Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe Crab Tachypleus tridentatusEggs
ITAYA Shinji, SHUUNO Mari, ONIKURA Norio, TAI Akira, and YANO Shinichiro
Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe CrabEggs
ITAYA Shinji1), 2), *, SHUUNO Mari2), ONIKURA Norio3), TAI Akira1), and YANO Shinichiro1)
1),,,,819-0395,2),811-3304,3),,,,819-0395,
Physical factors affecting the survival ofeggs were investigated by translocating their eggs between the high intertidal zone and the low intertidal zone of a known spawning site. The mean egg survival rates per day were highest in the mid intertidal zone (45.1%±25.4%) and the lowest in the low intertidal zone (13.3%±27.6%). Differences in the elevation, air exposure time, and water content of the spawning ground were significant factors determining the egg survival rates. Excessive or insufficient air exposure time resulted in inadequate water content at higher and lower intertidal zones and could reduce egg survival. On the other hand, moderate saturation and dehydration were repeated with each tidal movement in the mid intertidal zone. This dynamic is considered as one of the crucial factors for the survival of eggs and is considered optimal for spawning. Therefore, the protection of the mid intertidal zone is imperative for maximizing the egg survival rate in Tsuyazaki Cove where almost all suitable nesting sites have disappeared due to coastal development. By protecting these optimal sites for spawning and recovering other optimal sites on suitable beaches, a positive contribution can be made to future management and conservation. The study also suggests that translocating eggs from marginal to optimal spawning sites might be a recovery strategy for this globally endangered species.
air exposure time; globally endangered species; intertidal elevation; intertidal zone; optimal spawning site;Tachypleus tri- dentatus; translocated horseshoe crab eggs; Tsuyazaki Cove
1 Introduction
Horseshoe crabs are a group of chelicerate arthropods known as ‘living fossils’ because their morphological cha-racteristics have hardly changed for about 200 million years. They are important marine organisms in discussing the his-tory of biological evolution (Rudkin and Young, 2009). Four species of horseshoe crabs exist in the world, namely, the tri-spine horseshoe crab (), the At- lantic horseshoe crab (), the coastal horseshoe crab (), and the mangrove horse- shoe crab (). Horseshoe crab eggs play an important role in coastal ecosystems as food resources for migratory birds (Botton and Shuster, 2003; Botton, 2009). In addition, horseshoe crab blood is the only natural resource that can rapidly detect bacterial en- dotoxins and therefore has been attracting attention in re- cent developments of new coronovirus vaccines (Witten- berg, 2021). Thus, horseshoe crabs are not only biological- ly valuable but also medically and commercially impor- tant. However, many horseshoe crabs are currently decli- ning worldwide due to overfishing and coastal develop- ment (Akbar., 2018; Laurie., 2019).
There is only one species of the horseshoe crab, the tri- spine horseshoe crab (), in Japan, and Japan is the northern limit of the distribution of this species (Laurie., 2019). The species has various ha- bitat types throughout its life history in coastal areas, such as sandy mudflats for juveniles and sandy beaches in calmsemi-enclosed bays for spawning (Sekiguchi, 1999). There- fore,is recognized as an indicator of a heal- thy coastal environment (Hsieh and Chen, 2009).
was once widely distributed in the Seto inland sea and the northern part of Kyushu. How- ever, almost all populations have disappeared due to an- thropogenic activities such as landfill projects, and present- ly it inhabits only limited areas in Japan (Sekiguchi, 1999).Thusis designated as critically endangered in Japan (Ito, 2014). It has also been listed as a critically endangered species on the Red List of the International Union for Conservation of Nature (Laurie., 2019). Thus,is facing extinction both in Japan and the whole world, and it is imperative to undertake strate- gic research and take practical measures regarding the con- servation of this species.
Conservation and restoration of the sandy beach areas of the spawning grounds of T. tridentatus are one of the most important issues for the survival of this species, whose population is declining significantly (Hsieh and Chen, 2009;Wada., 2010; Itaya., 2019). T. tridentatus lay theireggs only on the calm sandy beaches of inner bays. The sp- ecies spawns in the intertidal zone between the extreme high tide line (EHTL) and the mean high water neaps (MHWN)(Sekiguchi, 1999). However, the species that have lost suit- able spawning sites due to coastal development may layeggs at lower elevations than the MHWN (Sekiguchi, 1999). In our study site, Tsuyazaki Cove, Japan, the number of T. tridentatus breeding pairs has decreased remarkably. One of the causes is the decrease of sandy areas suitable for spawning due to coastal development (Itaya., 2019). Nesting at lower elevations than the MHWN was also found in our study site (Itaya., 2020). Some studies point out that the survival rate of eggs in such an unfavourable environment is low (Penn and Brockman, 1994; Vasquez., 2015). On the other hand, there are no field-based ex- periments on the effects of different elevations from high to low tide levels on T. tridentatus eggs. Therefore, thereis a lack of quantitative data on the causal relationships be- tween egg survival and coastal development impacts forthe restoration and conservation of T. tridentatus spawn- ing grounds.
In this study, we translocated T. tridentatus eggs at dif- ferent intertidal elevations. Then, the relationship between the egg survival rate and environmental characteristics was identified to find out the most suitable nesting area within the intertidal zone. The conservation measures for the spe-cies which have lost most of the optimal spawning groundsdue to coastal development was suggested based on the findings.
2. Methods and Materials
2.1 Study Site
This study was conducted at Tsuyazaki Cove (33˚47΄N, 130˚27΄E) in Fukuoka, Japan (Fig.1a). Tsuyazaki Cove wasonce a vast inner bay and salt marsh, but the landfilling for salt farming began in the Edo period, and most of it was reclaimed (Hirowatari and Shimoyama, 1999). Today, the remnants of the salt farms and agricultural areas form the eastern hinterland of the cove, and the tiny mountainous coast is located on the west side of the cove. Tsuyazaki Cove is a lagoon-like inland sea. On the north side of the cove, a mudflat, which is a habitat forjuve- niles, has formed. There are no large rivers flowing intothis cove, and therefore it is considered that the cove is uni- que as a T. tridentatus habitat and that it is hardly affected by freshwater (Itaya., 2019). Spawning of T. tridenta- tus has been found in small sandy areas along Tsuyazaki Cove, of which about 70% of spawning was concentrated around Tsuyazaki Bridge (Wada., 2010). In Tsuyaza- ki Cove, the spawning ground has disappeared significant-ly due to coastal development after World War II, which is one of the factors for the rapid decrease in the number of breeding pairs. This intensive development has removed al- most all optimal sandy intertidal spawning grounds in Tsu- yazaki Cove (Itaya., 2019).
