High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
WANG Xueping, LO Hoi Shing, FU Yijian, WU Zhou, QIN Danmei, HUANG Xing,ZHU Jingmin, CHEUNG Siu Gin, 4), *, and KWAN Kit Yue, 5), *
High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
WANG Xueping1), 2), LO Hoi Shing3), FU Yijian1), WU Zhou1), QIN Danmei1), HUANG Xing1), 2),ZHU Jingmin1), 2), CHEUNG Siu Gin3), 4), *, and KWAN Kit Yue1), 5), *
1),,535011,2),535011,3),,,4),,,5),535011,
Microplastics (MPs) are ubiquitous in marine sedimentary environments. Their occurrence in horseshoe crabs and interactions with the sedimentary environment has not been determined. In this study, MPs, mostly microfibers, were found in all extracted gastrointestinal tract (GIT) samples of the juvenilefrom the northern Beibu Gulf, China. The MP concentrations (4–53itemsind−1) were higher than those in most marine benthic invertebrates (<15itemsind−1) reported in Chinese waters, despite their generally low level in habitat sediments (9–1818itemskg−1). The correlation between the juveniles and sediments was not evident, but the abundance in juvenile horseshoe crab GITs decreased with ages. The findings were relevant to the life-history characteristics of the species, typically with limited dispersal capability and their spending nine years or longer time living in mangrove wetlands during low tides, with apparent ontogenetic changes in their diets. These baseline data enable a better understanding of MP availability in benthic macroinvertebrates, and the ecological risks present in the ecosystems.
horseshoe crab; invertebrate; fiber; intertidal; cellophane
1 Introduction
Marine plastic pollution and its effects on ecosystems are recently of particular concern due to its ubiquitous pre- sence from polar to the equator regions (Amin., 2020; Sfriso., 2020). Microplastics (MPs), referred to plas- tic items with a diameter from 1μm to 5mm (Arthur., 2009), overlap with natural organic matter and plankton. Consequently, MPs are found to be ingested by myriad as- sortments of marine organisms with varying feeding modes (Wright., 2013), and transported along marine food webstrophic interactions (Carbery, 2018). MPingestion may represent physical and/or toxicological threats to marine organisms. Controlled laboratory studies report- ed MP-induced adverse effects on assimilation, develop- ment, regeneration, reproduction and behavior in various examples of marine species (Haegerbaeumer, 2019). Owing to the large surface area/volume ratio and hydro- phobic properties, MPs are important resource of organic pollutant contaminations (Lo., 2019), and may result in the bioaccumulation and biomagnification in the aqua- tic food webs.
The occurrence of MPs in marine sediments has been broadly reported (Yao., 2019; Harris, 2020; Zhou.,2020; Sun., 2021), and the densities were much high- er than those detected in the nearby waters (Haegerbaeu- mer., 2019). Therefore, benthic fauna should be more susceptible to the ecological risk caused by plastic pollu- tion. Among marine sedimentary environments, estuaries contain higher concentrations of MPs due to their greater sediment trapping efficiency (Harris, 2020). However, the distribution pattern of MPs in benthic species in response to habitat type, feeding mode and trophic level is current- ly unclear. Field and laboratory studies demonstrated that filter feeders suffered the most from MP pollution (Setälä., 2015; Scherer., 2017; Bour, 2018b); none- theless, in another study the count of MPs per individual in filter feeders was significantly lower than that in pre- dators (Bour., 2018a). Xu. (2020b) found a sig- nificantly higher quantity of MPs in gastropods indepen- dent of their feeding mode, indicating that MP levels in benthic invertebrates may be species-specific because of their great variation in feeding selectivity, gut retention time and egestion rate.
To date, the occurrence of MPs in horseshoe crabs and their interactions with the sedimentary environment has not been determined. Horseshoe crabs are important prey and predators in estuarine and coastal food webs (Botton, 2009). Their eggs are also vital sources of lipids and pro- teins to support shorebird population along the Atlanticcoast (Botton, 2009). Different from most other benthic in- vertebrates, horseshoe crabs utilize a wide variety of coas-tal habitats ranging from upper and mid-intertidal zones to shallow waters within 30m deep (John., 2018; Lau- rie, 2019; Wang., 2020). For the juveniles, they have restricted dispersal capability and may spend nineyears or longer time living in the intertidal areas (Hu., 2009, 2015; Xie., 2020). Their foraging activities fol- low the tidal cycle: burrowing in the sediment during the rising tide to avoid predators and emerging when the tide is receding. Horseshoe crabs forage on mixed diets of small benthic invertebrates, but predominantly assimilate nutri- ents from sedimentary organic matter and benthic plank- ton during their early growth stages (Gaines, 2002; Kwan., 2021). Such foraging behavior and life-his- tory characteristic may render them exposed to a higher abundance of MPs in the estuarine environment.
The northern Beibu Gulf is perceived as one of the leastdisturbed semi-enclosed areas in the southern China, which supporting the largest population of tri-spine horseshoe crab,in China (Liao and Li, 2001; Brockmann and Smith, 2009). Extensive historical har- vest records of adult horseshoe crabs for bleeding to pro- duce endotoxin tests in pharmaceutical products and for local food consumption have been well documented (Liao and Li, 2001; Fu, 2019; Liao., 2019). However, approximately 6.8 million coastal residents in the north- ern Beibu Gulf (Fu, 2019) and a lack of an efficient plastic recycling policy have made the widespread occur- rence of MPs in coastal seawater, mangrove sediment and marine organisms. MP concentration in the Beibu Gulf coastal water ranged from 399 to 5531itemsm−3(Li., 2020b), 273–6360itemskg−1 in mangrove sediment (Li, 2019, 2020a, b; Zhang, 2020b), 1–8itemsind−1in mangrove crab, 2–14itemsind−1 in mangrove fish (Koon-golla, 2020; Zhang, 2021a), 3–9itemsind−1 inoysters (Zhu, 2019), and 7–53itemskg−1 in mangrove snail, Ellobium chinense (Li et al., 2020b). Aquaculture ac-tivities along the coast with widespread plastic ropes and foam buoys are suggested to be the important source of MPs in the region (Zhang, 2020a), which can be con- firmed easily by the researchers’ observations.
