Removal of Basic and Non-Basic Nitrogen Compounds from Model Oil by FeCl3-Based Ionic Liquids
Li Wenshen; Liu Jie
(School of Petrochemical Engineering, Liaoning Petrochemical University, Fushun 113001, China)
Abstract: The low-viscosity FeCl3-based ionic liquids (ILs) prepared from the interaction of anhydrous FeCl3 and alkyl imidazolium bromide ([1-alkyl-3-methyl-imidazolium]Br, alkyl=ethyl, butyl, hexyl, octyl) are highly effective for the denitrogenation of model oil containing quinoline or indole. The results indicate that the chain length of the alkyl group on the imidazolium cation has little influence on the N-extraction efficiency. With the selected IL [Bmim]Br/FeCl3, up to 99.1% of N-extraction efficiency from model oil containing quinoline can be attained at an extraction temperature of 30 °C with an IL/oil mass ratio of 1/7 and an extraction time of 30 min. The indole extraction efficiency reaches 98.9% at an IL/oil mass ratio of 1:1. Moreover, the quinoline extraction efficiency remains as high as 92.3% after the IL has been recycled four times.
Key words: FeCl3-based ionic liquid; denitrogenation; quinoline; indole
1 Introduction
The removal of nitrogen compounds (N-compounds) is an important process in the fuel processing industry. The emission of nitrogen oxides during the combustion of N-compounds causes serious environmental effects, such as acid rain and haze[1-3]. N-compounds also have an inhibiting influence on the conventional hydrodesulfurization process through competitive adsorption and catalyst poisoning[4-6]. At present, the most popular denitrogenation method applied in industry is hydrodenitrogenation, which requires harsh operating conditions involving high temperatures and pressures, large quantities of hydrogen, and significant catalytic activity. Extraction techniques for denitrogenation[7-8]are more competitive because they require less equipment, have a simpler process, and involve lower operation costs and milder operating conditions (usually normal pressure and ambient temperature).
Extraction denitrogenation based on ionic liquids (ILs) has received considerable attention in recent years, due in part to the unique properties of ILs over traditional organic solvents, such as negligible vapor pressure, higher affinity to N-compounds, chemical and thermal stability, non-flammability, and good recyclability[9-12]. ILs bearing imidazolium or pyridinium cations are efficient extractants of N-compounds because of the π-π interaction between the cations and aromatic heterocyclic N-compounds. Compared with neutral anions such as [Cl]-, [BF4]-, and [N(CN)2]-, acidic anions such as the Lewis acidic [ZnCl3]-, [ZnCl2(EtSO4)]-, Brönsted acidic [HSO4]-, and [H2PO4]-exhibit better denitrogenation performance for basic N-compounds, attaining N-extraction efficiencies above 99% in a single extraction[13-24]. This level of efficiency can be attributed to the complex interaction between H+or the unoccupied orbitals on metal ions and the lone pair electrons of the N atom in the basic N-compound.
In our previous study[25]using FeCl3-containg imidazolium-based ILs, 1-butyl-3-methyl imidazolium bromide-FeCl3was found to be effective for the denitrogenation of shale diesel oil. However, there has been no general study on its removal performance for basic and non-basic N-compounds. In the present work, quinoline and indole (Figure 1) were selected as the representative basic and non-basic N-compounds, respectively, and the extraction ability of FeCl3-based ILs for different N-compounds from model oil was investigated in detail. This paper discusses the influence of the chain length of the 1-alkyl-3-methylimidazolium cation on the N-extraction efficiency. The results presented herein provide a reference for the application of FeCl3-based ILs.
Figure 1 N-compounds quinoline (a) and indole (b)
2 Experimental
2.1 Experimental reagent and apparatus
2.1.1 Reagent
As experimental reagents, 1-ethyl-3-methylimidazolium bromide ([Emim]Br, ≥99%), 1-butyl-3-methylimidazolium bromide ([Bmim]Br, ≥99%), 1-hexyl-3-methylimidazolium bromide ([Hmim]Br, ≥99%), and 1-octyl-3-methylimidazolium bromide ([Omim]Br, ≥99%) were purchased from Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences (China). Anhydrous FeCl3(AR), quinoline (99.5%), indole (AR),n-dodecane (99%), toluene (99.5%), and carbon tetrachloride (99.5%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China).
