Selection of Extraction Solvents for Bitumen from Indonesian Oil Sands through Solubility Parameters
Cui Wenlong; Zhu Qingqing,2; Zhao Chenze; Wang Cheli
(1. Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Advanced Catalysis and Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou 213164, China;2. Zhejiang Chemical Products Quality Inspection Company Ltd., Hangzhou 310023, China)
Abstract: Indonesian oil sands were systematically separated to investigate their basic composition. The extraction effects of the solvents with different Hilderbrand solubility parameters (HSPs) on the bitumen of Indonesian oil sands were compared. Furthermore, the Hansen solubility combination parameter (HSCP) and Teas triangle were used to explore rules in the separation of oil sands bitumen via solvent extraction. Finally, the saturates, aromatics, resins, and asphaltenes (SARA) fractions of the bitumen from Indonesian oil sands were analyzed. The results showed that the Indonesian oil sands were oil-wet with a bitumen content of 24.93%. The solvent extraction for bitumen could be accurately and conveniently selected based on the solubility parameter. When the HSPs of the extraction solvent were around 18–19 and the HSCPs were closer to a certain range (δd = 17.5–18.0, δp = 1–3.5, and δh = 2–6), the extraction effect of bitumen from Indonesian oil sands improved, and the primary component affecting the extraction rate of bitumen were asphaltenes.
Key words: oil sand bitumen; extraction solvent; solubility parameter; SARA fractions
1 Introduction
With diminishing petroleum reserves and increasing demands, the huge energy gap will largely be compensated by oil sands, shale oil, and other resources in the future. Oil sands are unconventional resources consisting of sands, bitumen, water, and clay, which are rich in the world and far greater than the proven reserves of petroleum[1-2]. It is important to develop bitumen from oil sands, which can be used to further process and produce synthetic crude oil. Compared with traditional crude oil, the extraction and refining cost of oil sands are somewhat expensive. However, oil prices have been priced high for some time in recent years, which makes the separation and analysis of oil sands widely valued[3-4].Oil sands can be divided into water-wet, oil-wet oil, and neutral types based on the different wettability and adsorption forms of the sand surfaces. The selection of oil sand separation methods is limited by the type of oil sand wettability[5-6]. For example, hot alkali water washing is used primarily to separate water-wet oil sands and has the disadvantages of large water consumption, incomplete separation, tailings pollution of the environment, and bitumen that needs to be refined[7-10]. For Indonesian oilwet type oil sands, it is suitable to use solvent extraction separation or pyrolysis dry distillation separation methods[11-12]. Nevertheless, a higher temperature is required for pyrolysis with dry distillation, which leads to a high energy consumption. Although the solvent extraction method has not yet achieved industrial application, it is regarded as a promising technique due to its advantages of zero water consumption, universality, and high efficiency[7,8,13].
The solubility parameter (δ) was first proposed by Hilderbrand for simple liquid molecules, which is defined as the square root of the ratio of the cohesive energy to the molar volume[14-15]. Hansen then subdivided the solubility parameter into the dispersion force parameter (δd), polar force parameter (δp), and hydrogen bond adhesion parameters (δh), ternary solubility combination parameter (δt)[13]. Later, Teas assumed that the values of the solubility parameters were the same and converted the three parameters of the Hansen solubility into fractions as percent dispersion force parameter (fd), percent polar force parameter (fp), and percent hydrogen bond adhesion parameter (fh). Teas designed the Teas triangle picture, which contains every solvent[16].
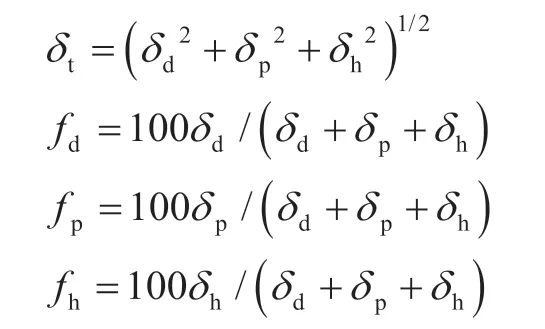
whereδd,δp,δh, andδtare the parameters for the dispersion force, polar force, hydrogen bond adhesion, and Hildebrand solubility; andfd,fp, andfhare the fractions for the dispersion force parameter, polar force parameter, and hydrogen bond adhesion parameter, respectively.
