Impact of Different Diets on Adult Tri-Spine Horseshoe Crab, Tachypleus tridentatus
YING Ziwei, BAO Yuyuan, LI Yinkang, YE Guoling, ZHANG Shuhuan,XU Peng, ZHU Junhua, and XIE Xiaoyong, 3), *
Impact of Different Diets on Adult Tri-Spine Horseshoe Crab,
YING Ziwei1), 2), 3), #, BAO Yuyuan4), #, LI Yinkang1), 2), YE Guoling1), ZHANG Shuhuan5),XU Peng6), ZHU Junhua6), and XIE Xiaoyong1), 2), 3), *
1);,,510300,2),201306,3),511458,4),510322,5),550025,6),,535011,
Effective culture and management of adult tri-spine horseshoe crab,can ensure that stock enhancement programs and aquaculture systems are maintained. To explore suitable feed for animalsduring the breeding season, Pacific oyster () (oyster group; OG) and frozen sharpbelly fish () (frozen fish group; FG) were selected to feed 20male and female pairs, respectively. At the end of the experiment, intestinal samples were obtained to measure digestive enzymes activities. The intestinal flora were determined by 16S rDNA sequencing. No eggs were observed in the FG and oneadult died. No animals died in the OG, and 9.7×104eggs were obtained. These results show that oysters are more suitable for the development and reproduction of adultthan frozen fish. Additionally, the digestive enzyme activity analysis revealed that animals in the OG exhibited higher protein digestibility than those in the FG, but no significant differences in lipid and carbohydrate uptake were observed between the groups. Furthermore, the intestinal flora analysis showed that operational taxonomic units (OTUs) and the Chao1 index were significantly higher in the OG than in the FG, but no significant difference was observed in the Shannon or Simpson indices between the groups. Our data indicate that the oyster diet improved the intestinal microbial diversity ofWe hypothesize that nutrients, such as oyster-based taurine, proteins, and highly unsaturated fatty acids, improve protease activity in thedigestive tract,alter the intestinal floral structure, and improve the reproductive performance of.
; diet; reproductive performance; digestive enzyme activity; intestinal flora
1 Introduction
Horseshoe crabs are known as ancient ‘living fossils’ as they have survived for 400 million years on the Earth(Van Roy., 2010; Kwan., 2018). The hemolymphfromcan be used to produceamebocyte lysate; therefore,is of uni- que value for national public health security (Xie., 2021). Human disturbance and environmental pollution have caused a sharp decline of theresource (Cai., 2021). In 2019, the International Union for Conser- vation of Nature Red List updatedto endangered status (Laurie., 2019), and the animal was listed as a Grade II protected species in the National Key Wildlife List of China in 2021. Due to the ongoing CO- VID-19 pandemic, the large-scale production of vaccines has led to increasing demand foramebocyte lysate and increased pressure on horseshoe crab conser- vation. Large-scale artificial breeding, larval culture, and field release measures are vital and reliableconser- vation modalities forresource management (Hong, 2011). Therefore, cultivation and management of adult horseshoe crab are of great importance to ensure en- hanced release and culture of. Food sources are crucial to the physiological activities of aquatic animals (Doxa., 2013). Mastering feeding demands and beha- viors are key to successful artificial breeding programs,such as improving growth performance (Tacon., 2002), reducing mortality (Espinosa and Allam, 2006), enhanc- ing adult fecundity (Pan., 2009), and replacing natu- ral diets with artificial compound feed (Millamena, 2002).
Horseshoe crabs have different dietary habits at differ- ent life stages. Mollusks, crustaceans, and polychaetes have been observed in gut samples from adultin India (Chatterji., 1992) and adultin Malaysia (John., 2012). How- ever, studies on the diet ofhave only been reported for juvenile and sub-adult animals. Hu(2013) studied the diet of juvenileat the third instar. Kwan(2014) investigated the health status of juve- nilefed with different diets at the eighth in- star, and Gao(2003) conducted preliminary research on the diet of juvenilewith a prosomal width of 10–20cm. There is a need for research on the diet of adultduring the breeding season, which has not been studied.
Different diets can lead to differences in intestinal microbial community structure, with intestinal microbiota af- fecting the host immune defense, digestion and absorption, nutritional metabolism, and other physiological functions (Chen., 2018). Little information is available on the intestinal microbiota diversity ofMiao(2020) analyzed the gut microbiota diversity of first and second instar, and reported that initial molt- ing rather than feeding has a significant effect on the in- testinal flora of juvenile. The effects of diet on intestinal microflora diversity in adultre- main unclear.