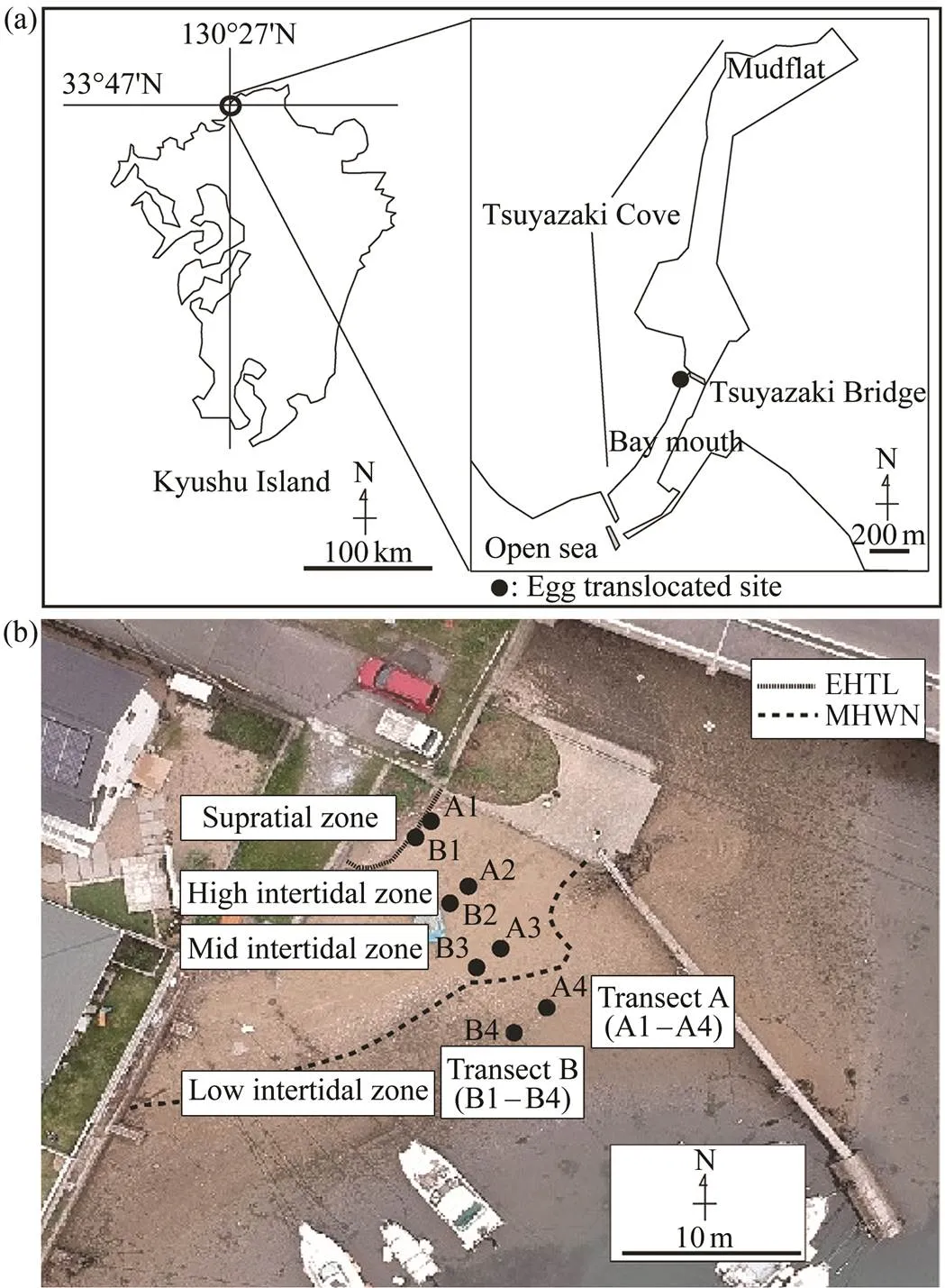
Fig.1 (a) Location of the study site in Tsuyazaki, Fukuoka, Japan. The dots indicate the sites whereTachypleus triden- tatuseggs were translocated in this study. (b) Translocation site ofTachypleus tridentatuseggs in Tsuyazaki, Fukuoka, Japan. The extreme high tide line (EHTL) and the mean highwaterneaps (MHWN) are shown in the broken lines. The dots indicate the translocation points (A1–A4 and B1–B4).
2.2 Egg Translocation Experiment and Analysis
We translocated T. tridentatus eggs to test the hypothe- sis that the egg survival rate is affected by the physical pa- rameters at different elevations of the intertidal zone with- in the spawning ground. The high tide and the low tide zones in the translocation site were decided as follows. First, the site’s elevation and latitude/longitude data were obtain- ed using the Real-Time Kinematic-Global Navigation Sa- tellite System (RTK-GNSS: Trimble R4 GNSS, Nikon- Trimble Co., Ltd.). Then these data were taken into Arc- GIS (ArcMap version 10.8, ESRI Japan Co., Ltd.) to createa digital elevation map of the site. Next, the EHTL and the MHWN were calculated from the local tide measured us- ing a portable water depth meter (COMPACT-TD ATD- HR, JFE Advantech Co., Ltd.). Finally, the position of the high intertidal zone to the low intertidal zone within the translocation site was determined by overlaying EHTL/MHWN and the digital map. In addition, to confirm theconsistency of the elevation map, aerial photographs of theEHTL and the MHWN at the translocation site were taken using a drone (Spark, Dji Co., Ltd.).
spawning activities take place at high tide during summer. A female crab digs holes in a sandy beach and lays eggs in the holes. Then a male crab releases sperm there to fertilize the eggs (Sekiguchi, 1999). The eggs laid by the same breeding pair on 3 July 2019 on the same beach in our study site were collected for translocation on 4 July 2019. Sincehas external fertilization, we as- sumed that fertilized eggs were distributed homogeneous- ly in all translocated eggs.Two transect lines, transect A (A1 to A4) and transect B (B1 to B4), were placed on the sandy area of the translocation site. Four translocation points (A1 to A4, B1 to B4) were set on each transect line. The translocation points were located in the high intertidal zone (near the EHTL), upper-mid intertidal zone (between the EHTL and MHWN), lower mid intertidal zone (near theMHWN), and low intertidal zone (below the MHWN). To- tally 100 eggs were translocated at each point of 8 locations (Fig.1b). For translocating eggs, we used a plastic hydro- ponic mesh pot (600mL) with a nylon net (mesh size 1mm) spread out inside the pot to prevent egg loss and hu- man disturbance as there are houses nearby, and many peo-ple use the site. The translocated eggs were placed in the pots together with the sand collected from the study site. The eggs were slightly separated from each other to pre- vent infection within the pot (Fig.2). Then, the hydropo- nic pots were buried 15cm below the surface of each trans- location point on the transects. The translocated eggs were buried at a depth at which horseshoe crabs lay eggs in the wild, as Maeda. (2000) and Chen. (2004) de-scribed.