The solubility parameters are physical constants that measure the compatibility of liquids and substances. These are also regarded as a quantification of the principle of “similarity compatibility”. Solvents with similar parameters should theoretically be able to dissolve the same substance; thus, it is generally believed that the degree of compatibility between substances is mastered when the solubility is mastered, which helps quickly and reasonably selecting solvents in practical applications[17-18]. The solubility parameters are now widely used to predict polymer-solvent system compatibility and solvent selection, such as for industrial rubbers, coatings, and plastics[19-20]. The solvent extraction method for bitumen from oil sands is based on the theory of similar compatibility. Bitumen can be regarded as a complex polymer mixture and the most important factor that affects oil extraction. Therefore, studying the selection law of solvents plays a vital role in the development of the oil sands separation industry. The degree of material polarity and its composition are the keys to measure the interactions between molecules and have the most direct relationship with solubility[21]. The solubility parameters of bitumen are studied here to summarize the law of extraction, and the composition of bitumen extracted by different solvents was analyzed to guide solvent selection.
2 Experimental
2.1 Materials
A representative oil sand was employed as the feedstock (obtained from Indonesia) and was smashed and sieved to obtain oil sands that were smaller than 40 mesh. The result was stirred evenly and sealed for storage. The utilized petroleum ether, xylene,n-heptane, dichloromethane, neutral alumina, cyclohexane, ethyl alcohol, 2-butanone, toluene, trichloromethane, ethyl acetate, acetone, and solvent oil were all analytically pure.
2.2 Experimental methods
2.2.1 Determination of water and bitumen contents
The distillation apparatus was composed of a round bottom flask, water content measuring tube, and condensation tube. The water content was calculated after three measurements. Then, the oil sands were wrapped in two oil filter papers and placed into a Soxhlet extractor. Toluene (130 mL) was added to an empty conical flask and the unit was connected. Next, the conical flask was heated in an oil bath, and the filter paper was repeatedly washed with regurgitant toluene until the effusion in the Soxhlet extractor was completely transparent. After that, the unit was removed, the solvent was distilled and recovered, and the filter paper bag and cleaned conical flask were vacuum dried for 4 h. Finally, the filter paper bag and conical flask were weighed, and the bitumen content was calculated based on the gravimetric method and averaged over several tests.
2.2.2 Solvent extraction method
The oil sands (8 g) were accurately weighed and placed in a 150 mL flask. The solvent (24 mL) was added to the flask and was fully mixed. The extractions were performed under stirring with 500 r/min at a temperature of 50 °C. The extractions lasted for 30 min. When complete, the systems were allowed to stand for 5 min. The bitumen solution was transferred to a conical flask, and the solvent was removed via evaporation. The dry bitumen was placed in a vacuum drying oven for 1 h and the weight was calculated. The bitumen was measured by the saturate, aromatic, resin, and asphaltene (SARA) analysis with reference to the SH/T 0509–1992.
3 Results and Discussion
3.1 Composition of Indonesian oil sands and bitumen
The basic composition of Indonesian oil sands and their bitumen are illustrated in Table 1. The oil sands contained 0.77% water, 74.30% solids, and 24.93% bitumen, in which there were 23.56% saturates, 23.08% aromatics, 21.61% resins, and 31.75% asphaltenes.

Table 1 Basic composition of Indonesian oil sands and bitumen
3.2 Effect of solubility parameters on the extraction rate of bitumen
The effect of different solvents on the extraction rate of bitumen is shown in Figure 1. The effect of HSP on the extraction rate of bitumen was typical. Under certain operating conditions, the extraction rates of bitumen by non-polar or weak polar solvents (such asn-heptane and petroleum ether) reached more than 15%. It is speculated that these solvents can only dissolve weak polar oil components but have dissolution difficulty for asphaltenes with strong polarity, which were adsorbed on the surface of oil sands. At a greater solvent polarity, the HSP increased, and the extraction rate of bitumen gradually increased. The extraction rates of bitumen for different HSP solvents between 18 and 19 were more than 24%. The asphaltenes were also possibly well extracted. According to the principle of similar solubility parameters, it is indirectly estimated that the solubility parameter of Indonesian oil sands asphaltenes is around 18–19[6]. However, even with the better extraction effect, such as the HSP of ethyl acetate at 18.1, the extraction rate of bitumen was only approximately 16%, which could be due to the HSCP separation. Finally, as the polarity of the solvent continues to increase, the extraction rate of bitumen drops below 10% because of the strong solvent polarity, which worsens the extraction ability of weak polar oil components and asphaltenes.

Figure 1 Effect of different HSPs on the extraction rate of bitumen
3.3 Analysis of HSPs and Teas triangle for solvents
The HSP is only a single data point used to reflect all the forces between molecules, so it cannot be fully fit with the theory that the closer the parameters are, the better the dissolution effect is. Different HSCPs and their fractions were subsequently analyzed[13,14]. It is seen from Table 2 that the closer HSCPs of the solvent are to a certain range (δd=17.5 – 18.0,δp=1 – 3.5,δh=2 – 6), the higher the extraction rate of bitumen.