In this study, juvenilewas chosen as the representative benthic macroinvertebrate species in coastal and estuarine ecosystems. The objectives were to under- stand whether MP abundance and composition in juvenile horseshoe crabs varied among growth stages and sam- pling sites. The baseline information is fundamental to a better understanding of MP availability to benthic macro- invertebrates, and the potential to accumulate and transfer across the benthic food webs.
2 Materials and Methods
2.1 Study Locations and Samplings
Six important nursery habitats forwere visited in summer (August–September 2018) according to the previous population studies along the coast of the nor- thern Beibu Gulf, China (Xie., 2020) (Fig.1). Yuzhou- ping (YZP), Tieshangang (TSG) and Shatiangang (STG) are located in regions with fast-growing industrial ports in Southwest China. Shaluoliao (SLL) is positioned along the coast of Qinzhou Bay and next to a new power station un- der construction. Zhongsandun (ZSD) is located within the estuary of Dafengjiang River and near the Sanniang Bay, a famous ecotourism spot for dolphin watching (Wu., 2020). Xiacun (XC) is situated within the Beihai National Wetland Park, nonetheless, small-scale intertidal beachcom-bing activities are commonly found (Wang., 2020). All these sampling sites are sandy-mudflats (grain size: 0.22–0.44mm) with sediment total organic carbon in the range of 0.14%–0.34% (Xie.,2020).
At each study site, two transects were set horizontal to the coastline at 1.6m and 1.3m above chart datum,., the lowest astronomical tide, to include only the intertidal areas with the high-density juvenile horseshoe crab populations (Kwan,2016; Xie, 2020). The first transect waslocated along the outer edges of the mangroves or the salt- marsh cordgrass, except that SLL was in front of the sea- wall (Fig.1). Lengths of each transect ranged 1.0–2.1km, according to the shore length or the determined core feed- ing area of the juveniles. Along each transect, five equal- distance quadrats (0.33×0.33m2) were placed. For each site, juvenile horseshoe crabs (=5–39; prosomal width, PW=33.1–77.3mm) found within the 10 quadrats were col- lected. Approximately 1–2kg of wet sediment was also sampled within each quadrat at the depth of 2–3cm dur- ing low tides with an aid of stainless-steel shovel. All sam-ples, including juvenile horseshoe crabs and sediment, were stored in separate cotton bags and transported to the la- boratory for microplastic analysis.
2.2 Microplastic Extraction
Sediment samples were dried naturally in the labora- tory for two months, sieved through 125μm and 5mm sieves, and weighed. The separation between MPs and se- diment particles followed the protocol of Lo(2018). In brief, 3L of ZnCl2solution (density of 1.6–1.7gmL−1) was added into a 5L glass beaker and stirred for 5min using a stainless-steel spatula. The mixture was covered with aluminum foil, settled overnight and the overflow su- pernatant was filtered through a 5μm glass microfiber fil- ter membrane (GF/C grade, binder-free, Millipore, UK). The separation process of each sediment sample was re- peated thrice to enhance the recovery rate. The surface of the beaker was rinsed thoroughly with distilled water to ensure all remaining plastic items were collected. Those on the filters were dried at 65℃, and stored in covered glass Petri dishes before microscopic examination. To avoid any possible MP contamination, all laboratory apparatus were repeatedly rinsed with distilled water prior to use.

Fig.1 Locations of study sites and sampling areas (highlighted in red) along the northern Beibu Gulf shoreline. The rela- tive position of mangrove patches is denoted in green. YZP, Yuzhouping; SLL, Shaluoliao; ZSD, Zhongsandun; XC, Xia- cun; TSG, Tieshangang; STG, Shatiangang.
For juvenile, they were frozen to death prior to dissection in the laboratory. The animals were thawed, and their PW and body weight were measured. Each individual was classified following their instar stagesas described by Hu(2015). The juveniles were rinsed with distilled water to eliminate any possible particles ad- hered to the body surface, then their gastrointestinal tracts (GITs) and gills were isolated and stored in glass beakers covered with aluminum foil at −20℃. Several early-instar juveniles were found at YZP, ZSD and STG, and their GITs were too fragile and not successfully isolated during dis- section. The GIT samples obtained from larger-instar ju-veniles were digested using 10% KOH solution at 40℃ for 48h (Li.,2019). For gill samples, however, their surface was covered with chitin and could not be digested by the alkaline solution, even the samples had been cut into small pieces. The use of strong acids for digestion proba- bly lead to the fragmentation or eventually the loss of mi- croplastics such as polyamide (nylon) and polyoxymethy- lene (Bürkle GmbH, 2021), which may end up with inac- curate or biased results. Therefore, the gill samples were not included in the experiment. The digested liquids of GIT samples were then filtered through 5μm glass microfiber membranes (GF/C, Whatman, France), and stored in clean Petri dishes with lids for further examination.
2.3 Qualification and Quantification of Microplastic
The filters were visually inspected and counted under a stereomicroscope (Olympus, Japan) at a magnification of 40× to 120×. All suspected items were recorded in images using CapStudio software (SC600 digital CMOS camera, Nanjing Lookout Photoelectric, China), and categorized based on their shapes and colors as described previously (Lo., 2018; Zhu., 2019). The length of items was quantified using ImageJ program (https://imagej.net/). To reduce the tremendous amount of work, only characteris- tics (., length, shape and color) of suspected MPs in GITs of juvenilewere quantified.
For the composition quantification, approximately 20% of the items on each filter paper were chosen at random (Xu.,2020b) and verified using micro-Fourier Trans- form Infrared Microscope (μFTIR; Thermo Fisher Nicolet iN5, USA) under attenuated total reflection mode. All sus- pected MPs would be subjected to the composition iden- tification if there were less than five items on a filter pa- per. In total, 440 out of 1301 suspected items in GIT and sediment samples were tested.
A matching between the suspected MP spectrum and the OMNIC standard spectra library of Hummel Polymer Sample Library was conducted. Any item with a matching score greater than 70% would be counted as a MP (Lo.,2018). The final abundance of MPs was revised by the ra- tio of the suspected plastics confirmed as MPs with 78.01% in GITs and 100% in sediments. A procedural control was implemented weekly with the inclusion of all the digestionprocedures, except for the sediment and biological samples, to assess possible air contamination during tissue digestion. No MP was detected during the control experiments (=12).