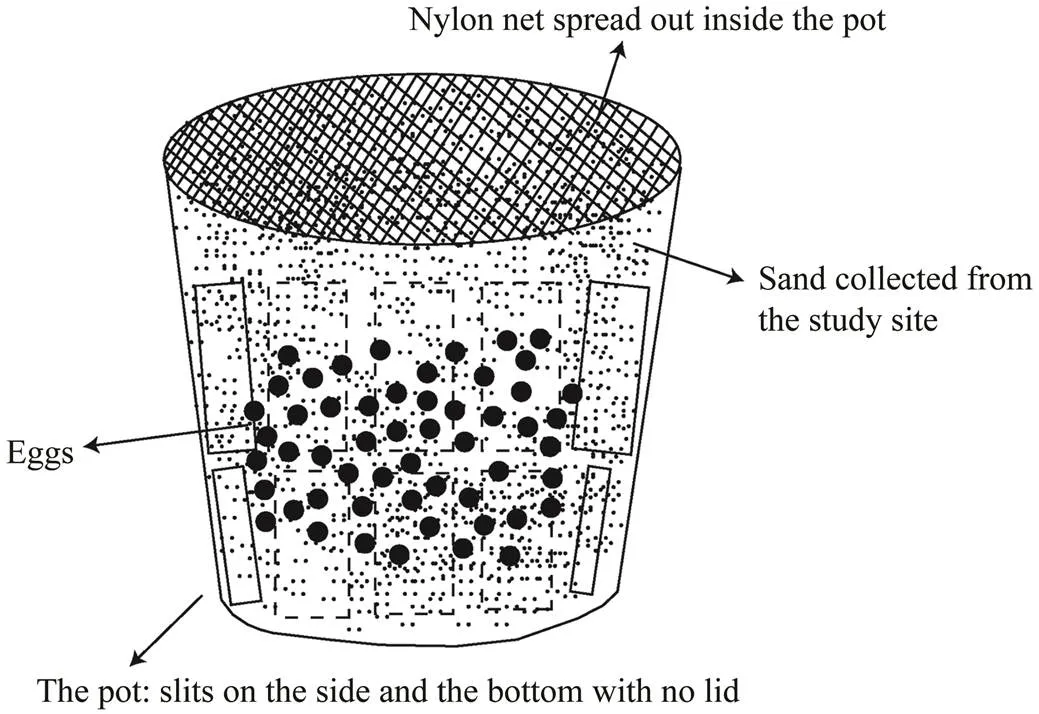
Fig.2 A diagram of a hydroponic mesh pot (600mL) used for translocating the eggs. A nylon net (mesh size 1mm) was placed inside the pot to prevent egg loss. Totally 100 horseshoe crab eggs were placed in each pot together with local sand, and they were buried 15cm below the ground surface, which is the depth at which the horseshoe crab spawns in the wild.
T. tridentatus eggs become rotating eggs as they approachhatching (Sekiguchi, 1999). After the stage of rotating eggs, the dispersion of the hatched larvae occurs, making it dif- ficult to count the surviving eggs accurately. Therefore, we observed the eggs during the period from eggs (stage 1) to late embryos or rotating eggs (stage 21) based on the classification by Sekiguchi (1990). There are some detail- ed reports on the development of T. tridentatus eggs in cap- tivity (Sugita and Sekiguchi, 1981; Sugita., 1985; Se- kiguchi, 1999). However, unlike in captivity, it was im- possible to observe the detailed developmental stages of horseshoe crab eggs in the field. Therefore, we did not ca- tegorize them; instead, we determined whether the eggs were alive or dead. Infected, decayed, and broken eggs were ju- dged as dead eggs. During the inspection, the eggs at each point were quickly transferred to a plastic tray full of sea- water to determine how many eggs were dead or alive. Af- ter counting the surviving eggs, we took their photographs and buried them in their original locations. Of the two tran- sect lines, we inspected transect B at about two weeks in- tervals until August 21, 2019 to observe the survival sta- tus of the eggs. For transect A, we counted the final survi- ving eggs without digging until August 21, 2019.
The survival rate of the translocated eggs was calculated as
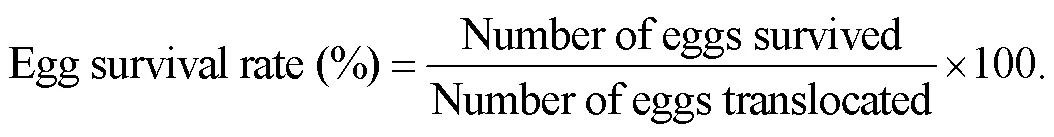
Since each hydroponic pot contained 100 eggs, the survi- val rate of eggs was equal to the number of surviving eggs. The survival rate of eggs at each translocation site during the experimental period was analyzed using only transect B. For the comparison of the egg survival rates at each translocation point from B1 to B4, the mean survival rate per day was calculated after interpolating the data using a linear regression approximation formula. A significant test of the mean survival of eggs per day between the translo- cation points was performed by one-way ANOVA. When<0.01, the difference was considered significant. If a sig- nificant difference was evident, then the Tukey-Kramer pro- cedure was used to examine where the significant differ- ence lay.
2.3 Measurement and Analysis ofPhysical Parameters
To help understand the relationship between egg viabi- lity and the physical characteristics of each of the translo- cation points, salinity, nest temperature, air exposure time, water content, and sediment types were measured and ana- lyzed. We measured seawater near the translocation site forsalinity every 30min over the study period (=2001 for each point) with an underwater electrical conductivity mea- surement data logger (HOBO U24, Onset Co., Ltd.). The nest temperature was measured every 30min over the study period (=2065 for each point) by burying an underwater temperature measurement data logger (HOBO U24, Onset Co., Ltd.) 15cm below the ground surface at each trans- location point.The air exposure time of the translocation points was estimated from the differences in the elevation of each point and the tide levels for all the time zones of the study period (=42 for each point). For the water con-tent, we selected the flood tide from August 17 to 18, 2019, and collected about 100g of soil that is 15cm below theground surface at each translocation point every hour. Then, the water content ratio in the soil was calculated as the dif- ference between wet weight and dry weight
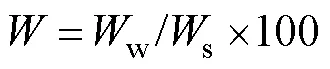
whereis water content,wis weight of water contain- ed in soil,sis weight of dried soil. Soil samplings were not possible when the sand was immersed about 10cm or more below the surface water because the soil mixed with seawater. Therefore, the soil samples for the water content were collected during low tides (=25 at B1,=21 at B2,=13 at B3,=7 at B4, respectively). About 100g of soil 15cm below the ground surface was collected at each trans- location point for the grain size analysis. We used seven types of sieves for the grain size analysis with a mesh size of 0.064 to 4mm. Dried soil samples were passed through each sieve, and the grain size composition was determin- ed from the weight of the particles remaining on each sieve and the ratio of the total weight. In addition, as represen- tative values of the sediment at each translocation point, the median diameter and the grain size-sorting were calcu- lated from the grain size distribution, and the silt-clay con- tent was calculated from the ratio of silt-clay to the totalweight. The display of physical data corresponding to each translocation point is described as B1 to B4 in this paper (Fig.1b).
2.4 Analysis of the Relationship Between Egg Viability and Physical Parameters
Generalized linear models (GLMs) were applied to find out the relationship between the egg survival rate per day and the physical parameters using R version 3.4.4. In thesemodels, we utilized the elevation, the mean salinity, the mean nest temperature, the mean air exposure time, the mean water content, the median diameter, the grain size- sorting and the silt-clay content as dependent variables (bi-nomial distribution) and the mean egg survival rate per day as a predictor variable. The square value of each physical parameter was also used as a predictor variable (ordinary least squares). The models with a significant-value (<0.01) were regarded as robust models, and thus these se- lected physical parameters were employed to estimate the mean egg survival rate per day.
3 Results
3.1 Egg Survival Rate
Rotating eggs were found only in the mid intertidal zone at both transects A and B (Fig.3). On transect A, the eggs in the mid intertidal zone became rotating eggs 49 days af- ter the translocation on August 21, 2019, but the survival rate was only 1%. On the other hand, 10% of eggs sur- vived as rotating eggs in the mid intertidal zone on tran- sect B. Although rotating eggs were confirmed on transect A, there was not enough data to analyze. Therefore, we only used the transect B data for analysis as described be- low.
On transect B, rotating eggs were found only in B3, the mid intertidal zone. These rotating eggs were first confirm- ed 44 days (August 16, 2019) after the translocation. By the 49th day (August 21, 2019), 10% of the rotating eggs in B3 were still alive. The lowest survival rate was found in the low intertidal zone (B4), where all eggs died within13 days after the translocation (July 16, 2019). In other in- tertidal elevations, more than 50% of eggs survived until day 13 (July 16, 2019), but in the high intertidal zone (B1) eggs had died out by day 28 (July 31, 2019). In the midintertidal zone, the survival rate of eggs on the 28th day(July 31, 2019) was 18% for B2 and 40% for B3. How- ever, on the 44th day (August 16, 2019), the eggs all died in B2and only survived in B3 (Fig.4a).
