperature. The synthesized [Fe3(μ3-O)(CH3COO)6] was washed with water and ethanol, followed by drying in vacuum. Then, the treated [Fe3(μ3-O)(CH3COO)6] (1.7 mmol), 3,3’,5,5’-azobenzene tetracarboxylic acid (H4ABTC, 2.8 mmol), and acetic acid (4 mL) were dissolved in DMF (40 mL). Finally, the obtained mixture was heated in an oven at 410 K for 12 h. After completion of the reaction, brown crystals were recovered by centrifugation. The recovered crystals were washed several times with DMF, water, and ethanol in sequence, followed by drying the PCN-250 product in vacuum at 350 K for 24 h.
Preparation of UiO-66:The synthesis of UiO-66 followed a similar procedure to that reported by Denny et al.[19]. First, zirconium tetrachloride (ZrCl4, 2.5 mmol), terephthalic acid (2.5 mmol), glacial acetic acid (4 mL), and DMF (50 mL) were mixed thoroughly at room temperature. Then, the mixture was transferred into a Teflon-lined vessel and heated at 410 K. After the system was cooled down to room temperature, white crystals were separated by centrifugation and washed with water and ethanol in sequence. Finally, the products were obtained after drying in vacuum at 350 K for 24 h.
Preparation of ZIF-8:ZIF-8 was synthesized according to a typical procedure[20]. Zinc nitrate hexahydrate (Zn(NO3)2·9H2O, 3.36 mmol) and 2-methylimidazole (6.72 mmol) were completely dissolved and stirred in DMF (30 mL) at room temperature. Then, the colorless solution was transferred into a Teflon-lined vessel and heated at 410 K for 24 h. After that, white crystals were separated by centrifugation and washed with water and ethanol. The ZIF-8 product was obtained after drying in vacuum at 350 K for 24 h.
2.2 Adsorption isotherm measurements
N2adsorption–desorption isotherms of the synthesized MOFs were measured in theP/P0range of 0.01–0.99 by a BSD-PS2 analyzer at 77 K. Before each measurement, the sample was activated under vacuum heating at 420 K for 12 h to remove solvent molecules trapped in the framework. Based on the N2adsorption isotherms, specific surface areas were calculated using the Brunauer–Emmett–Teller (BET) and Langmuir methods. Pore sizes were determined by the Horvath–Kawazoe (HK) method. Adsorption–desorption measurements of C2H6and C3H8light hydrocarbons on HKUST-1, Ni(bdc)(ted)0.5, PCN-250, UiO-66, and ZIF-8 were carried out at a high pressure of 150 kPa and 298 K on the BSD-PS2 analyzer.
2.3 Breakthrough experiments
Breakthrough curves were determined using a multicomponent adsorption breakthrough curve analyzer (BSD-MAB). In each experiment, a powder sample was evenly dispersed in a quartz cotton-filled column, thus preventing high-pressure drops. The column inner diameter was 0.6 cm and the loading height of the samples was approximately 8 cm. Prior to the measurements, all samples were activatedin situinside the absorbent by flowing helium for 120 min at 390 K. The gas flow rate was kept at 30 mL/min during the measurements. Three types of transient breakthrough curves were determined, i.e., single-component C2H6and C3H8, an equimolar twocomponent mixture of C2H6and C3H8, and an equimolar three-component mixture of C2H6, C3H8, and toluene. The gas at the outlet of the absorber was analyzed online by mass spectrometry.
3 Results and Discussion
3.1 N2 adsorption isotherms
The five microporous MOFs are made up of five different metal centers and organic linkers, and exhibit different types of pore structures. HKUST-1 has a paddle wheellike crystal structure, with open metal sites created by removing the water molecules axially bound to each copper ion. Ni(bdc)(ted)0.5contains Ni2+ions as the metal centers, with paddle wheel-type Ni2(COO)4(ted)2building units connected in a 2D network layer through bdc ligands; the resulting 3D porous structure contains two types of channels. PCN-250 has a regular octahedral shape with open metal sites[1,21]. UiO-66 exhibits channels of two different sizes and a highly symmetrical structure. ZIF-8 is a molecular sieve-like imidazole complex, whose topological structure is similar to that of a zeolite. The N2adsorption–desorption isotherms in Figure 1 reveal that the five MOFs showed similar adsorption behaviors. As their inflection points were located at very low pressures, all the N2isotherms exhibited typical type-I profiles, indicating microporous structures. In addition, the adsorption and desorption isotherms fully overlapped, proving that the adsorption process was completely reversible.