2.4 Statistical Analysis
Prior to the analyses, all data were subjected to the tests of normality and homogeneity of variance. Abundance data of MPs in juvenile GITs and sediments did not meet the normality requirements even though all possible arithme- tic transformations had been attempted. As a result, the dif- ference in sediments among sampling sites was tested us- ing non-parametric Kruskal-Wallis test. If a significant dif-ference was detected, multiple pair-wise comparisons withMann-Whitney U tests would be performed. Non-parame-tric Scheirer-Ray-Hare extension of Kruskal-Wallis test (site[fixed]×instar [fixed]) was used to examine MP level dif- ferences in juvenile GITs. Spearman rank-order correla- tion was employed to determine the possible relationship of MP abundance as well as length in GIT samples with the changes in juvenile prosomal width. The above calcu- lations were performed by SPSS statistics software (IBM version 22, New York, USA), and Scheirer-Ray-Hare ex- tension of the Kruskal-Wallis test was conducted follow- ing the modified SPSS protocol described by Shen. (2013).
3 Results
Microplastic items were found in all sediment samples obtained from the six study sites. The concentrations rangedfrom 8.8 to 1818.2itemskg−1dry weight (dw) with the mean value of (130.8±594.8)itemskg−1dw. SLL had the highest ((361±594.8)itemskg−1dw), in which the concentra- tion of MPs was significantly higher than those of the other sampling sites,., TSG ((152.1±87.1)itemskg−1dw)>YZP ((138.7±70.4)itemskg−1dw)>STG ((87.6±44.0)itemskg−1dw)>XC ((81.4±38.1)itemskg−1dw)>ZSD ((50.4±24.6)itemskg−1dw) (Fig.2).
MPs were also detected in all extracted GIT samples of juvenile horseshoe crabs. The abundance of GIT samples varied from 4 to 53itemsind−1(0–28.7itemsg−1, wet weight, ww). The mean value was (21.1±13.4)itemsind−1(3.2±5.4itemsg−1ww). Neither the sampling site nor instar stage of juveniles was found to influence the MP abundances in GITs (site:=0.154,=0.930; instar:=9.426,=0.090; site×instar:=0.160,=1.000). MP abundance in GITs was found tobe negatively correlated with prosomal width (size) of juvenile(Fig.3A, Spearman’s=−0.357,=0.014). However, a positive relationship was found between MP size and the juvenile body size (Fig.3B; Spearman’s=0.415,=0.004).

Fig.3 Relationships of (A) abundance and (B) mean length (size) of microplastics in gastrointestinal tract (GIT) with prosomal width of juvenile T. tridentatus.
Transparent MPs were the most commonly encounter- ed (86.2%) in GIT of(Figs.4–5). In terms of MP shape, only fiber and flake items were noted in all juvenile samples, and fiber contributed greater than 99% of all the samples (Figs.4–5). MPs that were greater than 1mm in length contributed about half in GIT samples (51.81%). Thirteen polymers were noted in sediments and GITs of juvenile, which included cellophane, polyamide (PA), polyacrylonitrile (PAN), polypropylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polyethylenimine (PEI), synthetic rubber (SR), polyvinyl acetate (PVA), polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC), polyethylacrylate acrylamide (PEA) and polyester (PES). Cellophane was the most dominant poly- mer in both sediments (41.3%) and juvenile horseshoe crab GITs (66.7%) (Fig.5). In addition to cellophane, PES (38.1%) and PA (9.5%) were the second and third most abundance. These three polymers accounted for >88.9% of total MPs in sediments. However, the composition ofremaining MPs in GIT samples greatly varied from PVDF (1.0%) to SR (10.1%) (Fig.5).
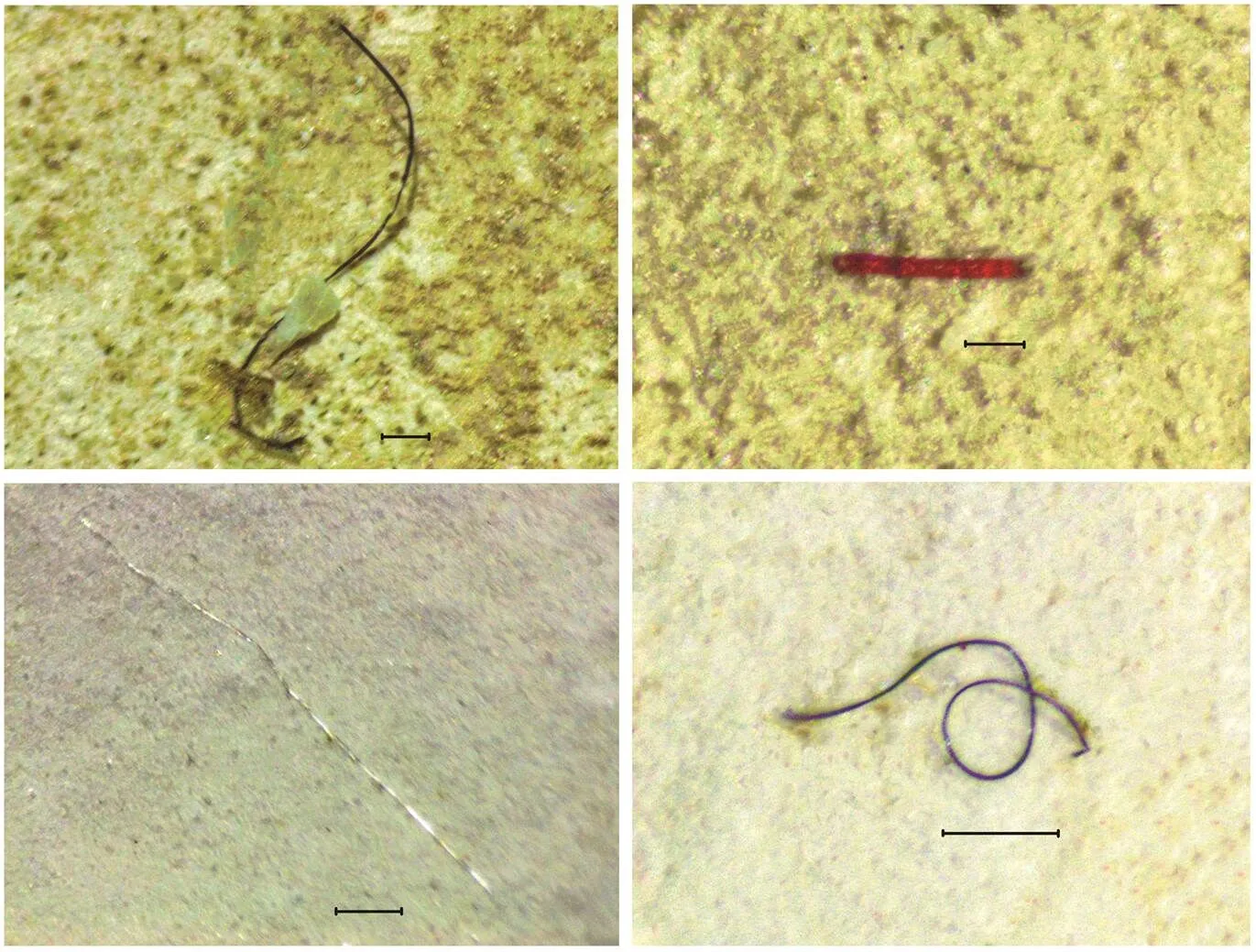
Fig.4 Microfibers found in gastrointestinal tracts of juvenile T. tridentatus with varying colors. The scale bars represent 200μm.