The mean survival rate of eggs per day was the highest in the mid intertidal zone and the lowest in the low inter- tidal zone (27.8%±33.9% at B1, 34.1%±30.8% at B2, 45.1%±25.4% at B3, 13.3%±27.6% at B4). A significant difference in the mean survival rate of eggs per day amongthe translocation points was demonstrated by one-way ANOVA (=9.87;=3192;=4.36×10−6). Multiple com- parisons based on the Tukey-Kramer test revealed that the survival rate of eggs was significantly lower in the low tide zone (<0.01) (Fig.4b).
3.2 Physical Parameters
We investigated air exposure time, water content, sali- nity, nest temperature and sediment to understand the re- lationship between egg viability and the physical charac- teristics of the translocation site. The results are described below. The comparison of physical parameters in this study and the previous studies is shown in Table 1.
The high intertidal zone (B1) was exposed to the air for almost 24h, whereas the low intertidal zone (B4) was sub- merged for almost 24h (Table 1). The mean water content was low at higher elevations and it was high at lower ele- vations (Table 1). The mean salinity of seawater near the translocation site was 28.5±3.7 (minimum 2.8, maximum 31.5,=2001) (Table 1). The mean nest temperature was the highest in the high intertidal zone and the lowest in the low intertidal zone (Table 1). The sediment characteristics at the translocation site are shown in Table 1. For the grain size composition, very fine sand accounted for about 90% at B1 and B2. At B3 and B4, medium sand and very fine sand each accounted for about 50%. Thus, all tranlocation points were composed of sand. The median diameters were 0.17mm for B1 and B2, and 0.26mm for B3 and B4, re- spectively. The grain size sorting was from=0.45 (B1) to 0.76 (B4). The silt-clay content was from 0.09% (B1) to 0.31% (B4).
3.3 Relationship Between Egg Survival Rate and Physical Parameters
The results of analyses of the mean egg survival rate per day and the physical parameters using generalized linear models (GLMs) are shown in Table 2. The follow- ing parameters significantly influenced the mean survival rate of the eggs: the elevation, the mean air exposure time, the mean water content, the mean nest temperatures and the grain size-sorting, respectively (Table 2). Of these fac- tors, the nest temperatures and the grain size-sorting were excluded from further analysis as these parameters were within the known range ofspawning grounds (Table 1). Thus, we selected elevation, air exposure time, and water content to predict the egg survival rate.

Fig.3 Egg survival status over the study period. (a) All eggs were dead in the low intertidal zone at transect B on July 16, 2019. (b) On July 31, 2019, no live eggs were found in the high intertidal zone at transect B, and reddish eggs were found in the high and mid intertidal zones. (c) On August 16, 2019, all eggs died in the upper mid intertidal zones, but the eggs in the lower mid intertidal zones became rotating eggs at transect B. (d) At transect B, in which the eggs were inspected regularly, 10% survived as rotating eggs on August 21, 2019. (e) On August 21, 2019, rotating eggs were found only in the mid intertidal zone at transect A, which was not inspected regularly. The survival rate in the mid intertidal zone at transect A was 1%.
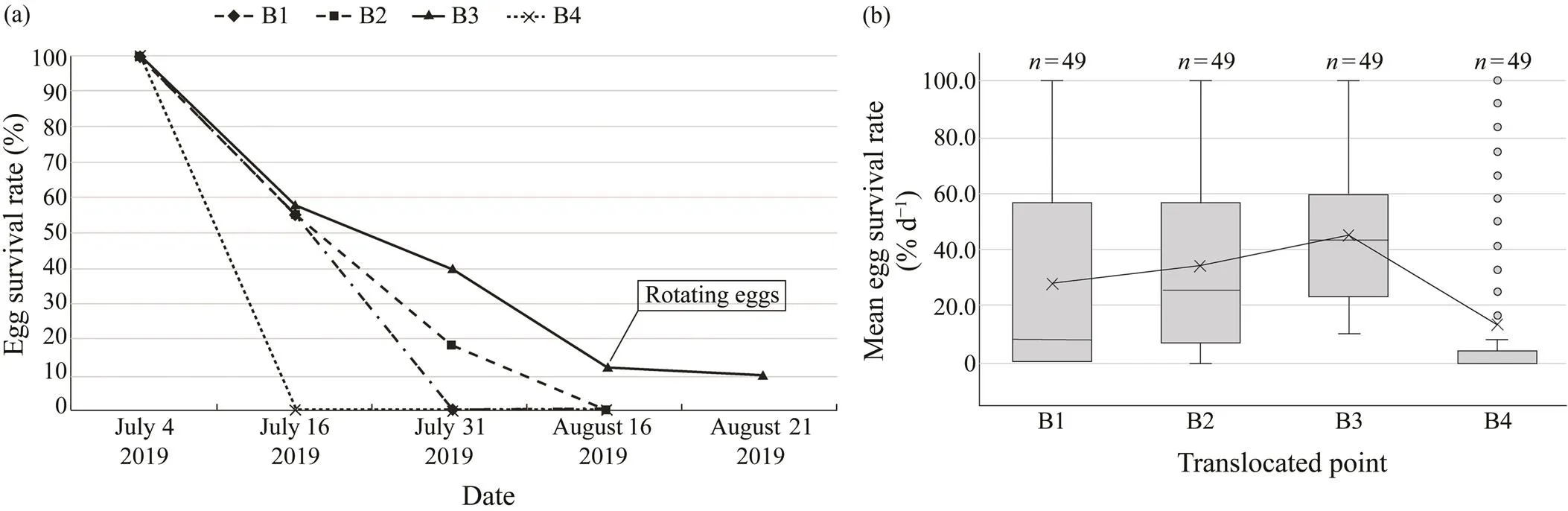
Fig.4 (a) Changes in the survival rate of translocated eggs at transect B over the study period. (b) Comparison of mean egg survival rates per day at transect B over the study period.