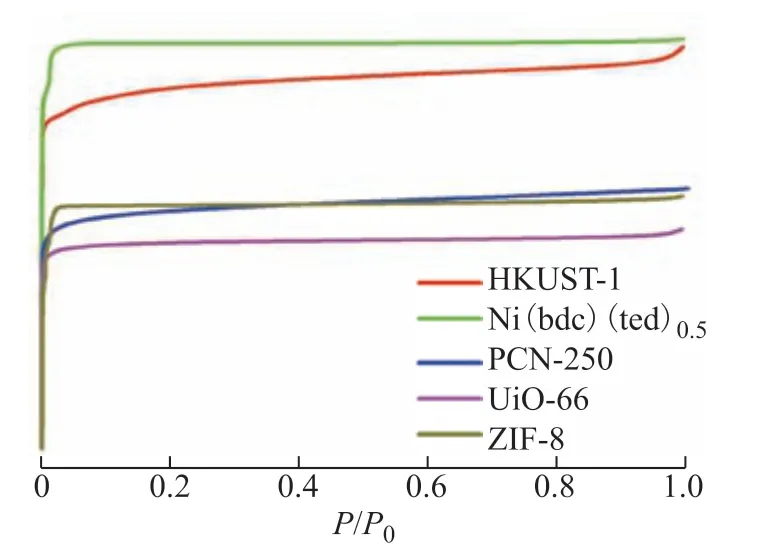
Figure 1 N2 adsorption–desorption isotherms of the five MOFs
Specific surface areas were calculated by BET and Langmuir methods. HKUST-1, Ni(bdc)(ted)0.5, PCN-250, UiO-66, and ZIF-8 had BET surface areas of 1936, 1884, 1209, 1101, and 1206 m2/g, respectively. The HK method was used to determine the pore size distributions of the five microporous MOFs; the total pore volumes and average pore sizes are shown in Table 1. The pore size distributions revealed that HKUST-1 had two main pore sizes of 0.56 and 0.63 nm, while Ni(bdc)(ted)0.5showed two main pore sizes of 0.59 and 1.05 nm. The size distribution of PCN-250 contained only a peak corresponding to 0.54 nm micropores, whereas that of UiO-66 displayed two peaks corresponding to 0.52 and 0.61 nm sizes. The distribution of ZIF-8 exhibited three peaks, corresponding to 0.67, 0.94, and 1.07 nm micropores. The pore sizes of the five MOFs were large enough to allow the access of light hydrocarbon molecules such as C2H6(3.8 Å) and C3H8(4.3 Å). The isotherms of Ni(bdc)(ted)0.5and ZIF-8 also exhibited small sub-steps at low relative pressures, which were mainly attributed to the filling of the two types of cages.

Table 1 Structural parameters of HKUST-1, Ni(bdc)(ted)0.5, PCN-250, UiO-66, and ZIF-8
3.2 C2H6 and C3H8 isotherms and adsorption mechanism
Figure 2 shows the adsorption isotherms of C2H6and C3H8on the five MOFs at 298.15 K and pressures up to 150 kPa. The curves show that the MOFs had different adsorption properties for C2H6and C3H8. Stronger C3H8than C2H6adsorption peaks were observed for HKUST-1, Ni(bdc)(ted)0.5, UiO-66, and ZIF-8, denoting a greater adsorption affinity of these frameworks for C3H8than C2H6. This could be due to the fact that the number of interaction sites in a linear alkane increases with the chain length, resulting in a stronger interaction with the MOFs[22]. The same phenomenon has been reported in other studies[6,23].