Based on the finding that major food sources for adultandare bivalves and fish (Guo, 2021; Halim, 2021), oysters and frozen fish were selected as diets for adultin this study. Oys- ters have more moisture, crude protein, crude fat, and ash content than frozen fish (Li, 2021). Moreover, oys- ters are rich in high-quality protein, glycogen, n-3 poly- unsaturated fatty acids, essential amino acids, trace ele- ments, and other nutrients (Li, 2021). We then ana- lyzed the dietary effects on reproductive performance and intestinal flora of adultto provide a scien- tific and theoretical basis for selecting an artificial breed- ing diet for these animals
2 Materials and Methods
2.1 Selection and Culture Management of Adult T. tridentatus
Horseshoe crabs (female, 4.21kg±0.68kg; male, 1.70kg±0.22kg) were obtained from the South China SeaFisheries Research Institute, Chinese Academy of FisheriesSciences in March 2019. Twentymale and female pairswere randomly selected for breeding studies (May–August). The average prosomal width of the females was 35.28cm±4.62cm, while that of males was 27.19cm±2.37cm.
Culture studies were conducted in two indoor cement ponds (4m×4m×0.8m) containing filter-disinfected seawater at 28–32℃, with 26%–30% salinity, pH of 7.4–7.8, and dissolved oxygen ≥4.0mgL−1. Ten animal pairs were randomly selected and placed in each pond, which was also equipped with a water circulating system, and a sand covered bottom (depth, 20–40cm; grain size, 0.5–2.0mm). The animals were fed once daily at 18:00 during culture. In one pond, animals were fed 20mm (length)×8mm(width)×5mm (height) oysters () (oyster group; OG), while the other pond was fed an equal portion of frozen fish () (frozen fish group; FG) with the same particle size, and both groups were fed at 3% of overall body mass. About 80% of the seawater in each pond was renewed daily. The sandy bottom and pondwalls were cleaned once every 15 days. Health parameters, including final body weight, mortality, weight gain rate, and specific growth rate, were observed in all animals du- ring the study. All horseshoe crabs were weighed at the beginning and the end of the experiment. Additionally, breeding activities were recorded in the groups, including egg number and hatching rate. Eggs were obtained by na- tural spawning. The total number of eggs spawned by the 10 pairs of horseshoe crabs was counted at the end of the experiment.
2.2 Sampling and Experimental Manipulation
After 120 days of feeding experiment, threemale and female pairs were randomly sampled from each pond. The digestive tract was dissected and stored at −80℃. Intestinal tissues (0.5g per sample) were ground twice in an automatic freezing grinding instrument (JXFSTPRP-32L, Shanghai Jingxin Industrial Development Co., Ltd., Shang- hai, China), and the supernatant was centrifuged at 4℃ (Thermo ST16, Shanghai, China) and stored to determine enzyme activities. Pepsin, trypsin, lipase, and α-amylase levels were determined using kits provided by Nanjing Jian- cheng Bioengineering Institute (Shanghai, China).
Intestinal samples (0.5g per sample) were also ground. Total intestinal bacterial DNA was extracted using the Tian-gen DNA extraction kit (DP308, Tiangen Biotech Co., Ltd., Beijing, China), and the V3 and V4 regions of 16S rRNA were amplified. The polymerase chain reaction (PCR) was carried out in a 30μL reaction system with 15μL of Phusion® High-Fidelity PCR Master Mix (New England Bio- labs, Ipswich, MA, USA), 0.2μmolL−1of the forward and reverse primers, and about 10ng of template DNA. The PCR conditions were initial denaturation at 98℃ for 1min,followed by 30 cycles of denaturation at 98℃ for 10s, annealing at 50℃ for 30s, and elongation at 72℃ for 60s, followed by a final elongation step at 72℃ for 5min. Oncesuccessful amplification was indicated using 1% agarose gel electrophoresis, high throughput sequencing of the 16S rRNA gene was entrusted to Mingke Biotechnology Co., Ltd. (Hangzhou, China). The PCR primers were: 515F (5’- GTGCCAGCMGCCCGG-3’) and 907R (5’-CCGTCAAT TCMTTTRAGTTT-3’) DNA was amplified using Trans- Start Fastpfu DNA Polymerase (TransGen AP221-02, Bei- jing, China) with a PCR instrument (ABI GeneAmp® 9700,ABI, Foster City, CA, USA). Three replicates of each sam- ple were mixed, and the PCR products from the same sam- ple were recovered by 2% agarose gel electrophoresis. The PCR products were purified using the AxyPrepDNA Gel Recovery Kit (Axygen®, Tewkesbury, MA, USA) and eluted in Tris-HCl (pH 7.4).