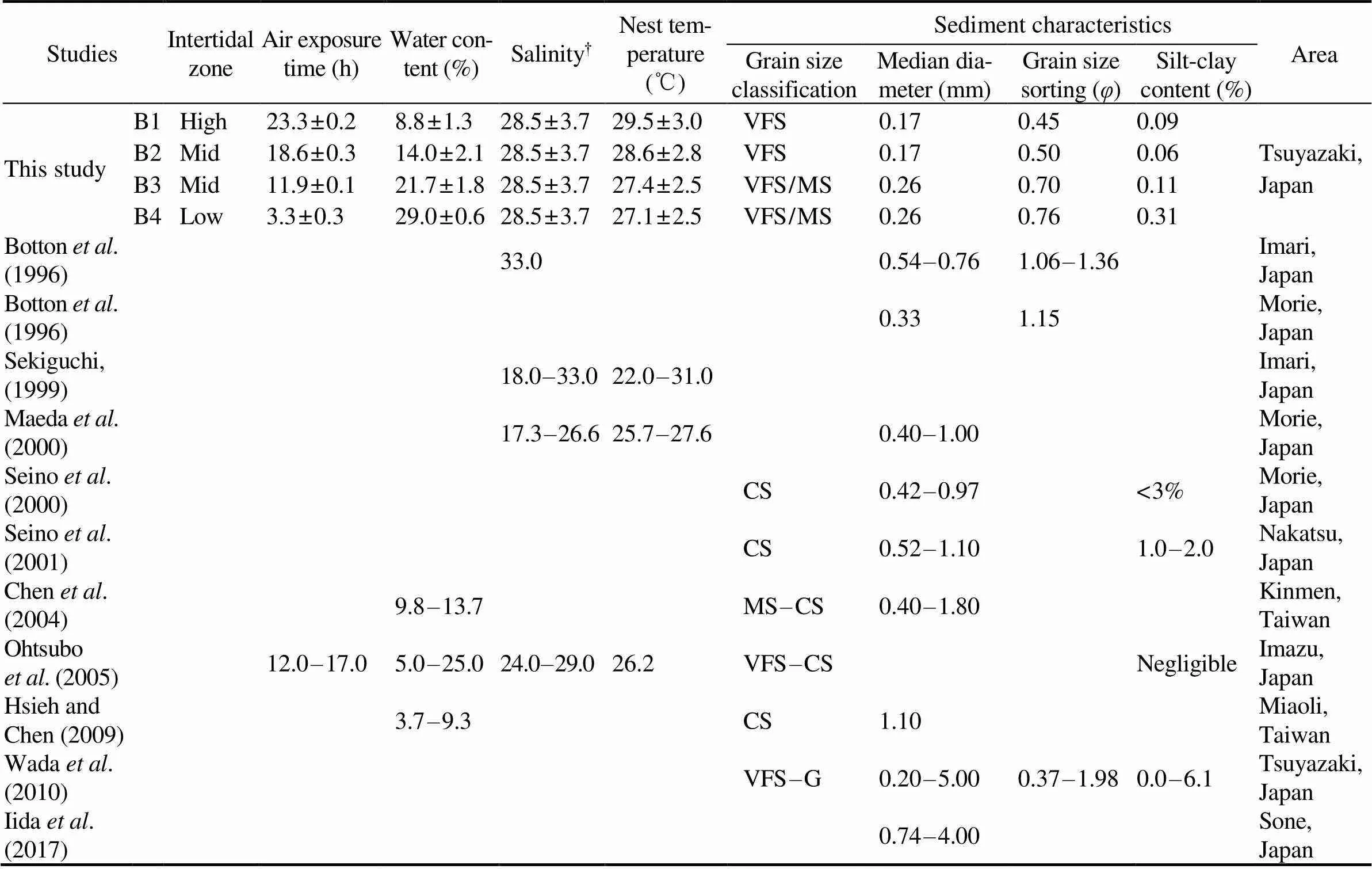
Table 1 Comparison of the physical parameters of T. tridentatus spawning grounds in this study and previous studies
Notes: B1 to B4 are the egg translocation points in this study.†Seawater salinity near the survey site. G, granule; CS, coarse sand; MS, medium sand; VFS, very fine sand. The blank spaces indicate no data available.
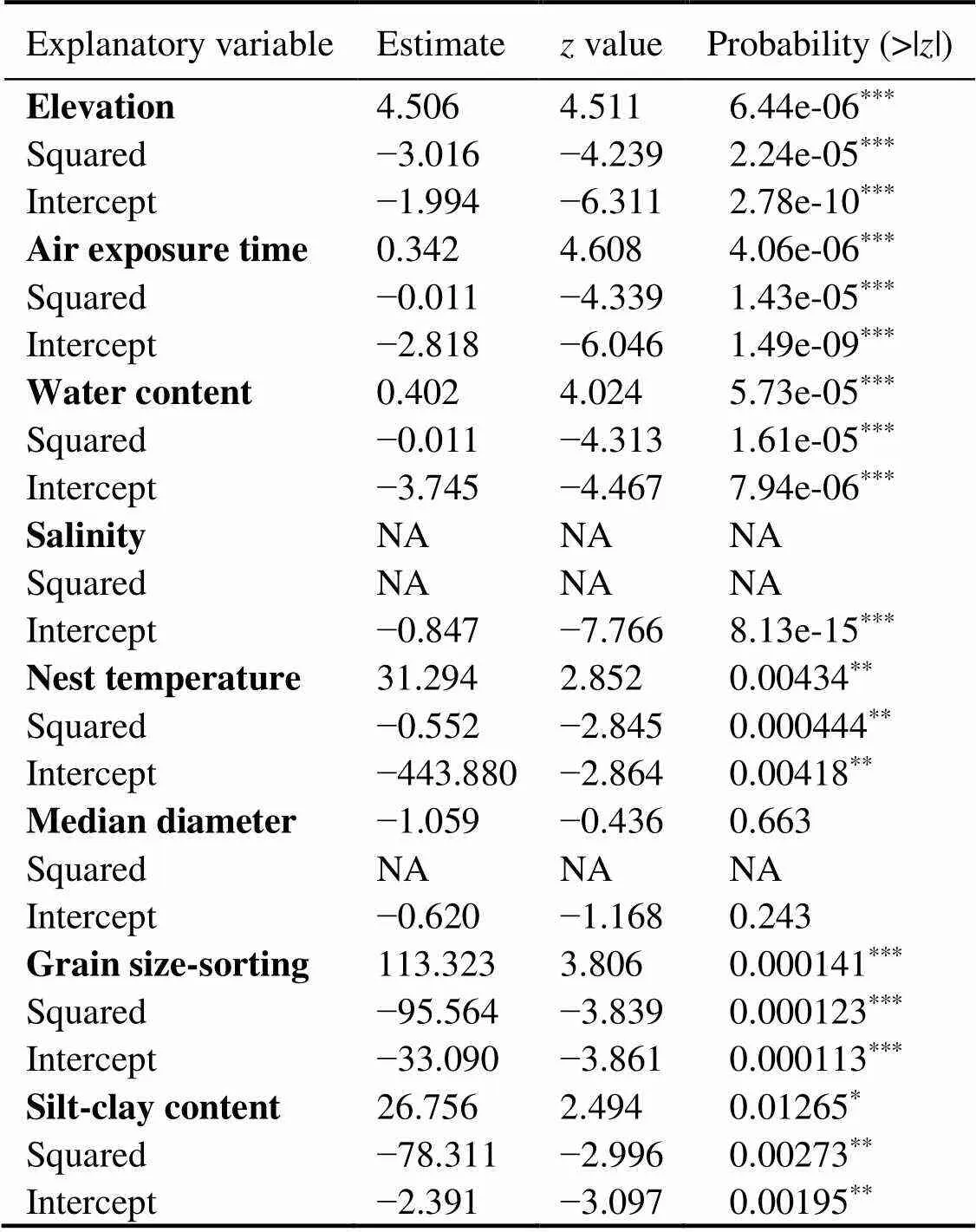
Table 2 GML analysis results for the relationship between mean egg survival per day and physical parameters
Notes: NA, not available.***<0.001,**<0.01,*<0.05.
The change in the predicted values of the mean egg sur- vival rate per day with elevation, air exposure time, and water content are shown in Fig.5. Of all these three phy- sical factors, the estimated egg survival rate per day was the highest in the mid intertidal zone (B2 and B3) and the lowest in the low intertidal zone (B4) (Fig.5).