The van der Waals attractive interactions of C2H6and C3H8gas molecules can be described in terms of their polarizability[21,24]. C3H8is thus expected to have stronger van der Waals interactions with the MOFs, because its polarizability (6.29×10-24cm3) is higher than that of C2H6(4.47×10-24cm3)[25]. These strong host–guest interactions resulted in more evident hysteresis characteristics in the C3H8than C2H6isotherms. In the case of PCN-250, the adsorbed amount of C2H6exceeded that of C3H8when the pressure was greater than 120 kPa, while a higher adsorbed amount of C3H8was observed in the lowpressure region of the isotherm. This suggests that the effect of the adsorbent at high pressure was different from that at low pressure. At high pressure, the molecules interact not only with the surface but also with other molecules, owing to the occupation of a large number of preferred adsorption sites on the surface.
A high specific surface area provides a large number of adsorption sites. Among the five MOFs, HKUST-1 and Ni(bdc)(ted)0.5exhibited a higher adsorption capacity (see Figure 3). The C2H6and C3H8adsorption capacities of HKUST-1 were up to 122.8 and 170.6 cm3/g, respectively, at 150 kPa and 298 K. The open Cu2+sites enhanced the binding between framework and adsorbate[26-27]. In the case of Ni(bdc)(ted)0.5, the C2H6and C3H8adsorption capacities were up to 119.4 and 135.4 cm3/g at 150 kPa and 298 K, respectively. The PCN-250 and ZIF-8 MOFs showed similar BET specific surfaces. However, PCN-250 adsorbed significantly larger amounts of C2H6and C3H8(91.2 and 89.0 cm3/g at 150 kPa and 298 K, respectively) than ZIF-8 (51.7 and 72.0 cm3/g under the same conditions). This may be due to the availability of open metal sites