2.1.2 Experiment apparatus
The experimental devices used in this study include a
constant-temperature magnetic heating stirrer DF-101S (Gongyi City Instrument Co., Ltd., China); TSN-5000 series fluorescence nitrogen/sulfur analyzer (JiangFen Electroanalytical Co., Ltd., China); electronic balance FA2104N (0.0001 g, Shanghai Jingke Scientific Instruments Co., Ltd., China); vacuum oven ZK-82J (Shanghai Experimental Instrument Factory, China); and Cary 600 Series FTIR Spectrometer (American Agilent Technologies).
2.2 Synthesis of FeCl3-based ILs
ILs containing FeCl3, [Emim]Br/FeCl3, [Bmim]Br/FeCl3, [Hmim]Br/FeCl3, and [Omim]Br/FeCl3were prepared by mixing anhydrous FeCl3and corresponding alkyl imidazolium bromide at a molar ratio of 1:1 in a roundbottomed flask. The mixture was heated to 100°C and maintained at this temperature while being stirred for 4 h. All Fe-containing ILs were liquid and had a low viscosity at room temperature.
2.3 Preparation of model oil
Two model oils containing a basic or non-basic N-compound were prepared by dissolving 0.4639 g of quinoline or 0.4196 g of indole, respectively, in 100 g of n-dodecane/toluene mixture (mass fraction of toluene: 0.2). The N-content in both model oils was ~500 μg/g.
2.4 Denitrogenation experiment procedure and N-content analysis
In a typical experiment, the model oil and IL were placed at a certain mass ratio in a 50 mL beaker, and then magnetically stirred for a specified time at a specified temperature before settling for 2 h at room temperature for phase separation. The N-content in the upper oil phase was analyzed using a TSN-5000 series fluorescence nitrogen/sulfur analyzer equipped with a liquid auto sampler. The extraction efficiency (E, %) and distribution coefficient (D) were calculated using the following equations:

whereC0andC1denote the initial and final N-content in the model oil, whilem1andm2refer to the mass of the model oil and IL, respectively.
3 Results and Discussion
3.1 FTIR spectroscopy analysis of FeCl3-based ILs
The structures of the FeCl3-based ILs, i.e., [Emim]Br/FeCl3(C2), [Bmim]Br/FeCl3(C4), [Hmim]Br/FeCl3(C6), and [Omim]Br/FeCl3(C8), were characterized by FTIR spectroscopy. The results are shown in Figure 2.
In Figure 2, the peaks near 3151 cm-1and 2960 cm-1come from the stretching vibrations of C-H in the imidazolium ring and aliphatic chain, respectively. The C=N stretching vibration appears near 1566 cm-1. The peak near 1164 cm-1is generated by the stretching vibrations of the imidazolium ring. The peaks near 830 cm-1and 740 cm-1are the in-plane and out-of-plane ring vibrations, respectively. Obviously, the cationic structures of the ILs [Cnmim]Br/FeCl3(n=2, 4, 6, 8) are basically consistent with that of [Bmim]Br[25], implying that the [Bmim]+cation does not participate in the complexation, and the complexation only occurs between Br-and FeCl3, similar to previous results[26].