2.3 Intestinal Microbiome Analysis Determination of Taurine Content in Two Diets
Based on the Illumina PE250 sequencing tool, fast length adjustment of short reads and paired-end reads were fil- tered and spliced according to the overlapped relation- ships to generate good quality data. Operational taxono- mic units (OTUs) were clustered to analyze differences in species abundance and the α- and β-diversity indices between the groups. Linear discriminant analysis effect size (LEfSe) was used to identify significant differences in the relative abundance of the bacterial taxa.
Muscle samples were collected randomly and locally fromand(six sam- ples for each). Taurine concentrations in muscle samples were measured using taurine assay kit (Cell Biolabs, San Jose, CA, USA).
2.4 Data Analysis
Data were tested for normality and homogeneity of va- riance using the Shapiro-Wilk test, and the-test was used to analyze differences between groups. The results are ex- pressed as mean±standard deviation (SD). Duncan’s mul- tiple comparison test was used to analyze differences between groups, and<0.05 was considered significant. Prin- cipal component analysis (PCA) was performed in R soft- ware (Version 4.0.5; The R Foundation for Statistical Com- puting, Vienna, Austria) to identify differences in the mi- crobial structure between the groups.
3 Results
3.1 Effects of Different Diets on Adult T. tridentatus Growth and Reproductive Performance
No eggs were observed in the FG, and one adultdied. No horseshoe crabs died in the OG, and 9.7×104eggs were obtained. These eggs were pale yellow spherical, with diameters of 2.98mm±0.15mm, and a hat- ching rate of 89%. The final body weight, weight gain rate,and specific growth rate of the OG group were higher than those of the FG group (Table 1).

Table 1 Influence of the different diets on growth of adult Tachypleus tridentatus
Notes: IBW, initial body weight; FBW, final body weight; WGR, weight gain rate; SGR, specific growth rate. Different superscript letters indicate a significant difference between the treatment groups (0.05).
3.2 Effects of the Different Diets on Adult T. tridentatus Intestinal Enzyme Activity
As shown (Table 2), pepsin activity was significantly higher in the OG crabs than in the FG crabs (<0.01,=−3.038), while lipase, α-amylase, and trypsin activities were not significantly different (>0.05).
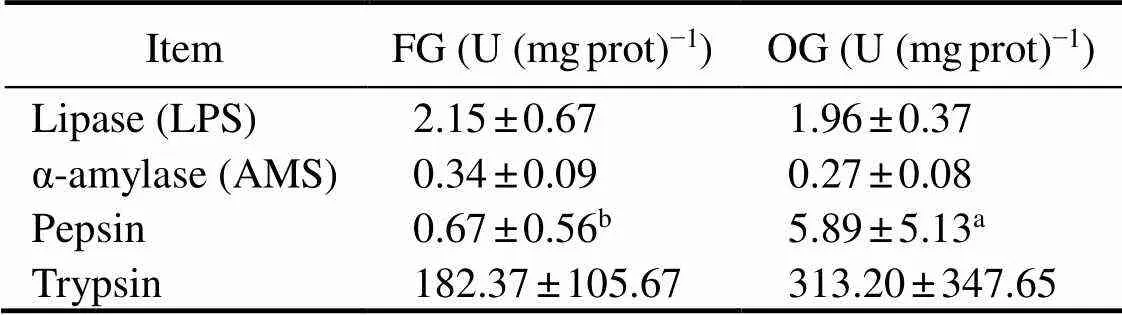
Table 2 Influence of different diets on intestinal enzyme activities of adult Tachypleus tridentatus
Note: Different superscript letters indicate a significant difference between the treatment groups (0.01).