4 Discussion
4.1 Egg Survival Rate
The eggs translocated at the mid intertidal zone became rotating eggs (stage 21) after 44days (August 16, 2019) at 27.40℃±2.52℃ and salinity of 28.5±3.6 (Table 1, Fig.4a). Our results showed a similar development rate as a labo- ratory experiment conducted by Sekiguchi (1999). In his ex- periment, the captive eggs reached stage 21 in 43 days at 30℃ and salinity of 20 to 35 (Sekiguchi, 1999). It is thought that egg development proceeds at the same rate in the field as in captivity when conditions such as temperature andsalinity are similar (Maeda., 2000). The ‘wild eggs’ that were laid at the mid intertidal zone on the same beach, where our experiment was conducted, also became rotatingeggs during the same period. It was observed that the ‘wild eggs’ were laid on July 2, 2019 and became rotating eggs on August 16, 2019. Thus, our experiment may reflect the average development rate of. tridentatus eggs.
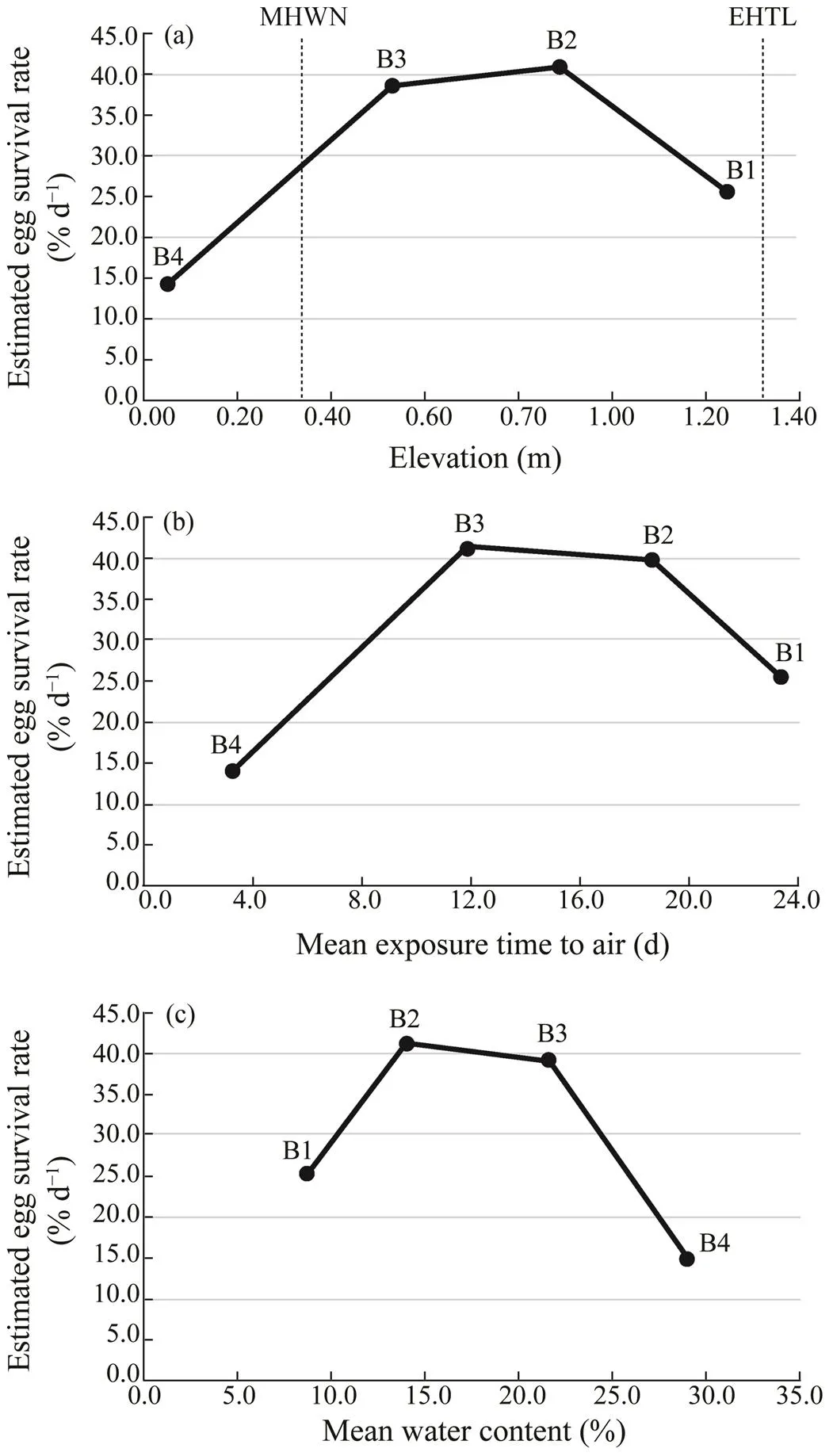
Fig.5 Change in estimated mean egg survival rate per day with significant physical parameters at the translocation site. (a), change in mean egg survival rate per day with differ- ent elevations;(b), change in mean egg survival rate per daywith different mean air exposure time per day;(c), change in mean egg survival rate per day with different mean wa- ter contents.
The translocated eggs had the highest survival rate in the mid intertidal zone. Survival of eggs decreased at higher and lower elevations, especially ‘inferior’ in the low inter- tidal zone (Figs.3 and 4). Ideally speaking, more replica- tions should have been prepared for the experiment. How- ever, we had difficulty preparing enough eggs for the ex- periment because of the rapid decline of T. tridentatus and thus a minimal number of breeding pairs came to our study site (Itaya., 2019). Therefore, care should be taken in interpreting the results of the egg survival rate as sufficient statistical analysis could not be performed with the small number of replications.A follow-up experiment with more replications of translocated eggs is necessary to verify the current findings. Nevertheless, we can still conclude that there were differences in egg survival rate at different ele-vation points (Fig.4). In addition, the GLM modeling show- ed that elevation from high tide to low tide was an impor- tant factor in determining egg survival rate (Fig.5a), which is worthy with more attentions. The egg survival rate was the highest in the mid intertidal zone (B2 and B3). Of theseeggs, 10% of B3 eggs finally developed to rotating eggs (Fig.4a). There is almost no field-based research on the survival rate ofeggs in the wild. In Taiwan of China, Hsieh and Chen (2009) found that the hatching rate ranged from 0 to 88.5%, with an average of 33.9%, and a decline in hatching rates occurred in places where the ti- dal amplitude was small. In the experiment of Hsieh and Chen (2009), there were three spawning areas, A, B, and C on the same beach, but the above survival rate was cal- culated for only 4 nests in area A where hatching was con- firmed (the reason for the exclusion of areas B and C is unknown). However, if we included areas B and C where hatching was not confirmed, the total number of nests would be 12. Then, the average survival rate of 33.9% found by Hsieh and Chen (2009) could be divided by12 nests in- stead of 4 nests, and then the survival rate would be about 11%. The egg survival rate in the mid intertidal zone (B3) of this study was 10% (Fig.4a), which is almost the same as their conversion value. In addition, when we consider all of the translocated eggs in transect B, the number of sur- viving eggs is 10 out of 400 eggs, the survival rate is 2.5%. In summary, the range of egg survival rate in our study is from 2.5% (minimum) to 10% (maximum), which is not essentially different from the example reported by Hsieh and Chen (2009). Therefore, it is assumed that the survi- val rate ofeggs obtained in this study may reflect the survival rate in the wild.
On the other hand, we found some reddish eggs show- ing bacterial infection in our translocated eggs (Fig.3). Se- kiguchi (1999) removed infected eggs every time in his la-boratory experiment. However, removing the infected eggs in the hydroponic pots was not performed to reduce human influence as much as possible. It is more likely that infec- tion can spread quickly within the closed environment of the hydroponic pots. Thus, this may have led to a decrease in the survival rate of the translocated eggs.