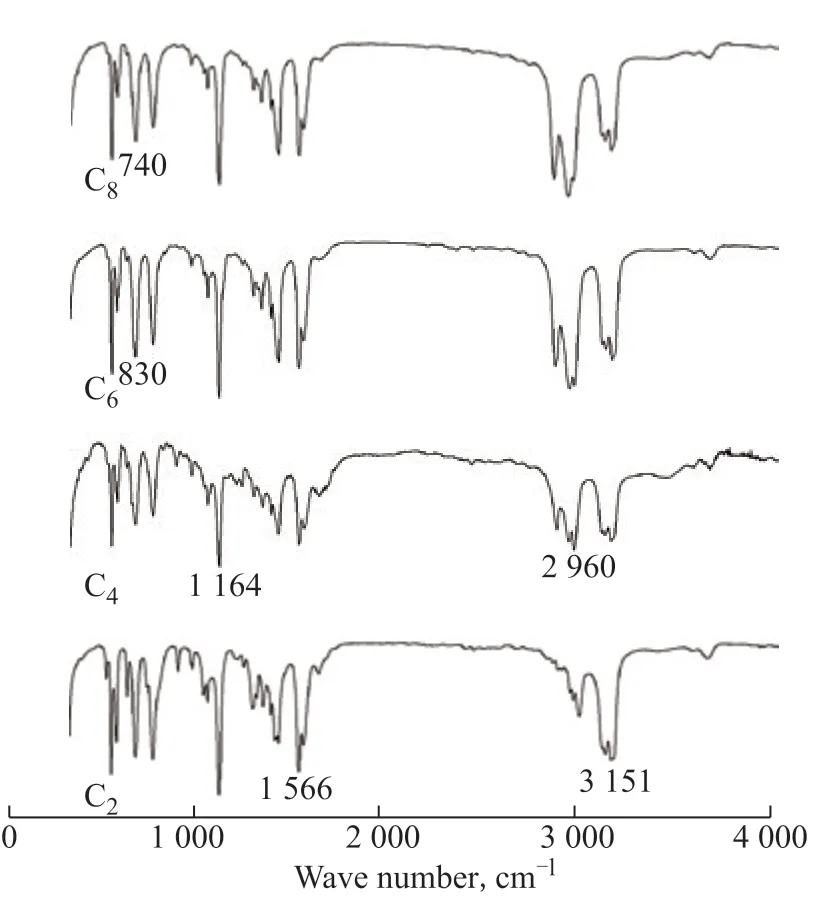
Figure 2 FT-IR spectroscopy of FeCl3-based ILs
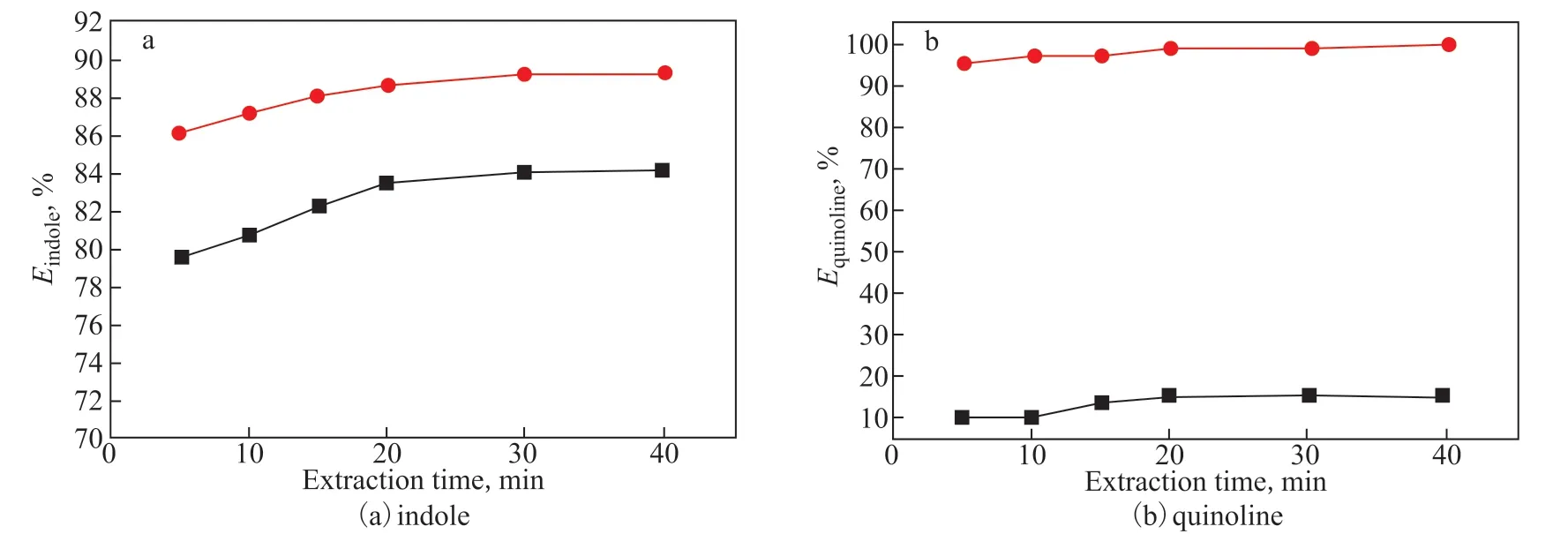
Figure 3 Extraction efficiency for indole (a) and quinoline (b) by [Bmim]Br (black) and [Bmim]Br/FeCl3 (red)
3.2 Comparison of denitrogenation performance between [Bmim]Br and [Bmim]Br/FeCl3
The denitrogenation performance of the ILs [Bmim]Br and [Bmim]Br/FeCl3for quinoline and indole was compared at an extraction temperature of 30 °C and IL/oil mass ratio of 1/7 to clarify the influence of FeCl3on the denitrogenation performance of ILs. The results are presented in Figure 3. As can be seen from Figure 3, [Bmim]Br achieves moderate efficiency for the removal of indole (Eup to 84.0%), but has a poor ability in terms of removing quinoline (E<20%) under these experimental conditions. The π-π interaction between the imidazolium cation and aromatic N-compounds is conducive to the removal of N-compounds by [Bmim]Br. In particular, a hydrogen bond, N-H…Br, can form between the anion of the IL and the neutral indole molecule. Nevertheless, quinoline, which is a basic N-compound, cannot form the hydrogen bond with the anion as there is no hydrogen atom connected to the nitrogen atom[27]. Compared with [Bmim]Br, which exists as a solid at room temperature, [Bmim]Br/FeCl3is a less-viscous liquid and has better fluidity, which is beneficial for ensuring sufficient contact between the IL and N-compound. The extraction efficiency of indole with [Bmim]Br/FeCl3increases from 84.1% to 89.3%, while the extraction efficiency of quinoline is significantly improved by the co-presence of FeCl3, resulting in an N-extraction efficiency of above 99.0%. It can be concluded that the anion of the IL plays a pivotal role in the removal of quinoline with [Bmim]Br/FeCl3, supposedly through the strong complex interaction between the lone pair of electrons on the N atom of the basic quinoline molecule and the vacant orbitals of the FeⅢ-containing anion. The above results suggest that Fecontaining ILs are promising N-compound extractants for practical applications.
Figure 3 also presents the effect of the extraction time on the N-extraction efficiency of [Bmim]Br/FeCl3. The N-extraction efficiency rises as the extraction time increases, e.g., the N-extraction efficiencies of quinoline and indole are 95.4% and 86.1% after 5 min, respectively, but reach 99.1% and 89.3% after 30 min. The N-extraction efficiencies of quinoline and indole remain almost constant after 30 min, indicating that the extraction equilibrium can be achieved in 30 min. Therefore, we consider 30 min to be a suitable extraction time.
3.3 Effect of chain length of the 1-alkyl-3-methylimidazolium cation
The various FeCl3-containing ILs, i.e., [1-alkyl-3-methylimidazolium]Br/FeCl3(alkyl=ethyl, butyl, hexyl, octyl), were used to investigate how the chain length of the imidazolium cation affects the extraction efficiency. The results are presented in Figure 4.