in PCN-250[28]. UiO-66 had the lowest C2H6and C3H8adsorption capacities (48.1 and 57.4 cm3/g, respectively) among all studied MOFs. Owing to their higher specific surface areas, the total amounts of C2H6and C3H8adsorbed on HKUST-1 and Ni(bdc)(ted)0.5were slightly higher than those adsorbed on the other three MOFs. Thus, HKUST-1 and Ni(bdc)(ted)0.5showed a great potential for the adsorption of light hydrocarbons. Among the five MOFs, only PCN-250 had a higher adsorption capacity for C2H6than C3H8, which was consistent with its small microporous channels, providing a suitable host environment for ethane adsorption.
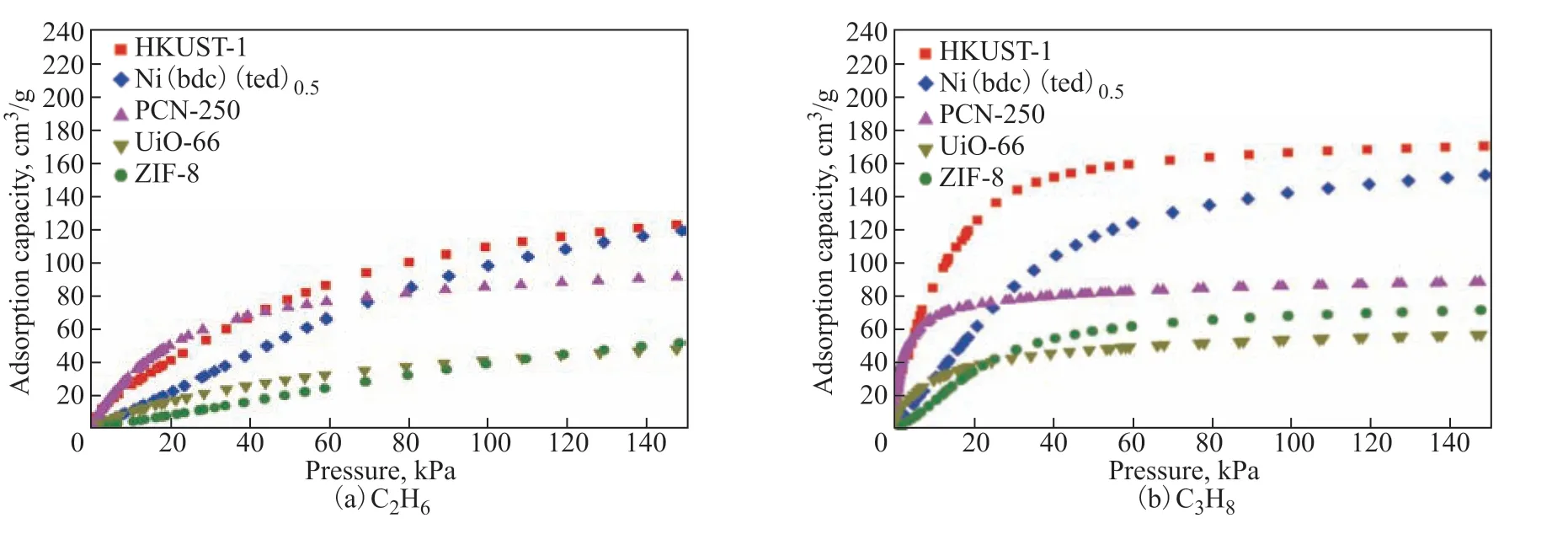
Figure 2 C2H6 (a) and C3H8 (b) adsorption isotherms of the five investigated MOFs
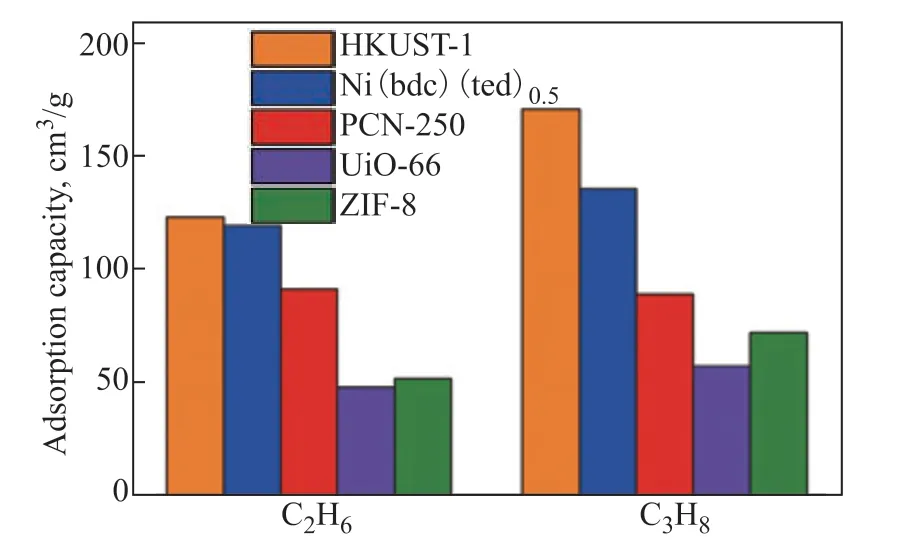
Figure 3 Comparison of C2H6 and C3H8 adsorption capacities of the five investigated MOFs
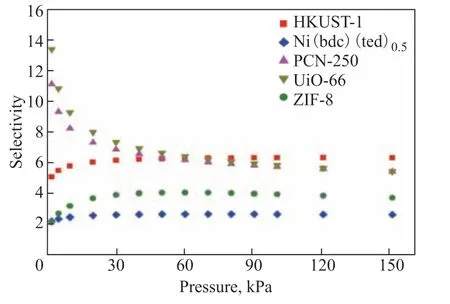
Figure 4 IAST-predicted selectivities for C2H6/C3H8 (1:1) mixtures of the investigated MOFs at 298 K
For comparison, the C2H6/C3H8adsorption capacities of the present MOFs and other reported adsorbents at 298 K and 100 kPa are summarized. Among previously reported adsorbents, some microporous MOFs exhibited good adsorption properties for light hydrocarbons. JLU-Liu38 (8.39 mmol/g)[29]had the largest adsorption capacity for C3H8, while PCN-224 (8.25 mmol/g)[15], UiO-67 (8.2 mmol/g)[30], JLU-Liu47 (8.12 mmol/g)[31], and JLU-Liu37 (7.95 mmol/g)[29]showed good C3H8adsorption capacities at 298 K and 100 kPa. Mg-MOF-74 (6.6 mmol/g)[12]had the largest C2H6adsorption capacity at 298 K and 100 kPa. Moreover, JLU-Liu47 (5.58 mmol/g)[31]and Fe-MOF-74 (5 mmol/g)[32]exhibited good C2H6adsorption capacities at 298 K and 100 kPa.