3.3 Effects of the Different Diets on the Adult T. tridentatus Intestinal Flora
3.3.1 OTU cluster and species diversity analyses
In total, 237969 valid sequences were identified across the sample groups, including 114155 from the FG and 123814 from the OG. The OTUs belonged to 11 phyla, 17 classes, 32 orders, 50 families, 57 genera, and 55 species. OTU similarity and overlap between the groups were in- vestigated using a Venn diagram (Fig.1A). In total, 1487 OTUs were identified in both groups, of which the num- bers of OTUs in the FG and OG were 775 and 1272, re-spectively. A total of 560 OTUs were common between the groups, while there were 215 (FG) and 712 (OG) unique OTUs. These data indicate that oysters improved the in- testinal microbial diversity of
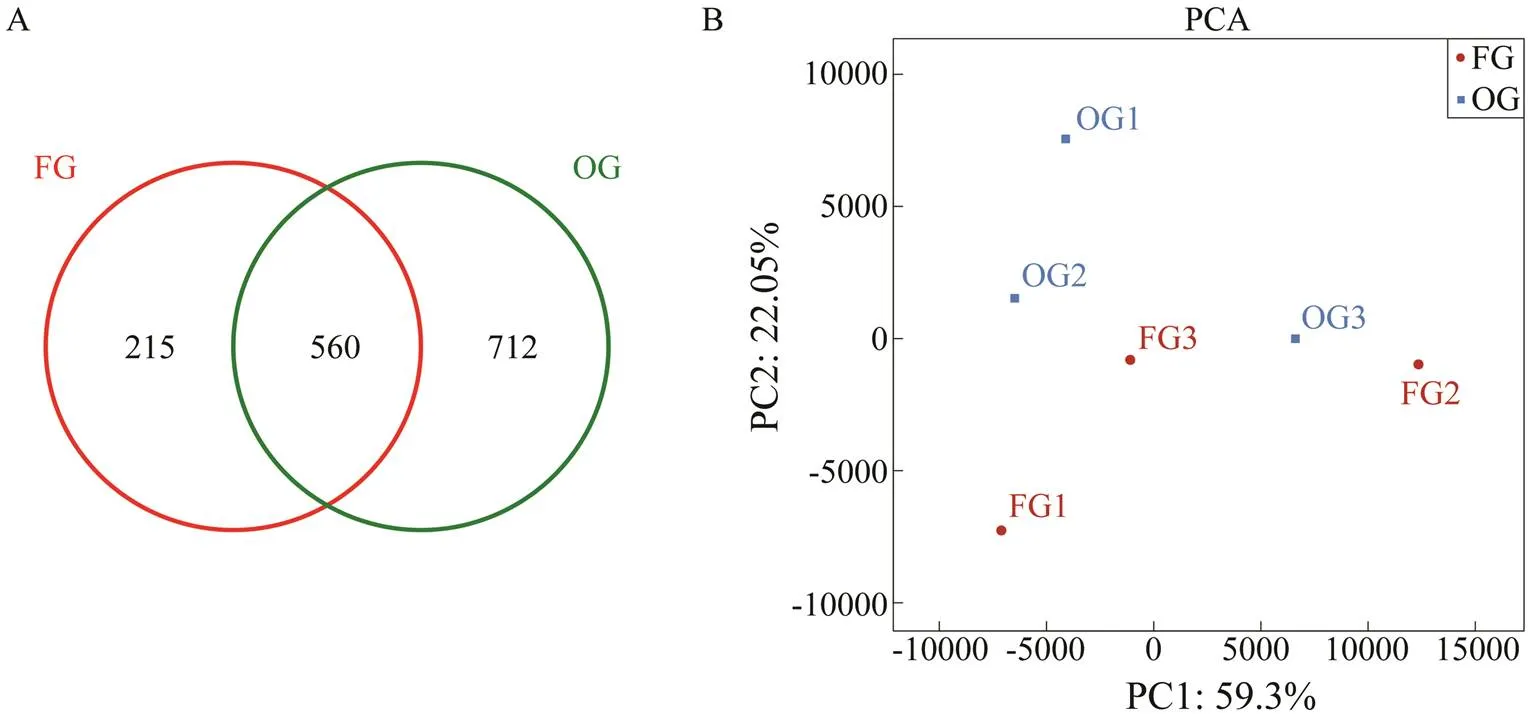
Fig.1 (A)Venn diagram used to count the number of shared and unique OTUs in different samples; the red circle represents the FG group, the green circle represents the OG group, and the overlap represents the number of shared OTUs between the two groups; (B) PCA of adult Tachypleus tridentatus intestinal flora. FG, frozen fish group; OG, oyster group.
The Chao1 index was used to evaluate the richness of the intestinal flora. The Shannon and Simpson indices have been commonly used to assess intestinal flora diversity; a higher Shannon index and a lower Simpson index indicate higher diversity in bacterial communities (Liu and Peng, 2021). Our results show that the coverage value of both groups was >0.99, suggesting that the results were reliable. The abundance (OTUs and Chao1 index) and diversity (Shannon index) of the intestinal flora were higher in the OG than those in the FG (Table 3). The OTUs and Chao1 indices were significantly different between the groups (<0.05), while the Shannon and Simpson indices were not significantly different (>0.05) (Fig.2).