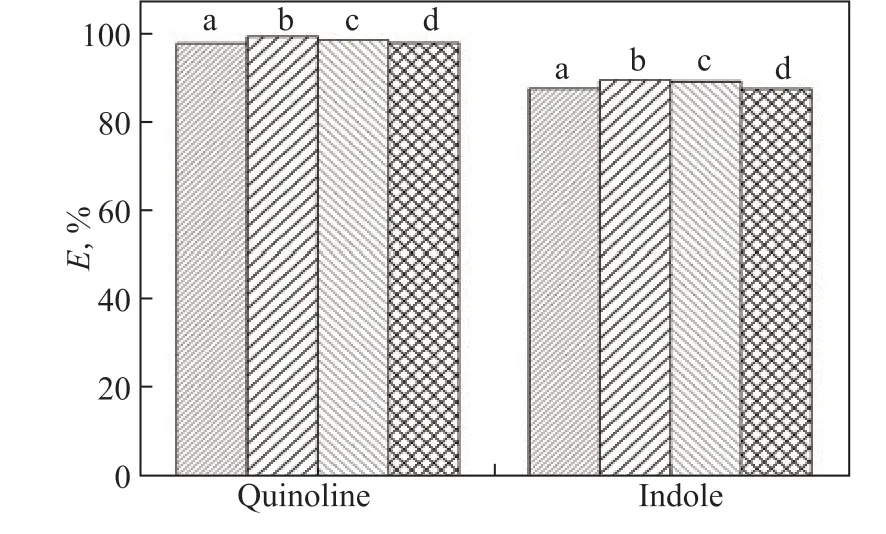
Figure 4 Effect of different alkyl chain lengths on extraction efficiency (E) of quinoline and indole
The N-extraction efficiencies of [Emim]Br/FeCl3, [Bmim]Br/FeCl3, [Hmim]Br/FeCl3, and [Omim]Br/FeCl3are 97.6%, 99.1%, 98.3%, and 97.7% for quinoline and 87.4%, 89.3%, 88.9%, and 87.3% for indole, respectively. Clearly, the chain length of the alkyl group on the imidazolium ring has little effect on the N-extraction efficiency, confirming previous results[20]. The above results are caused by multiple factors. The increase in chain length of the alkyl group on the imidazolium ring can increase the electron cloud density of the imidazole ring, which enhances the π-π interaction between the imidazolium cation and N-compounds. Additionally, the viscosity of the ILs increases as the chain becomes longer, resulting in insufficient contact between the ILs and N-compounds. For the removal of quinoline with IL [1-alkyl-3-methyl-imidazolium]Br/FeCl3, the limited influence of the chain length of the imidazolium cation on the N-removal efficiency supports the important role of the FeCl3-containing anion during denitrogenation.
[Bmim]Br/FeCl3was selected as a representative IL to investigate the influence of extraction conditions such as the extraction temperature and mass ratio of IL to model oil on the extraction process. The results are described in detail in the following subsections.
3.4 Effect of extraction temperature
The influence of the extraction temperature was investigated in detail through single-factor experiments. Five temperatures from 30–70 °C were selected for the extraction of quinoline and indole. The extraction was conducted with a mass ratio of 1:7 for 30 min. The results are presented in Figure 5.
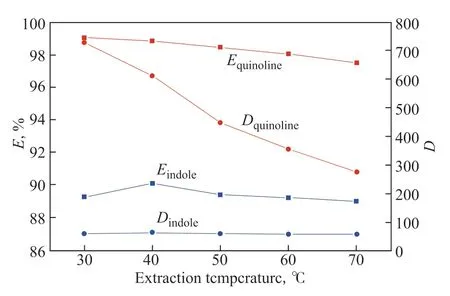
Figure 5 Effect of temperature on extraction efficiency (E) and distribution coefficient (D) of quinoline and indole
The extraction efficiency and distribution coefficient of quinoline decrease as the extraction temperature increases. For example, the extraction efficiency and distribution coefficient are 99.1% and 729.8 at 30 °C, respectively, and decrease to 97.5% and 275.3 at 70 °C. This might imply that the interaction between the IL and basic N-compounds is exothermic. The extraction temperature seems to have no notable influence on the extraction of indole by the IL. The extraction efficiency and distribution coefficient are 89.3% and 58.4 at 30 °C, respectively, and vary only slightly as the extraction temperature increases. These results demonstrate that denitrogenation with [Bmim]Br/FeCl3can be performed at or around room temperature, which is favorable in terms of energy consumption. Thus, the optimum extraction temperature was set to 30 °C in the subsequent experiments.
3.5 Effect of IL/oil mass ratio
Next, the denitrogenation of quinoline and indole by [Bmim]Br/FeCl3was investigated at different IL/oil mass ratios. The extraction experiments were carried out at 30 °C for 30 min. The results are presented in Figure 6. As can be seen, the distribution coefficient and extraction efficiency for both quinoline and indole exhibit the same trend, namely, they increase with increasing IL/oil mass ratio. In the case of indole, the N-removal efficiency and distribution coefficient increase from 89.3% and 58.4 to 98.9% and 89.1 as the IL/oil mass ratio changes from 1/7 to 1/1. Note that the residual nitrogen content in the model oil was below 5 μg/g at an IL/oil mass ratio of 1/1, demonstrating that excellent removal of indole can be achieved at higher IL/oil mass ratios. As expected, the extraction efficiency and distribution coefficient of quinoline reach high values of 91.8% and 223 at an IL/oil mass ratio of 1/20, proving that good removal performance for basic N-compounds can be realized by [Bmim]Br/FeCl3at a low IL/oil ratio.