The adsorption capacities of HKUST-1 and Ni(bdc)(ted)0.5measured in this work exceeded those of most reported MOFs. At 298 K and 100 kPa, the amounts of C2H6and C3H8adsorbed on HKUST-1 reached 4.86 and 7.45 mmol/g, while the corresponding adsorption capacities of Ni(bdc)(ted)0.5were up to 4.38 and 6.38 mmol/g, respectively. The other three materials studied in this work had lower adsorption capacities. The C2H6and C3H8uptakes of PCN-250 were 3.79 and 3.88 mmol/g, whereas those of UiO-66 were 1.83 and 2.42 mmol/g, respectively; finally, the C2H6and C3H8adsorption capacities of ZIF-8 were 1.75 and 3.05 mmol/g, respectively.
3.3 Adsorption selectivity
The Langmuir−Freundlich (L-F) model was used to fit the C2H6and C3H8adsorption isotherms of the present MOFs. The L-F equation can be written as follows:

whereqis the adsorption capacity (mmol/g).pis the pressure of the bulk gas at equilibrium with the adsorbed phase (kPa),ais the saturation capacity (mmol/g),bis the affinity coefficient, andnis the corresponding deviation from an ideal homogeneous surface. Table 2 lists the fitting parameters of the L-F model for the pure C2H6and C3H8isotherms of the MOFs.
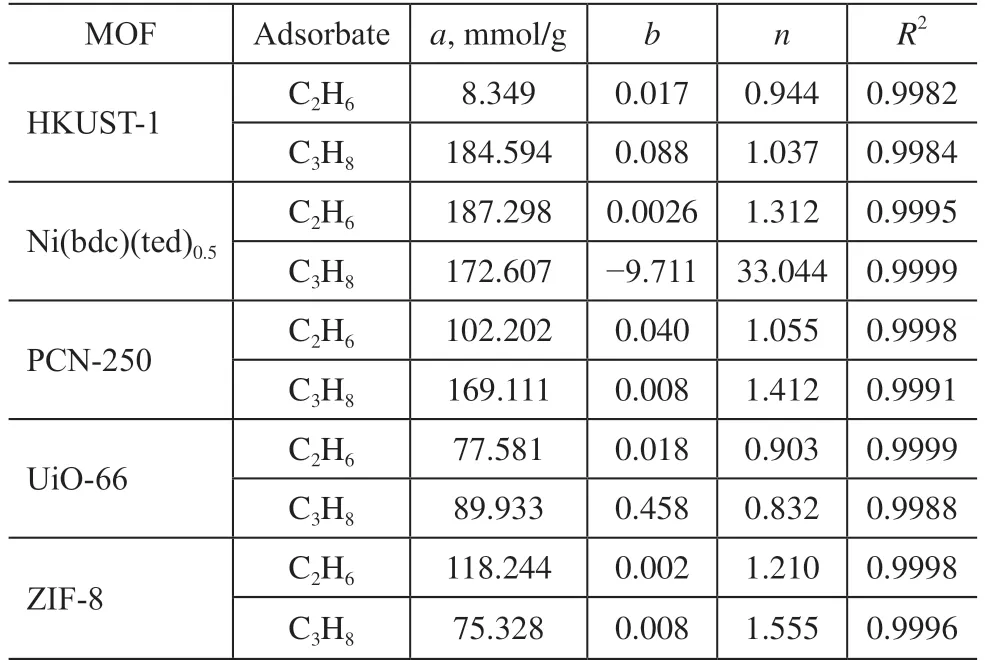
Table 2 Parameters of L-F model equation
Then, the ideal adsorbed solution theory (IAST)[33]was used to predict the adsorption isotherms of gas mixtures and calculate the selectivities of the MOFs for C3H8/C2H6adsorption. The formula employed to calculate the selectivity was as follows:
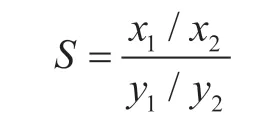
wherexiandyiare the molar fractions of componenti(i= 1, 2) in the adsorbed and gas phases, respectively.