Fig.5 Characteristics (color, shape and size) and compositions of microplastics in the sediments and juvenile T. tridentatus. PA, polyamide; PAN, polyacrylonitrile; PP polypropylene; PE, polyethylene; PET, polyethylene terephthalate; PEI, polyethylenimine; SR, synthetic rubber; PVA, polyvinyl acetate; PVDF, polyvinylidene fluoride; PVC, polyvinyl chloride; PEA, polyethylacrylate acrylamide; PES, polyester; GIT, gastrointestinal tracts.
4 Discussion
The northern Beibu Gulf is regarded as one of the un- disturbed wilderness areas retaining its relatively pristine state compared to other coastal regions in China (Zhang.,2020b). Nevertheless, there are recently increasing studies confirming that the gulf has been polluted by va- rious MPs, although their abundances in coastal water and sediment samples were highly variable even in the same or adjacent area. For example, Qiu(2015) reported that the MP abundance of mangrove sediment on a beach (site name: Beihai) close to XC in this study was 6080itemskg−1dw. In another adjacent sampling site (site name: Da-guan Sha) within 1km from XC, however, Zhang(2020b) found that the concentration was only approxi- mately one-seventh ((873±63)itemskg−1dw) of that re- ported in Qiu(2015). Similar cases were found in the MP abundance in mangrove sediments at Yong An (>500itemskg−1dw; Zhang., 2020b) and Guangxi station 7 (87.8itemskg−1dw; Zhou.,2020), in which both sites are in the vicinity of STG in this study ((138.7±70.4)itemskg−1dw). Overall, the abundance of MPs in sediments from the important nursery habitats ofin this study was relatively low<(400itemskg−1dw per site). These inconsistencies may be due to the cur- rently limited environmental baseline data regarding MP occurrence in the Beibu Gulf region.
For MPs in juvenile horseshoe crabs, the abundance(4–53itemsind−1or 0–28.7itemsg−1ww) was higher than that in most marine benthic invertebrates reported in Chi- nese waters (Table 1). In the coastal and offshore areasof Beibu Gulf, the count of MPs in oysterand various mangrove crabs were recorded in the range of 2–9itemsind−1(Table 1, Zhu., 2019; Zhang., 2021a). In other coastal regions, those in bi- valves, gastropods and