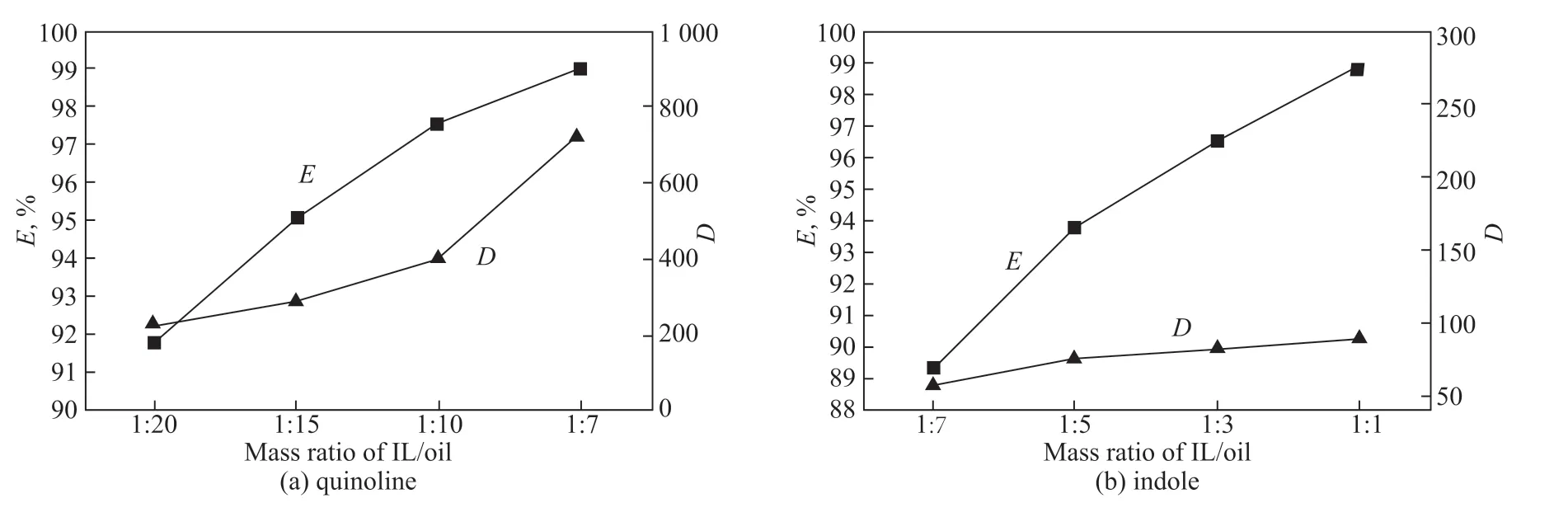
Figure 6 Effect of IL/oil mass ratio on extraction efficiency (E) and distribution coefficient (D) of quinoline (a) and indole (b)
3.6 Regeneration and recycling of ILs
The recycling of ILs is very important for industrial applications. The [Bmim]Br/FeCl3used for the extraction of quinoline was regenerated using carbon tetrachloride as a back extractant. In a typical run, the oil phase was separated from the IL phase using a separating funnel after denitrogenation. The IL phase was stirred with an equal volume of carbon tetrachloride for 30 min and allowed to settle for 1 h. The process was repeated five times, in which quinoline was transferred from the IL phase to the carbon tetrachloride phase. Finally, the residual carbon tetrachloride was removed from the IL phase by vacuum drying at 50 °C until the mass of the IL was constant. The regenerated IL was reused to extract quinoline from the model oil under the same extraction conditions. Figure 7 shows the extraction efficiency of quinoline using regenerated [Bmim]Br/FeCl3. There is a slight decrease in the extraction efficiency of quinoline after each recycling phase; nevertheless, the extraction efficiency still reaches 92.3% after four recycling phases, demonstrating the strong recyclability of this IL in the denitrogenation process. The regenerated IL was checked by FT-IR spectroscopy. As shown in Figure 8, comparing the spectra of the regenerated IL with that of the fresh IL, there are no significant differences in the absorption peaks.