Figure 4 presents the IAST-predicted selectivities for C2H6/C3H8(1:1) mixtures of the five MOFs at 298 K. The selectivities ranged between 2 and 14 at pressures below 150 kPa. The C2H6/C3H8selectivities of PCN-250 and UIO-66 at 298 K decreased with increasing pressure and remained constant at higher pressures. In contrast, the C2H6/C3H8selectivities of HKUST-1, Ni(bdc)(ted)0.5, and ZIF-8 at 298 K increased with increasing pressure over the studied range and remained constant at higher pressures. Moreover, PCN-250 and UIO-66 showed a higher selectivity at low pressure than the other three MOFs, while at pressures above 70 kPa the selectivity of HKUST-1 exceeded that of PCN-250 and UIO-66.
3.4 Dynamic adsorption performance
Figure 5 shows the measured breakthrough curves of C2H6and C3H8adsorption on HKUST-1, activated carbon, and molecular sieve 13X. Figure 5(a) shows that the molecular sieve had the shortest initial and complete breakthrough times of C2H6among the three materials. HKUST-1 showed a shorter initial and longer complete breakthrough time than activated carbon. This indicated that, among the three adsorbent materials, the molecular sieve had the lowest dynamic adsorption capacity for C2H6, while activated carbon and HKUST-1 showed similar performances in this context. Figure 5(b) shows that the initial and complete breakthrough times of the three materials increased in the order molecular sieve < activated carbon < HKUST-1, reflecting the dynamic adsorption capacity of the three materials for C3H8. In addition, the comparison of Figures 5(a) and 5(b) clearly shows that the C3H8breakthrough time of the three adsorbents was longer than the C2H6one, which indicated that C3H8had a higher affinity for the substrate materials than C2H6.
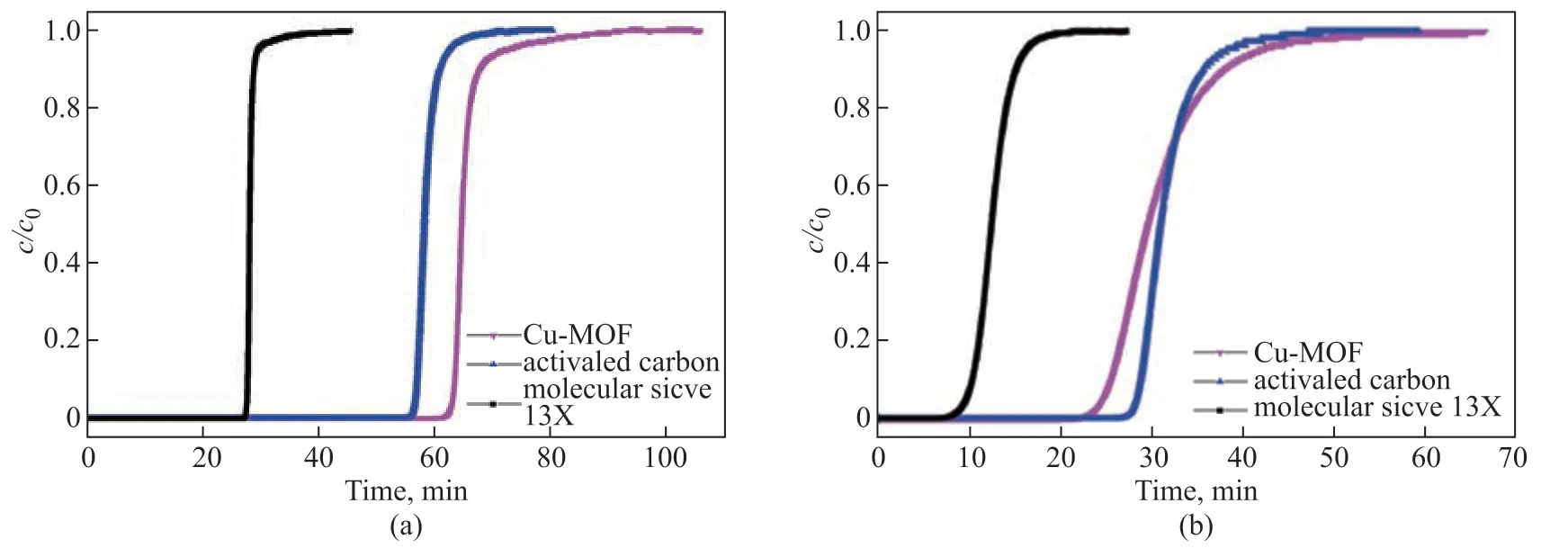
Figure 5 Breakthrough curves of (a) C2H6 and (b) C3H8 adsorption on HKUST-1, activated carbon, and molecular sieve
Both single-component and binary mixture adsorption capacities can be calculated from the breakthrough data using the following relation[34]:
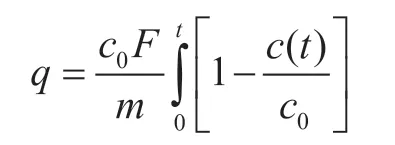
whereqis the adsorption capacity (mmol/g),C0is the concentration of C2/C3in the feed gas andC(t) is the concentration of C2/C3at the exit of the column at timet.F(m3/min) represents the total gas flow rate at 298 K and atmospheric pressure.m(g) is the weight of adsorbent sample in the packed column.