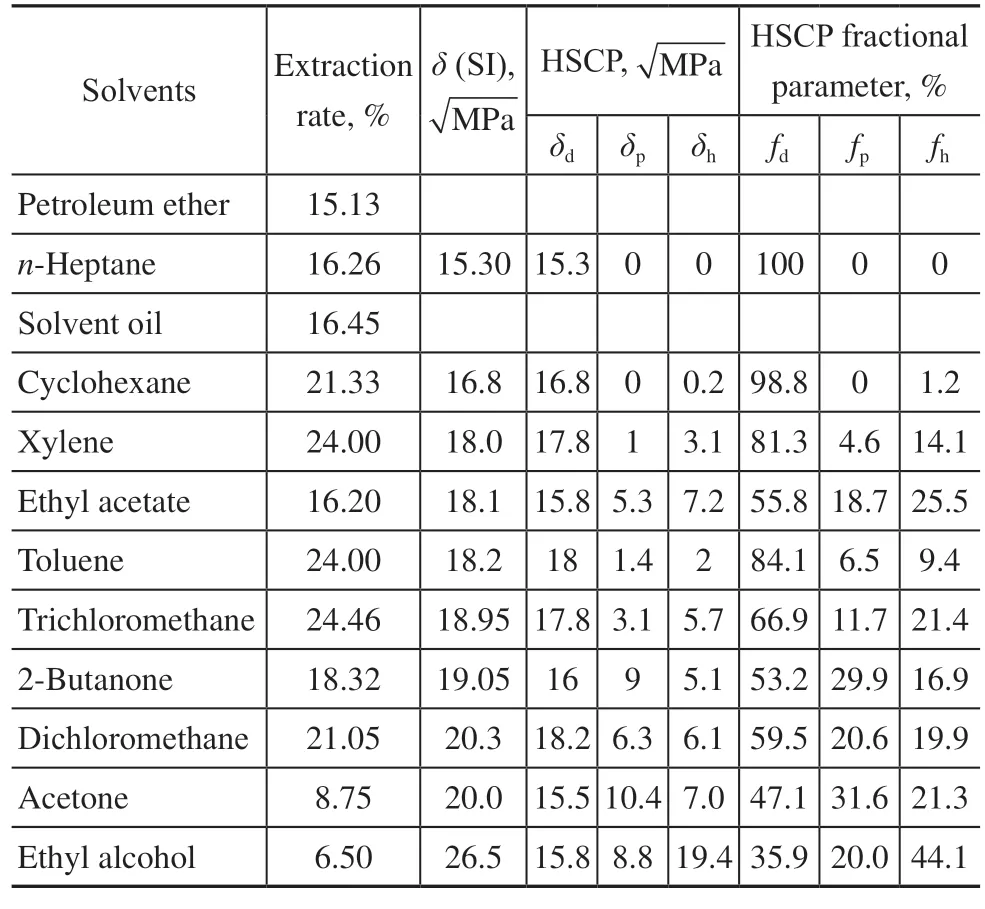
Table 2 Different HSCPs and their solvent fractions
The Teas triangle of the fractions of the solvent solubility parameter is shown in Figure 2 to more intuitively investigate the relationship between the HSCPs and the extraction rate of bitumen. The darker color of the point indicates a higher extraction rate of bitumen in the experimental results. The closer HSCP fractional parameters of the solvent point were to a certain range (fd= 60%–85%,fp= 15%–12%,fh= 9%–22%), the better the extraction effect on the bitumen. For example, the points of trichloromethane, toluene, and xylene with extraction rates above 24% were concentrated in this area. According to the principle of the solubility parameter similarity, the HSCP of the oil sand bitumen mixture should also fall within this range. It was found that although the HSP of ethyl acetate was very close to that of toluene, xylene, etc., the position on the Teas triangle was somewhat far from the high-efficiency area, which explains the poor extraction effect.

Figure 2 Teas triangle of the fractions for the solvent solubility parameter
3.4 Analysis of bitumen fractions extracted by different solvents
The extracted bitumen is a heavy oil composed of nonpolar alkanes, naphthenes, and aromatic hydrocarbons with a certain polarity, as well as highly polar nonhydrocarbons. Heavy oil can be analyzed from the perspective of polarity. Liquid-solid adsorption chromatography is widely used to separate heavy oils into saturates, aromatics, resins, and asphaltenes, known as SARA fractions. Differences in the extraction effects were analyzed from the bitumen composition, and the SARA fractions of the bitumen extracted from different solvents are shown in Table 3.