crustaceans were reported at 2–15itemsind−1(0.2–7.1itemsg−1ww), 1.5–5.4itemsg−1ww and 0–10itemsind−1(0–22.7itemsg−1ww), respectively, except for the considerably high MP levels occurred in clam(about 49itemsind−1)and scal- lop(57.2itemsind−1) obtained from a fishery market in Shanghai (Li., 2015). The relatively higher MP abundance in juvenilewas believed to be related to their life-history char- acteristics with limited dispersal capability, spending at least nine years feeding in the intertidal mangrove areas at low tides (Hu, 2015; Kwan., 2021; Xie., 2020).
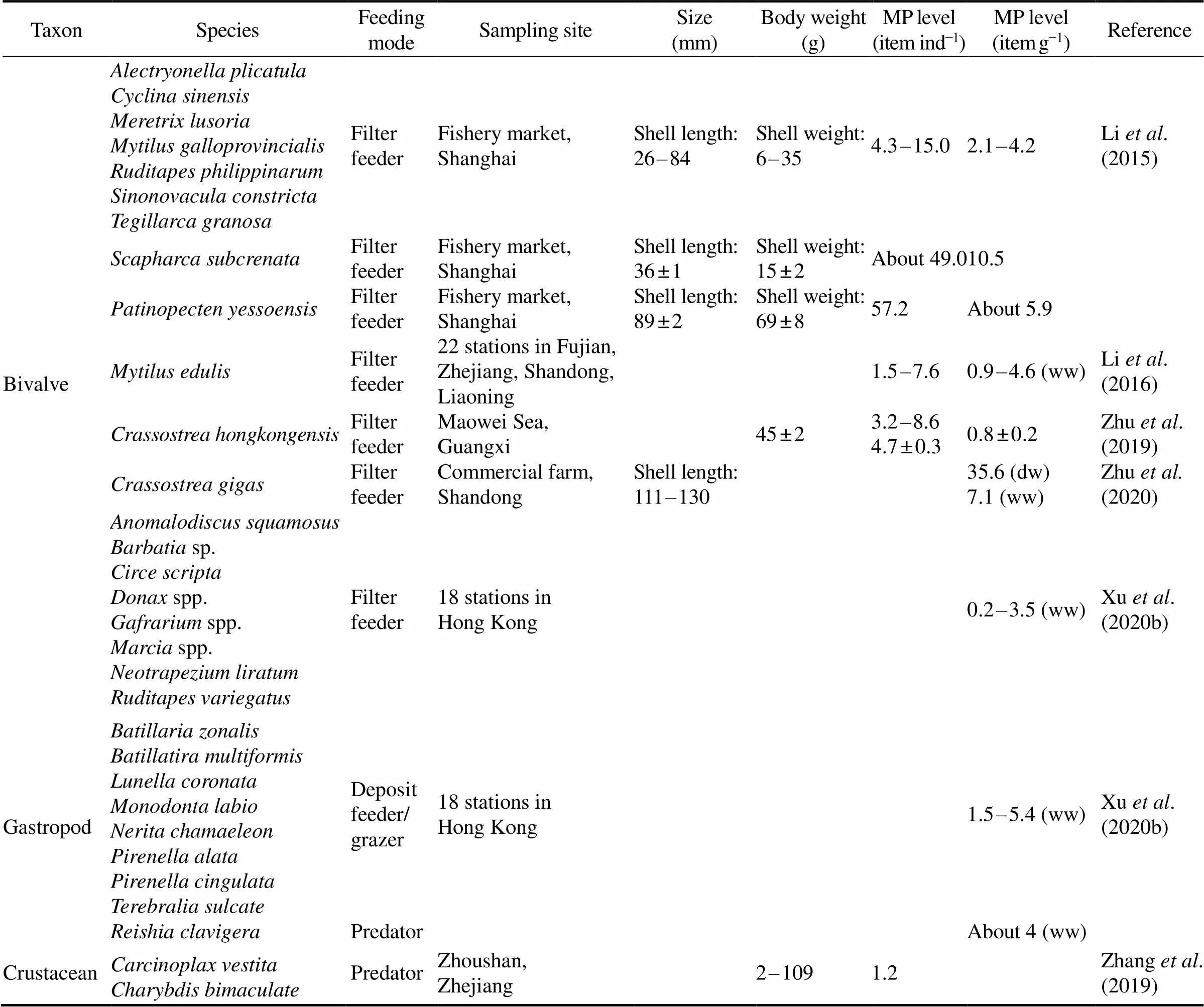
Table 1 Microplastic (MP) contamination in marine benthic invertebrates in Chinese waters
()
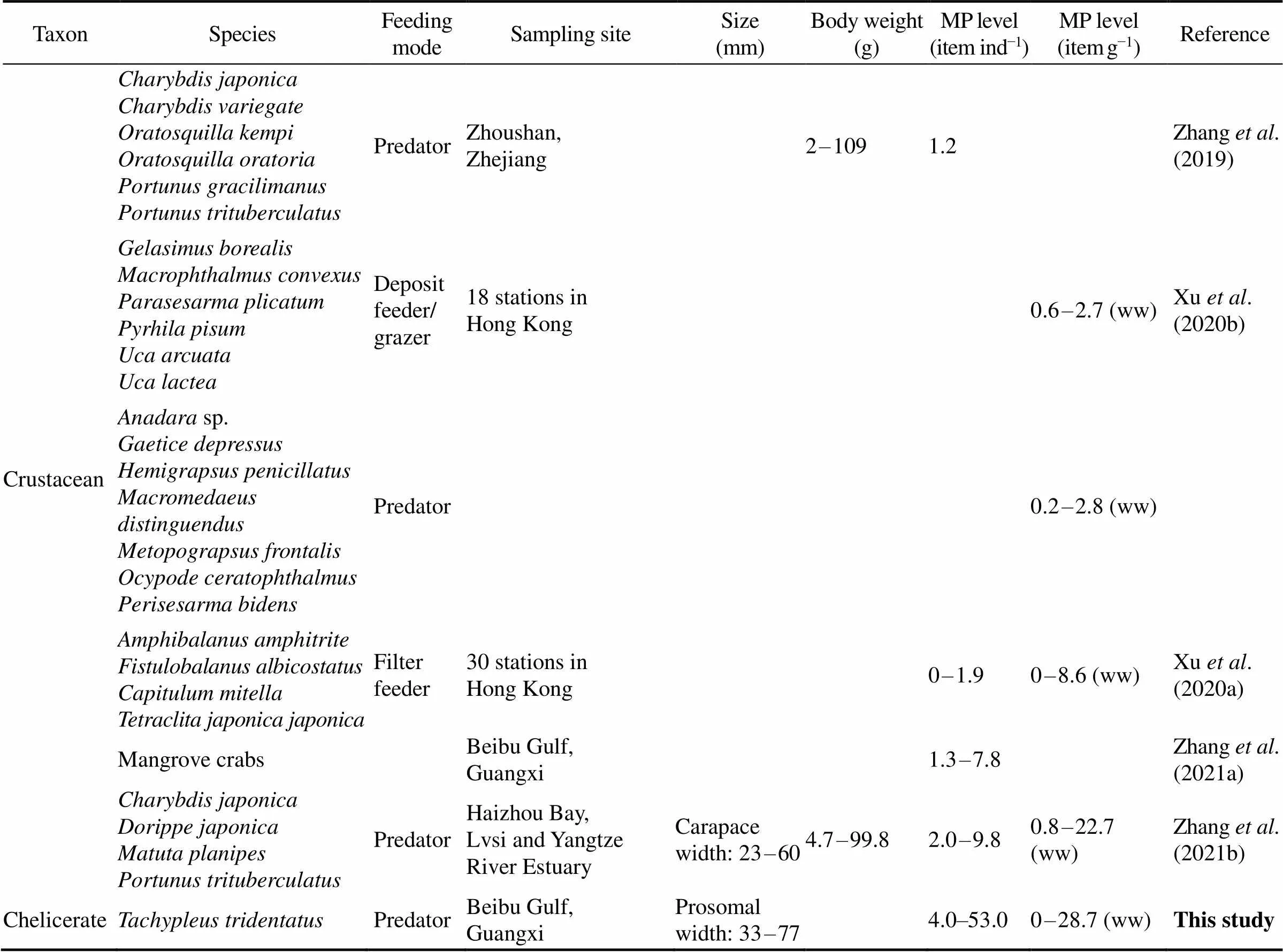
(continued)
Notes: Reported MP levels are represented in item per individual (mean±S.D., range) and/or item per tissue weight. dw, dry weight; ww, wet weight.
Consistent with the previous reports on other marine in- vertebrates, the relationship between the MP levels in se- diments and organisms was not evident (Hipfner., 2018; Li., 2020b; Xu., 2020b). Despite the fact that a higher level of MPs in habitats may increase the pro- bability of passive intake by the organisms, recent studies demonstrated that marine organisms such as juvenile palm ruff,(Ory., 2018), and blue mus- sel,(Woods, 2018; Chae and An, 2020) can effectively excrete most of the MPs in the form of feces or psedofeces. Zhu. (2018) also documented the avoidance behavior against MP ingestion in the collem- bolans. It is intuitive that the older an organism, the high- er the abundance of MPs in the body. The present find- ings, however, found a weak but significant negative rela- tionship between the quantity of MPs and the body size of. Such correlation is possibly related to the apparent ontogenetic changes in juvenile horseshoe crab, while their diets change from primarily sedimentary orga- nic matter to small-sized invertebrates with aging (Gaines., 2002; Kwan., 2021). The marked difference in MP compositions between sediments and the juvenile GITsalso suggest that the MPs in GIT samples were mainly derived from other organisms and transferred through ben- thic food webs.