There are several reports on the spawning location of horseshoe crabs within the intertidal zone. 97% of T. tri- dentatus spawning was found between the EHTL and the MHWN in Imari, Japan, but most were concentrated near the mid intertidal zone (Sekiguchi, 1999). In Malaysia as well, more than 50% of T. tridentatus spawning was con- centrated in the mid intertidal zone (Mohamad., 2019). The Atlantic horseshoe crab (Limulus polyphemus) laid theireggs between the high intertidal and the mid intertidal zones. Increased egg development was evident in both zones but decreased in the low intertidal zone (Vasquez., 2015). Another study pointed out that L. polyphemus spawned in the mid intertidal zone which maximized their egg deve- lopment rate by avoiding the dry and hot environment in the high intertidal zone and the oxygen-deficient conditionin the low intertidal zone (Penn and Brockman, 1994). Our results were consistent with these previous studies. The translocated eggs had the highest survival rate in the mid intertidal zone (Figs.4 and 5). Although no other research was found on investigating. tridentatusegg developmentat different elevations from high tide to low tide, it is thought that T. tridentatus selectthe mid intertidal elevations of the intertidal gradient for spawning.
4.2 Physical Parameters Contributing to Egg Survival Rate
Sincespawning occurs near the MHWN, the total time the eggs are submerged is relatively short. The air exposure time of eggs at the translocated site was long- er at higher elevations of the intertidal zone. Consequent- ly, the water content was lower at higher elevations and higher at lower elevations (Table 1).However, due to the water storage capacity of the sandy beach and the waterabsorptive function of the egg itself, seawater is retained in the egg even when the beach is exposed to air (Sekiguchi,1999). Penn and Brockman (1994) found that the water con- tent of sand is one of the factors that influences egg deve- lopment in horseshoe crabs. In this study, the water con- tent of the eggs at each translocated point was found to be determined by the tidal movement and the degree of eleva- tion of the intertidal zone.
In the mid intertidal zone (B2, B3), where egg survival was higher, the mean air exposure time was 11.9 to 18.6h per day (Table 1). Although very few studies quantified the air exposure time of spawning grounds, it was 12.0 to 17.0h per day in Imazu, Japan (Otsubo., 2005), which is almost the same as the results of this study (Table 1). On the other hand, in the high intertidal zone (B1) and the lowintertidal zone (B4) where egg survival was relatively poor, B1 was exposed to air for almost 24h and B4 was flooded almost all day (Table 1).
In this study, the egg survival rate was the highest in the mid intertidal zone (B2 and B3) and the lowest in the low intertidal zone (B4) (Fig.4). This trend was also evident in the GLM models, which showed that the air exposure time and water content contributed significantly to the egg sur- vival rate (Table 2; Figs.5b and c). Otsubo. (2005) po-inted out that the low intertidal zone is not suitable for spawning due to the amount of dissolved oxygen in the porewater is low in moist sand. A sandy beach takes in abun-dant oxygen at low tide when exposed to the air. Then, whenit immerses in seawater at high tide, the oxygen in the sand dissolves into the seawater and finally is absorbed by the eggs. Therefore, T. tridentatus eggs develop better when the seawater is completely removed at a certain frequency thanwhen they are constantly immersed in seawater (Sekiguchi,1999). Thus, the low egg survival rate in the low intertidalzone can be explained by the lack of air exposure time, re- sulting in excessive water content (Table 1), which causes insufficient oxygen for the eggs.
The water content in the high intertidal zone (B1) in thisstudy was within the range of other studies (Table 1). How- ever, in the high intertidal zone, the water content tempo- rarily increased during high tides but remained low over the other tidal periods. Furthermore, while other translo- cated points were submerged into seawater at least once a day, there were three times when the high intertidal zone was not submerged for more than a week during the study period. It accounted for 62% of the total time when the high intertidal zone was not immersed in seawater. In a la- boratory experiment with the coastal horseshoe crab(gigas), Faizul. (2013) showed that eggs in sand develop well when immersed in seawater at a fre- quency of 1 to 3 days. It was suggested that when the eggs are not submerged in seawater for more than a week, desi-ccation may cause a poor egg development rate (Faizul., 2013). Therefore, it is possible that dissolved oxygendid not reach the eggs sufficiently in the high intertidel zoneas the immersion time and its frequency were extremely low in this study.
Significantly, the mid intertidal zone (B2 and B3) was covered in seawater at a moderate frequency and it was ex- posed to air for about 30% to 50% each day. This, in turn, would have made it possible for a sufficient supply of oxy- gen for egg development. Thus, the mid intertidal zone is considered the most suitable spawning zone for T. triden- tatus with respect to air exposure frequency and the water content.
4.3 Impact of Coastal Development on T. tridentatus Spawning Ground and Conservation
The results of this field-based study can be applied to the restoration and conservation of the spawning ground for thecriticallyendangered horseshoe crab where the suitable spawning grounds are limited because of coastal develop- ment. The study revealed that the egg survival rate in the low intertidal zone was meager (Figs.4 and 5). Some breed- ing pairs of the area laid eggs in the low intertidal zone,suggesting there was probably a shortage of suitable spawn- ing habitat (Itaya., 2020). Hence, the egg survival rate of some of the breeding pairs is insufficient to sustain a viable population of horseshoe crabs in Tsuyazaki Cove. It may be necessary to translocate eggs laid in the low in- tertidal zone to the mid intertidal zone to increase their sur- vival rate. However, as described below, the translocation of the eggs alone may not be sufficient for the recovery of the natural population in Tsuyazaki Cove.
The sand supply to suitable sandy spawning grounds has decreased due to the coastal development in Tsuyazaki Cove(Itaya., 2019). As a result, the number of breeding pairs has decreased yearly with this habitat deterioration. Over the last decade, more than 90% of breeding pairs havedisappeared from our study site due to the development. Currently, the number of spawning pairs coming to the beachevery year is only 4 to 10 pairs (Itaya., 2019). Thus, the spawning grounds at Tsuyazaki Cove have degraded over time, and natural recovery of the spawning grounds and thepopulation cannot be expected. Therefore it is urgent to pro- tect the remaining suitable spawning sites and restore those sites which are degraded. We believe it is urgent to secure the mid intertidal zone for maximizing the egg survival ratein Tsuyazaki Cove by protecting what remains of it and by recovering other suitable sites for spawning. It is even possible to design and build artificial spawning sites. For these practical conservation measures, further research studies will be necessary to estimate the remaining suit-able spawning grounds and those degraded ones. This is es- pecially important for the recovery strategies of specific spe- cies.
5 Conclusions
In this study, the relationship betweenegg survival and the physical characteristics of thespawning ground was revealed for the first time. It wasfound that the difference in elevation from high tide to lowtide was a key factor in determining the egg survival. Withmedium elevation, air exposure time, water content and se-diment types were all suitable for egg survival. Under such conditions, the duration of the beach exposed to air and the water content in the sand caused by the difference in ele- vation was essential for the eggs to survive, suggesting that the mid intertidal zone is the most suitable for spawning. In higher and lower intertidal zones, excessive or insuffi- cient air exposure time reduced egg survival. In the mid intertidal zone, moderate saturation and dehydration were repeated with each tidal movement and this dynamic is con- sidered as one of the crucial factors for the survival of the translocated eggs in Tsuyazaki Cove. Thus, the protection of mid intertidal zones as optimal spawning sites will con- tribute positively to the future management and conserva- tion of this globally endangered species. Moreover, trans- locating eggs to the optimal spawning sites from marginal sites can also be a recovery strategy.