It is seen from Table 3 that the main component affecting the extraction rate of bitumen is asphaltenes. The more extracted asphaltene from the solvent, the higher the extraction rate of bitumen. Combined with the analysis in Table 2, in the range of suitable solubilities (δt=16–20), when the solvent had no polar or hydrogen bonding force, the saturates, aromatics, and resins could be well extracted, but the asphaltenes could not. As the petroleum ether and solvent oil were mixed with a small number of components with polar and hydrogen bonding forces, a small amount of asphaltene can be extracted. With increased polar and hydrogen bonding forces in the solvent, the extraction effect on asphaltenes gradually improved. However, excessive polar and hydrogen bonding forces weaken the extraction effect of solutes and asphaltenes. Therefore, it is necessary to select solvents with a suitable HSCP ratio, polar force, and hydrogen bonding force in the selection of oil sand extractants to ensure the SARA fractions in oil sand bitumen can be well separated.
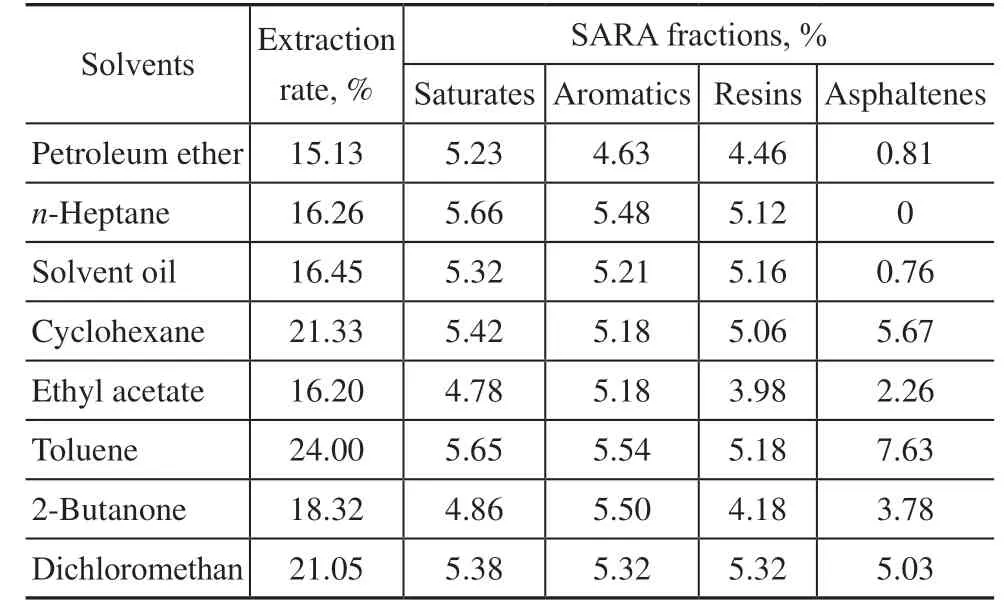
Table 3 SARA fractions of bitumen extracted by different solvents
4 Conclusion
Indonesian oil sands are oil-wet with a bitumen content of 24.93%. The solubility parameter has a good guiding effect on the selection of oil sand extractants. Within a certain solubility range, the primary component affecting the extraction rate of bitumen is asphaltenes. When the HSPs of the extraction solvent were approximately 18–19 and the HSCPs were closer to a certain range (δd=17.5–18.0,δp=1–3.5, andδh=2–6), the extraction effect of bitumen from Indonesian oil sands improved. The HSCP of the bitumen mixture should also fall within this range. These studies can help oil sand separation research to more accurately and quickly select the optimal extractant, which has great application value and prospects.
Acknowledgments:This work was financially supported by the Natural Science Foundation of Jiangsu Province (Grant number: BK20140260), Joint Project of Industry-University-Research of Jiangsu Province (Grant number: BY2018158, BY2021590), and State Key Laboratory of Heavy Oil Processing.
- 中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Microporous Metal Organic Frameworks for Light Hydrocarbon Adsorption
- Removal of Basic and Non-Basic Nitrogen Compounds from Model Oil by FeCl3-Based Ionic Liquids
- Synthesis of Hierarchical Porous Fe2O3/Al2O3 Materials and Study on Catalytic Viscosity Reduction of Heavy Oil
- Activated Carbon from Rice Husk with One-Step KOH Mechanical Mixing Activation as Adsorbent for Treating Phenolic Wastewater
- Effect of Particle Shape on Catalyst Deactivation during 2-Butene and Isobutane Alkylation of Liquid Phase in Fixed-Bed Reactor Using Particle-Resolved CFD Simulation
- Experimental and Numerical Investigation on Erosion Corrosion of the Air Cooler Tube Bundle in a Residue Hydrotreating Unit