Coastal anthropogenic activities, including tourism, aqua- culture and industrial production, have been identified as the primary contributors to the distribution of MPs in ma- rine sediments (., Nor., 2014; Zhang., 2020b; Zhang, 2021a). The pattern is consistent with the present results that, the sampling sites near the industrial ports (TSG, STG and YZP) had a relatively higher MP abundance (88–152itemskg−1dw) in sediments comparedto those located within the wetland park and/or away fromintensive human activities (XC and ZSD, 50–81itemskg−1dw). The presence of circulating water pipelines outside the power station near SLL may have lowered the ex- change rate of coastal water, consequently enhanced the sedimentation and MP accumulation (Willemsen., 2016; Zhang., 2020b).
Apart from industrial activities, the direct sources of MP pollution at these sampling sites were possibly due to tra- ditional intertidal aquaculture (Zhu., 2019), small- scale seafood harvesting activities (Wang, 2020) and/ or domestic wastewater inputs from the coastal popula- tions in this region (Zhang., 2020a). The ubiquitous presence of MP fibers (>99%) in both previous and pre- sent studies could also explain the major impacts of aqua- culture and fishing activities, which involved a tremen- dous amount of plastic waste discarded or lost in the ma- rine environment (Andrady, 2011). Microfibers derived fromclothing were also suspected to be a primary source of MPs in the coastal and estuarine environment. Consequent- ly, a great number of studies reported the presence of MP fibers in marine fishes (Jabeen., 2017; Garcés-Or- dóñez., 2020; Koongolla., 2020) and benthic in- vertebrates (Xu, 2020a, b; Zhang., 2021). La- boratory studies also suggested that MPs in the form of fibers were more prone to ingestion by marine living or- ganisms (Hämer., 2014; Rochman., 2015).
In terms of MP compositions, the occurrence of poly- mers such as PP, PE, PET and PS were frequently re- ported in the Beibu Gulf region (Qiu., 2015; Li., 2018; Zhang., 2020a). Our current findings, however, indicated the considerably higher percentage of cellophanefound in the sediments and juvenile horseshoe crabs (Fig.4).Cellophane is an organic cellulose-based polymer, and has been classified as microplastic (Woodall., 2014). As a typical raw material for food packaging as well as a re- leasing agent in fiberglass and rubber production, cello- phane was documented as one of the most commonly available polymers in the sediments of the Yellow Sea, the East China Sea and Ma’an Archipelago marine ranching area of China (Zhang., 2020a, b), in surface water from Qin River within the Beibu Gulf, as well as in ma- rine fishes (Jabeen, 2017) and edible oysters (Zhu., 2020). The abundant cellophane in the nursery ha- bitats of horseshoe crabs in the northern Beibu Gulf may originate from the weathering of fiberglass products and/ or cellophane wrappers, which suggest the primary con- tribution of land-derived MPs, particularly the beaches near the river mouth.
To conclude, MPs can be widely detected in both sedi- ment and juvenilefrom the northern Beibu Gulf shores. Most identified microplastics in the juveniles were transparent microfibers with length>1mm, and atrelatively higher concentrations compared to those in othermarine benthic invertebrates found in Chinese waters. The MP abundance decreased with horseshoe crab ages, but the MP size increased with their growth. These results de- monstrated that the influence of MP abundance in marine benthic invertebrates can be species-specific, which may be governed by their feeding behavior and selectivity, in- gestion and egestion rates, as well as life-history charac- teristics. As a result, baseline studies in marine inverte- brates are necessary to obtain a clearer picture regarding the fate and trophic interactions of MPs in benthic sedi- mentary environments. Owing to the limitations in this study, which included 1) MP occurrence in juvenile GIT samples was not completely determined, and 2) only about 34% of the suspected MPs were analyzed by μ-FTIR, the level of MPs in juvenile horseshoe crabs could be under- estimated. Given the high ecological importance of con- servingpopulation, future studies to enhance our knowledge regarding the source and sink of MPs in the region are therefore necessary. It is also dire to deter- mine the MP egestion rate of juvenile, and their potential physical and/or toxicological impacts on the organisms.
Acknowledgements
We thank Dr. Paul Shin and many other researchers who provided feedback and information which consider- ably enhanced the quality of this manuscript. The assis- tance from Dr. Chun-Chieh Wang from Guangxi Acade- my of Sciences for preparing the maps is much appre- ciated. This research was supported bythe National Na- tural Science Foundation of China (No. 41907320), the Natural Science Foundation of Guangxi Region (No. 2019 JJA150043), the Guangxi BaGui Youth Scholars Program- me, and Guangxi Recruitment Program of 100 Global Ex- perts.
Amin, R. M., Sohaimi, E. S., Anuar, S. T., and Bachok, Z., 2020. Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea., 150: 110616.
Andrady, A. L., 2011. Microplastics in the marine environment., 62: 1596-1605.
Arthur, C., Baker, J., and Bamford, H., 2009.. NOAA Technical Me- morandum NOS-OR&R-30, 528pp.
Botton, M. L., 2009. The ecological importance of horseshoe crabs in estuarine and coastal communities: A review and specula- tive summary. In:. Tanacredi, J. T.,., eds., Springer, Massachusetts, Boston, 45-63.
Bour, A., Avio, C. G., Gorbi, S., Regoli, F., and Hylland, K., 2018a. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level., 243: 1217-1225.
Bour, A., Haarr, A., Keiter, S., and Hylland, K., 2018b. Environ- mentally relevant microplastic exposure affects sediment-dwell-ing bivalves., 236: 652-660.
Brockmann, H. J., and Smith, M. D., 2009. Reproductive compe-tition and sexual selection in horseshoe crabs. In:. Tanacredi, J. T.,., eds., Springer, Massachusetts, Boston, 199-221.
Bürkle GmbH, 2021. Chemical resistance of plastics. https://www. buerkle.de/en/chemical-resistance. Accessed on 13 August 2021.
Carbery, M., O’Connor, W., and Palanisami, T., 2018. Trophic transfer of microplastics and mixed contaminants in the ma- rine food web and implications for human health., 115: 400-409.