The single-component equilibrium capacities (atc/c0= 1.0) of the molecular sieve calculated from the breakthrough data for C2H6and C3H8were 12.74 and 28.56 mL/g, whereas those of activated carbon were 31.99 and 59.05 mL/g, and the corresponding values measured for HKUST-1 were 31.40 and 66.04 mL/g, respectively. The molecular sieve showed a weaker adsorption of both C2H6and C3H8. The dynamic adsorption capacities of activated carbon and HKUST-1 for C2H6were very similar.
The results obtained by different experimental techniques showed large differences. The equilibrium adsorption capacities of HKUST-1 in the breakthrough experiments were much lower than those obtained from the isotherm measurements at 150 kPa and 298 K, which yielded C2H6and C3H8adsorption capacities of 122.8 and 170.6 cm3/g, respectively. The equilibrium adsorption capacity is known as working adsorption capacity, and has more direct relevance for the practical applications of an adsorbent.
Multicomponent breakthrough experiments were performed to investigate the dynamic separation performances of the adsorbents for C2H6/C3H8(1:1) and C2H6/C3H8/toluene (1:1:1) mixtures. Figure 6 shows the two-component breakthrough curves of the three adsorbents for the C2H6/C3H8mixture. In agreement with the single-component isotherms, the breakthrough curves of the three adsorbents showed a clear preference for C3H8. C2H6was first detected at the outlet of the adsorber, and its concentration in this region exceeded the feed concentration. This so-called “roll-up” behavior is caused by the displacement of C2H6by the more strongly adsorbed C3H8. Based on the breakthrough curves of the C2H6/C3H8mixtures (Figure 6), the calculated single-component equilibrium adsorption capacities of HKUST-1 for C2H6and C3H8were 25.21 and 61.16 mL/g, respectively; these results were very close to the adsorption capacities calculated from the single-component breakthrough curves. The separation factors can be calculated from the integrated areas of the breakthrough curves; for instance,α=q(C3H8)/q(C2H6) = 2.43 for HKUST-1. Similarly, the single-component equilibrium adsorption capacities of activated carbon for C2H6and C3H8, calculated from the breakthrough data, were 20.58 and 48.389 mL/g, whereas the values obtained for the molecular sieve were 9.99 and 25.79 mL/g, respectively. The separation factors calculated for activated carbon and molecular sieve were 2.35 and 2.58, respectively. Thus, the three studied materials showed a promising potential for the separation of C2H6/C3H8mixtures.