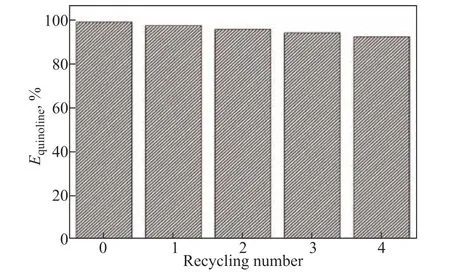
Figure 7 Extraction efficiency with respect to recycling number of IL for quinoline
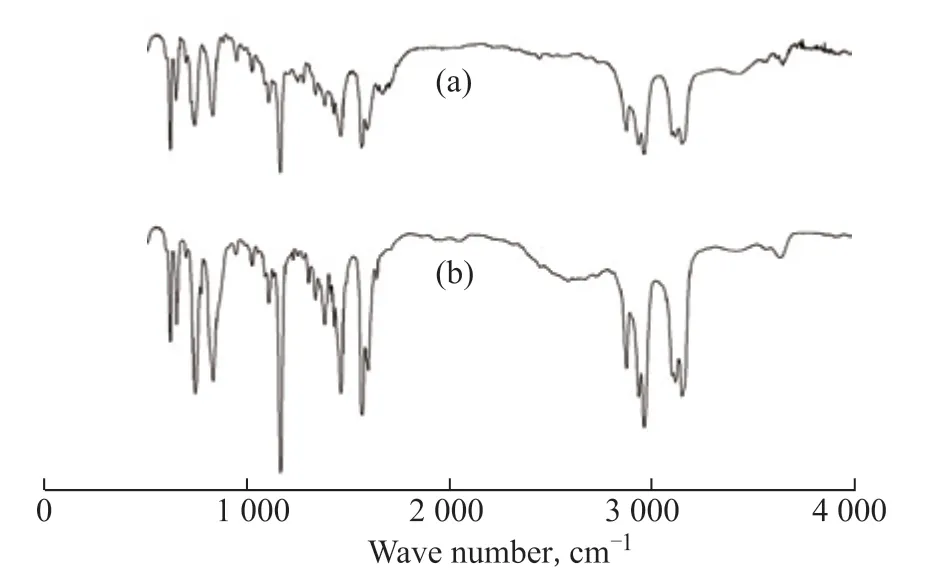
Figure 8 FT-IR spectra of fresh [Bmim]Br/FeCl3 (a) and regenerated [Bmim]Br/FeCl3 (b)
4 Conclusions
In this study, FeCl3-containing ILs prepared from the interaction of anhydrous FeCl3and alkyl imidazolium bromide were used to extract quinoline and indole from model oils. In particular, the performance of alkyl imidazolium bromide for the extraction of quinoline was significantly improved by the co-presence of FeCl3, which can be attributed to the complexing interaction of FeⅢspecies with the basic quinoline molecule, as well as the good fluidity of the FeCl3-based ILs. For indole, the imidazolium cation seems to play a major role. With the selected IL [Bmim]Br/FeCl3, the extraction efficiencies of quinoline and indole at an extraction temperature of 30 °C, IL/oil mass ratio of 1/7, and extraction time of 30 min are 99.1% and 89.3%, respectively. Moreover, the extraction efficiency of quinoline can still reach 92.3% after the IL has been subjected to four recycling phases.
Acknowledgments:The authors are grateful for financial support from the Doctoral Research Funds of Liaoning Petrochemical University (2019×JJ-006).
- 中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Microporous Metal Organic Frameworks for Light Hydrocarbon Adsorption
- Selection of Extraction Solvents for Bitumen from Indonesian Oil Sands through Solubility Parameters
- Synthesis of Hierarchical Porous Fe2O3/Al2O3 Materials and Study on Catalytic Viscosity Reduction of Heavy Oil
- Activated Carbon from Rice Husk with One-Step KOH Mechanical Mixing Activation as Adsorbent for Treating Phenolic Wastewater
- Effect of Particle Shape on Catalyst Deactivation during 2-Butene and Isobutane Alkylation of Liquid Phase in Fixed-Bed Reactor Using Particle-Resolved CFD Simulation
- Experimental and Numerical Investigation on Erosion Corrosion of the Air Cooler Tube Bundle in a Residue Hydrotreating Unit