Chae, Y., and An, Y. J., 2020. Effects of food presence on mi- croplastic ingestion and egestion in., 240: 124855.
Fu, Y., Huang, S., Wu, Z., Wang, C. C., Su, M., Wang, X.,., 2019. Socio-demographic drivers and public perceptions of con- sumption and conservation of Asian horseshoe crabs in nor- thern Beibu Gulf, China., 29: 1268-1277.
Gaines, E. F., Carmichael, R. H., Grady, S. P., and Valiela, I., 2002.Stable isotopic evidence for changing nutritional sources of juvenile horseshoe crabs., 203: 228-230.
Garcés-Ordóñez, O., Mejía-Esquivia, K. A., Sierra-Labastidas, T., Patiño, A., Blandón, L. M., and Díaz, L. F. E., 2020. Preva- lence of microplastic contamination in the digestive tract of fishes from mangrove ecosystem in Cispata, Colombian Ca- ribbean., 154: 111085.
Haegerbaeumer, A., Mueller, M. T., Fueser, H., and Traunspur- ger, W., 2019. Impacts of micro-and nano-sized plastic parti- cles on benthic invertebrates: A literature review and gap ana- lysis., 7: 17.
Hämer, J., Gutow, L., Köhler, A., and Saborowski, R., 2014. Fate of microplastics in the marine isopod., 48: 13451-13458.
Harris, P. T., 2020. The fate of microplastic in marine sedimen- tary environments: A review and synthesis., 158: 111398.
Hipfner, J. M., Galbraith, M., Tucker, S., Studholme, K. R., Do- malik, A. D., Pearson, S. F.,., 2018. Two forage fishes as potential conduits for the vertical transfer of microfibres in northeastern Pacific Ocean food webs., 239: 215-222.
Hu, M., Kwan, B. K. Y., Wang, Y., Cheung, S. G., and Shin, P. K. S., 2015. Population structure and growth of juvenile horse- shoe crabsand(Xiphosura) in southern China. In:. Carmichael, R. H.,., eds., Springer, Zug, Cham, 167-180.
Hu, M., Wang, Y., Chen, Y., Cheung, S. G., Shin, P. K. S., and Li, Q., 2009. Summer distribution and abundance of juvenileChinese horseshoe crabsalong an in- tertidal zone in southern China., 7: 107-112.
Jabeen, K., Su, L., Li, J., Yang, D., Tong, C., Mu, J.,., 2017. Microplastics and mesoplastics in fish from coastal and fresh waters of China., 221: 141-149.
John, B. A., Nelson, B. R., Sheikh, H. I., Cheung, S. G., War- diatno, Y., Dash, B. P.,., 2018. A review on fisheries and conservation status of Asian horseshoe crabs., 27: 3573-3598.
Koongolla, J. B., Lin, L., Pan, Y. F., Yang, C. P., Sun, D. R., Liu, S.,., 2020. Occurrence of microplastics in gastrointesti- nal tracts and gills of fish from Beibu Gulf, South China Sea., 258: 113734.
Kwan, B. K. Y., Hsieh, H. L., Cheung, S. G., and Shin, P. K. S., 2016. Present population and habitat status of potentially threatened Asian horseshoe crabsandin Hong Kong: A proposal for marine protected areas., 25: 673- 692.
Kwan, K. Y., Bopp, J., Huang, S., Chen, Q., Wang, C. C., Wang, X.,, 2021. Ontogenetic resource use and trophic dyna- mics of endangered juvenileamong diversified nursery habitats in the northern Beibu Gulf, China., 16 (6): 908-928, https://doi.org/10.1111/ 1749-4877.12495.
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H. L., John, A.,, 2019.(Errata Version Pub- lished in 2019). e.T21309A149768986. IUCN, Gland, Switzerland, 60pp.
Li, J., Lusher, A. L., Rotchell, J. M., Deudero, S., Turra, A., Bråte, I. L. N.,., 2019. Using mussel as a global bioindicator of coastal microplastic pollution., 244: 522-533.
Li, J., Yang, D., Li, L., Jabeen, K., and Shi, H., 2015. Micro- plastics in commercial bivalves from China., 207: 190-195.
Li, J., Zhang, H., Zhang, K., Yang, R., Li, R., and Li, Y., 2018. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China., 136: 401-406.
Li, J. N., Qu, X. Y., Su, L., Zhang, W. W., Yang, D. Q., Koland- hasamy, P.,., 2016. Microplastics in mussels along the coastal waters of China., 214: 177- 184.
Li, R., Yu, L., Chai, M., Wu, H., and Zhu, X., 2020a. The distri- bution, characteristics and ecological risks of microplastics in the mangroves of Southern China., 708: 135025.
Li, R., Zhang, S., Zhang, L., Yu, K., Wang, S., and Wang, Y., 2020b. Field study of the microplastic pollution in sea snails () from mangrove forest and their relation- ships with microplastics in water/sediment located on the north of Beibu Gulf., 263: 114368.
Liao, Y., Hsieh, H. L., Xu, S., Zhong, Q., Lei, J., Liang, M.,., 2019. Wisdom of Crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China., 53: 222-229.
Liao, Y. Y., and Li, X. M., 2001. Present situation of horseshoe crab resources in the sea area of China and tactics of preser- vation., 23: 53-57 (in Chinese with English abstract).
Lo, H. S., Wong, C. Y., Tam, N. F. Y., and Cheung, S. G., 2019. Spatial distribution and source identification of hydrophobic organic compounds (HOCs) on sedimentary microplastic in Hong Kong., 219: 418-426.
Lo, H. S., Xu, X., Wong, C. Y., and Cheung, S. G., 2018. Com- parisons of microplastic pollution between mudflats and sandy beaches in Hong Kong., 236: 208- 217.
Nor, N. H. M., and Obbard, J. P., 2014. Microplastics in Singa- pore’s coastal mangrove ecosystems., 79: 278-283.
Ory, N. C., Gallardo, C., Lenz, M., and Thiel, M., 2018. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish., 240: 566-573.
Qiu, Q., Peng, J., Yu, X., Chen, F., Wang, J., and Dong, F., 2015. Occurrence of microplastics in the coastal marine environ- ment: First observation on sediment of China., 98: 274-280.
Rochman, C. M., Tahir, A., Williams, S. L., Baxa, D. V., Lam, R.,Miller, J. T.,., 2015. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption., 5: 14340.