Acknowledgements
We would like to express our gratitude to Dr. John A. Harris (former Head, School of Environmental Science, University of Canberra, Australia) for his suggestions du- ring his visit to our study site and for editing the draft of this paper. We would like to show our gratitude to Dr. Aki- hiko Koyama at the Fisheries Laboratory of the Graduate School of Kyushu University for his useful advice regard- ing this paper’s structure and data analyses. Mr. Katsuji Motojima of Towa Technology Co., Ltd. gave us many en- couragements. We would also like to thank Mr. Ayumu Sa- kurada, Mr. Yu Ono, Mr. Kazuya Maeda, Mr. Daiki Ma- suda of the Faculty of Engineering, Kyushu University, and Mr. Hiroyuki Oyama of the limited liability companyMorisho for their cooperation in the survey of this research.
Akbar, J. B.A., Nelson, B.R., Sheikh, H.I., Cheung,S. G.,War- diatno, Y.,Dash, B. P.,.,2018. A review on fisheries and conservation status of Asian horseshoe crabs., 27: 3573-3598, DOI: 10.1007/s10531-018-1633-8.
Botton, M. L., 2009.. Springer, New York, 45-63.
Botton, M. L., and Shuster, C. N., 2003.. Harvard University Press, Cambridge, 133-153.
Botton, M. L., Shuster, C.N., Sekiguchi, K., and Sugita, H., 1996.Amplexus and mating behavior in the Japanese horseshoe crab,., 13(1):151-159, DOI: 10.2108/zsj.13.151.
Chen, C. P., Yen, H., and Lin, P., 2004. Conservation of the horse- shoe crab at Kinmen, Taiwan: Strategies and practices., 13: 1889-1904, DOI: 10.1023/B:BIOC.0000035868.11083.84.
Faizul, M. I. M., Faizal, M. M., Christianus, A., and Amin, S. M. N., 2013. Incubation and hatching of(Mul- ler, 1785) eggs in sand and water media., 8: 333-340, DOI: 10.3923/ajava.2013.333.340.
Hirowatari, F., and Shimoyama, S., 1999.. Tsuyazaki, Fukuoka, 107-144 (in Japanese).
Hsieh, H., and Chen, C., 2009.. Springer, New York, 417-438.
Iida, K., Yonetani, M., Nakamura, R., Kondo, Y., Hayashi, O., Ta-kahashi, S.,., 2017. Density of juvenile of trispine horse- shoe crabin the Sone Estuary, Kita- kyushu, Japan with notes on sediment particle sizes in habi- tats and breeding areas., 15: 1-7, DOI: 10.15027/44128 (in Japanese).
Itaya, S., Seino, S., Shuuno, M., Sakurada, A., and Koshiguchi, R., 2020..Springer, Singapore, 959-963.
Itaya, S., Seino, S., Wada, T., and Shuuno, M., 2019. Rapid de- cline in the number of breeding pairs of Tachypleus tridenta- tus and the degradation of sandbanks for spawning due to cu- mulative bay mouth modifications at the Tsuyazaki Cove in Fukuoka, Japan., 24: 157-170,DOI: 10.18960/hozen.1817 (in Japanese with Eng-lish abstract).
Ito, T., 2014.https://ikilog.biodic.go.jp/rdbdata/files/envpdf/Arthropods_008.pdf, accessed on 24 April 2021(in Japanese).
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H., John, A.,., 2019.. https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T21309A149768986.en, accessed on 13 July 2021.
Maeda, K., Seino, S., Nishihara, S., and Hino, A., 2000. Ecology of hatched larvae of the horseshoe crab(Leach) in relation to the physical environment., 55: 12-24, DOI: 10.5179/benthos.55.15 (in Japanese with English abstract).
Mohamad, F. M., Sofa, M. F. A. M., Manca, A., Ismail, N., Cob, Z. C., and Ahmad, A. B., 2019. Nests placements and spawn- ing in the endangered horseshoe crab(Leach, 1819) (Merostomata: Xiphosurida: Limulidae) in Sabah,Malaysia.,39 (6): 695-702, DOI:10.1093/jcbiol/ruz070.
Ohtsubo, M., Ishida, H., Minei, H., Yamaoka, S., and Higashi, T., 2005. Physical and chemical properties of the nest-site beach of the horseshoe crab rehabilitated by sand placement. Japa- nese Society of Soil Physics,99: 55-63(in Japanese with Eng- lish abstract).
Penn, D., and Brockmann, H. J., 1994. Nest-site selection in the horseshoe crab,., 187: 373-384.
Rudkin, D. M., and Young, G. A., 2009.. Springer, New York, 25-44.
Seino, S., Shiozaki, M., Uda, T., Goto, T., Kuroki, T., and Naka-mura, T., 2001. Investigation of the habitat and spawning groundof horseshoe crab by combination of reading of micro-geo-morphology on tidal flat from aerial photographs and field ob-servation., 45: 1021-1026(in Japanese with English abstract).
Seino, S., Uda, T., Tsuchiya, Y., Maeda, K., and Minami, T., 2000. Field observation of geomorphological features of the spawn- ing site and disperson of hatchlings of the horseshoe crab Ta- chypleus tridentatus–Towards mittigation planning for the rarespecies., 3 (1): 7-19(in Japa-nese with English abstract).
Sekiguchi, K., 1999..Seisaku Doujin-sha, Tokyo, 45-177(in Japanese).
Sugita, H., and Sekiguchi, K., 1981. Swelling mechanism of the embryo of the Japanese horseshoe crab,., 90: 271-282.
Sugita, H., Murakami, K., and Sekiguchi, A., 1985. Effect of sa- linity on horseshoe crab embryogenesis., 34: 1-9.
Vasquez, M. C., Johnson, S. L., Brockmann, H. J., and Julian, D.,2015. Nest site selection minimizes environmental stressor ex-posure in the American horseshoe crab,., 463: 105-114, DOI: 10.1016/j.jembe.2014.10.028.
Wada, T., Itaya, S., and Shuuno, M., 2010. Spawning sites and annual variability of the number of reproductive visiting pairs of the horseshoe crab Tachypleus tridentatus along the Tsuya- zaki coast in Fukuoka, Japan., 15: 163-171, DOI: 10.18960/hozen.15.2_163 (in Japanese with English abstract).
Wittenberg, A., 2021. Got your COVID-19 vaccine? Thank a horse- shoe crab. https://www.eenews.net/stories/1063723555?fbclid=IwAR2K2e7RKguyVmOBb2HYlhRY6Pj0AlCJyMZseaG2l3quDlP4270Vxl6DRiU, accessed on 31 January 2021.
J. Ocean Univ. China(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5357-8
ISSN 1672-5182, 2022 21 (3): 601-610
(February 22, 2022;
March 21, 2022;
April 11, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
Corresponding author. Tel: 08190-3418-0784 E-mail: ecological-identity@nethome.ne.jp
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
- Experimental Investigation on the Interactions Between Dam-Break Flow and a Floating Box
- Variational Solution of Coral Reef Stability Due to Horizontal Wave Loading
- High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
- Influence of Autonomous Sailboat Dual-Wing Sail Interaction on Lift Coefficients
- Nonlinear Dynamic Analysis and Fatigue Study of Steep Wave Risers Under Irregular Loads