As shown in Figure 7, the three materials exhibited similar adsorption characteristics to the ternary mixture of C2H6, C3H8, and toluene. The breakthrough experiments for the three materials showed that C2H6was the first to emerge from the column, followed by C3H8, while toluene was eluted at a much later time. The C2H6, C3H8, and toluene breakthrough times on the molecular sieve were 2.13, 9.13, and 41.62 min, respectively. The corresponding times measured for activated carbon were 16.62, 42.52, and 127.55 min, while those obtained for HKUST-1 were 8.2, 19.77, and 41.53 min, respectively. The adsorption capacity of the three materials for the three gases followed the order toluene > C3H8> C2H6.
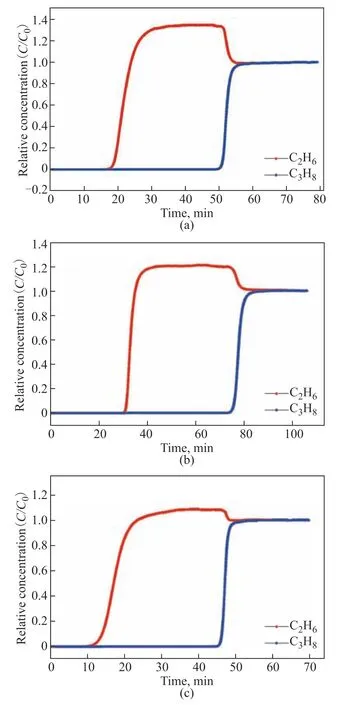
Figure 6 Breakthrough curves of equimolar mixtures of C2H6 and C3H8 on (a) HKUST-1, (b) activated carbon, and (c) molecular sieve

Figure 7 Breakthrough curves of equimolar C2H6/C3H8/toluene mixtures on (a) HKUST-1, (b) activated carbon, and (c) molecular sieve
4 Conclusions
In this work, five microporous MOFs were synthesized and tested as adsorbents for C2H6and C3H8. HKUST-1 and Ni(bdc)(ted)0.5, with larger specific surface areas, exhibited a greater potential for light hydrocarbon adsorption. The higher adsorption capacities and longer breakthrough time for C3H8indicated that the latter had a stronger interaction with the adsorbents than C2H6. The adsorption properties of HKUST-1 for C2H6and C3H8were superior to those of traditional adsorbents. Furthermore, multicomponent breakthrough experiments showed that HKUST-1 could effectively separate binary C2H6/C3H8and ternary C2H6/C3H8/toluene mixtures.
Acknowledgement:This work was financially supported by the National Natural Science Foundation of China (grant number: 21701189).
- 中国炼油与石油化工的其它文章
- Activated Carbon from Rice Husk with One-Step KOH Mechanical Mixing Activation as Adsorbent for Treating Phenolic Wastewater
- Removal of Basic and Non-Basic Nitrogen Compounds from Model Oil by FeCl3-Based Ionic Liquids
- Selection of Extraction Solvents for Bitumen from Indonesian Oil Sands through Solubility Parameters
- Synthesis of Hierarchical Porous Fe2O3/Al2O3 Materials and Study on Catalytic Viscosity Reduction of Heavy Oil
- Effect of Particle Shape on Catalyst Deactivation during 2-Butene and Isobutane Alkylation of Liquid Phase in Fixed-Bed Reactor Using Particle-Resolved CFD Simulation
- Experimental and Numerical Investigation on Erosion Corrosion of the Air Cooler Tube Bundle in a Residue Hydrotreating Unit