Scherer, C., Brennholt, N., Reifferscheid, G., and Wagner, M., 2017. Feeding type and development drive the ingestion of micro- plastics by freshwater invertebrates., 7: 17006.
Setälä, O., Norkko, J., and Lehtiniemi, M., 2015. Feeding type affects microplastic ingestion in a coastal invertebrate com- munity., 102: 95-101.
Sfriso, A. A., Tomio, Y., Rosso, B., Gambaro, A., Sfriso, A., Cora- mi, F.,, 2020. Microplastic accumulation in benthic in- vertebrates in Terra Nova Bay (Ross Sea, Antarctica)., 137: 105587.
Shen, X., Qi, H., Liu, X., Ren, X., and Li, J., 2013. Two-way non-parametric ANOVA in SPSS., 30: 913-914 (in Chinese with English abstract).
Sun, X., Wang, T., Chen, B., Booth, A. M., Liu, S., Wang, R.,, 2021. Factors influencing the occurrence and distribution of microplastics in coastal sediments: From source to sink., 410: 124982.
Wang, C. C., Kwan, K. Y., Shin, P. K. S., Cheung, S. G., Itaya, S., Iwasaki, Y.,., 2020. Future of Asian horseshoe crab conservation under explicit baseline gaps: A global perspec- tive., 24: e01373.
Weng, Z. H., Xie, Y. J., Xiao, Z. Q., Huang, L. M., Li, J., Wang, S. H.,., 2012. Survey on resource distribution of Chinese horseshoe crab () in Fujian and other coast water of China., 47: 40-48 (in Chinese with English abstract).
Willemsen, P. W. J. M., Horstman, E. M., Borsje, B. W., Friess, D. A., and Dohmen-Janssen, C. M., 2016. Sensitivity of the sediment trapping capacity of an estuarine mangrove forest., 273: 189-201.
Woodall, L. C., Sanchez-Vidal, A., Canals, M., Paterson, G. L. J., Coppock, R., Sleight, V.,., 2014. The deep sea is a major sink for microplastic debris., 1: 140317.
Woods, M. N., Stack, M. E., Fields, D. M., Shaw, S. D., and Ma- trai, P. A., 2018. Microplastic fiber uptake, ingestion, and eges- tion rates in the blue mussel ()., 137: 638-645.
Wright, S. L., Thompson, R. C., and Galloway, T. S., 2013. The physical impacts of microplastics on marine organisms: A re- view., 178: 483-492.
Wu, H., Peng, C., Huang, H., Jefferson, T. A., Huang, S. L., Chen, M.,., 2020. Dolphin-watching tourism and Indo-Pacific humpback dolphins () in Sanniang Bay, China: Impacts and solutions., 66: 1-9.
Xie, X., Wu, Z., Wang, C. C., Fu, Y., Wang, X., Xu, P.,., 2020. Nursery habitat for Asian horseshoe crabs along the nor- thern Beibu Gulf, China: Implications for conservation ma- nagement under baseline gaps.:, 30: 260-272.
Xu, X., Wong, C. Y., Tam, N. F. Y., Liu, H. M., and Cheung, S. G., 2020a. Barnacles as potential bioindicator of microplastic pollution in Hong Kong., 154: 111 081.
Xu, X., Wong, C. Y., Tam, N. F. Y., Lo, H. S., and Cheung, S. G., 2020b. Microplastics in invertebrates on soft shores in Hong Kong: Influence of habitat, taxa and feeding mode., 715: 136999.
Yao, P., Zhou, B., Lu, Y., Yin, Y., Zong, Y., Chen, M. T.,., 2019. A review of microplastics in sediments: Spatial and tem- poral occurrences, biological effects, and analytic methods., 519: 274-281.
Zhang, L., Liu, J., Xie, Y., Zhong, S., Yang, B., Lu, D.,., 2020a. Distribution of microplastics in surface water and se- diments of Qin River in Beibu Gulf, China., 708: 135176.
Zhang, L., Zhang, S., Guo, J., Yu, K., Wang, Y., and Li, R., 2020b. Dynamic distribution of microplastics in mangrove sediments in Beibu Gulf, South China: Implications of tidal current ve- locity and tidal range., 399: 122849.
Zhang, S., Sun, Y., Liu, B., and Li, R., 2021a. Full size micro- plastics in crab and fish collected from the mangrove wetland of Beibu Gulf: Evidences from Raman Tweezers (1–20μm) and spectroscopy (20–5000μm)., 759: 143504.
Zhang, T., Sun, Y., Song, K., Du, W., Huang, W., Gu, Z.,., 2021b. Microplastics in different tissues of wild crabs at three important fishing grounds in China., 271: 129479.
Zhao, S., Zhu, L., Wang, T., and Li, D., 2014. Suspended micro- plastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution., 86: 562-568.
Zhou, Q., Tu, C., Fu, C., Li, Y., Zhang, H., Xiong, K.,., 2020. Characteristics and distribution of microplastics in the coastal mangrove sediments of China., 703: 134807.
Zhu, D., Bi, Q. F., Xiang, Q., Chen, Q. L., Christie, P., Ke, X.,., 2018. Trophic predator-prey relationships promote trans- port of microplastics compared with the singleand., 235: 150-154.
Zhu, J., Zhang, Q., Li, Y., Tan, S., Kang, Z., Yu, X.,., 2019. Microplastic pollution in the Maowei Sea, a typical maricul- ture bay of China., 658: 62- 68.
Zhu, X., Qiang, L., Shi, H., and Cheng, J., 2020. Bioaccumula- tion of microplastics and itsinteractions with trace me- tals in edible oysters., 154: 111079.
J. Ocean Univ. China(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5163-3
ISSN 1672-5182, 2022 21 (3): 521-530
Corresponding authors. E-mail: bhsgche@cityu.edu.hk E-mail: kityuekwan@bbgu.edu.cn
(August 16, 2021;
October 15, 2021;
December 14, 2021)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe Crab Tachypleus tridentatusEggs
- Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
- Experimental Investigation on the Interactions Between Dam-Break Flow and a Floating Box
- Variational Solution of Coral Reef Stability Due to Horizontal Wave Loading
- Influence of Autonomous Sailboat Dual-Wing Sail Interaction on Lift Coefficients
- Nonlinear Dynamic Analysis and Fatigue Study of Steep Wave Risers Under Irregular Loads