PCA showed that diet (59.3%) was the main factor responsible for the difference of the intestinal content samples fromin the FG and OG groups (Fig.1B).

Table 3 Influence of the different diets on the intestinal microbial diversity index of Tachypleus tridentatus
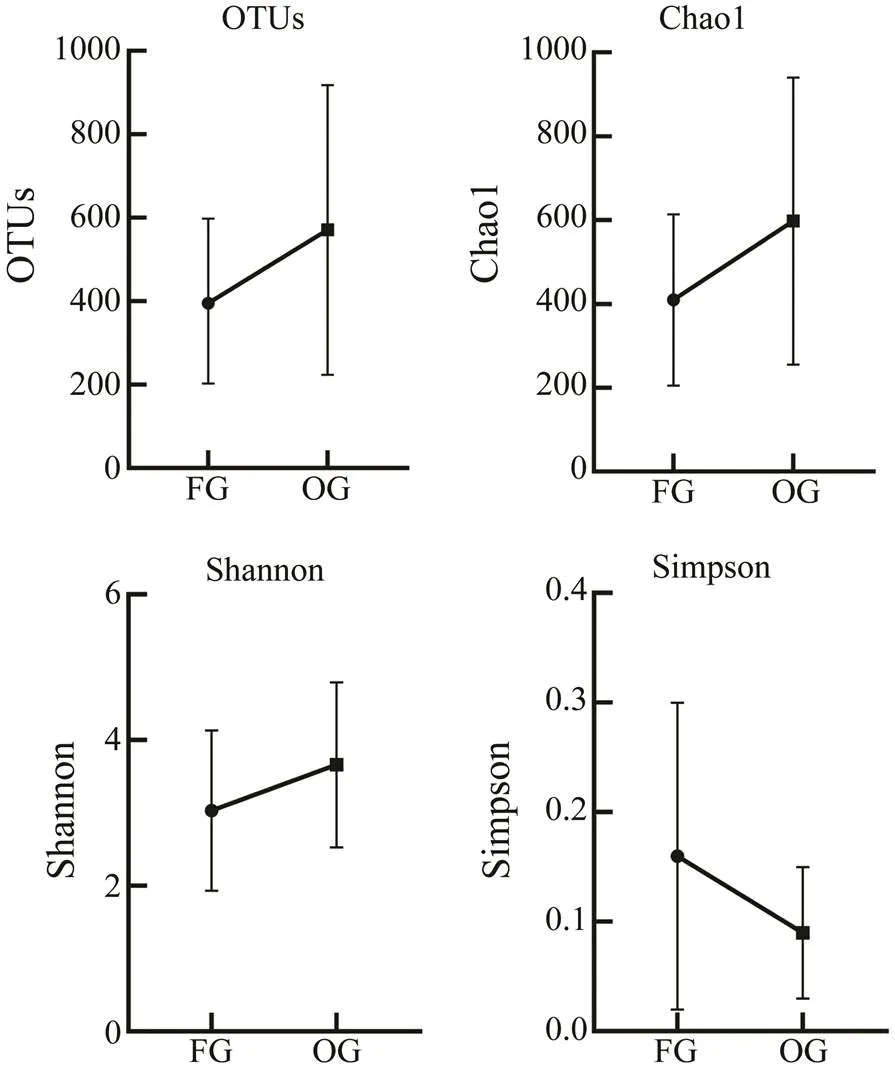
Fig.2 α-Diversity of the bacterial communities: The Chao1 index estimates richness; the Shannon and Simpson indi- ces estimate diversity. FG, frozen fish group; OG, oyster group.
3.3.2 Community composition and intestinal flora abundance
The dominant phyla and genera in thein- testinal samplesare shown in Fig.3. Proteobacteria (FG: 37.59% and OG: 20.59%), Tenericutes (FG: 34.89% and OG: 22.64%), Firmicutes (FG: 14.76% and OG: 21.62%), and Bacteroidetes (FG: 9.81% and OG: 16.85%) were the common dominant phyla. In addition, Fusobacteria (6.35%)was a unique dominant phylum in the OG, and the remain- ing abundances were<3% (Fig.3A).(14.02%),(5.73%),(5.49%),(4.25%),(3.96%), and(3.93%) were the dominant genera in the FG, while(7.08%),(6.34%),(5.99%), and(5.68%) were the dominant genera in the OG (Fig.3B).
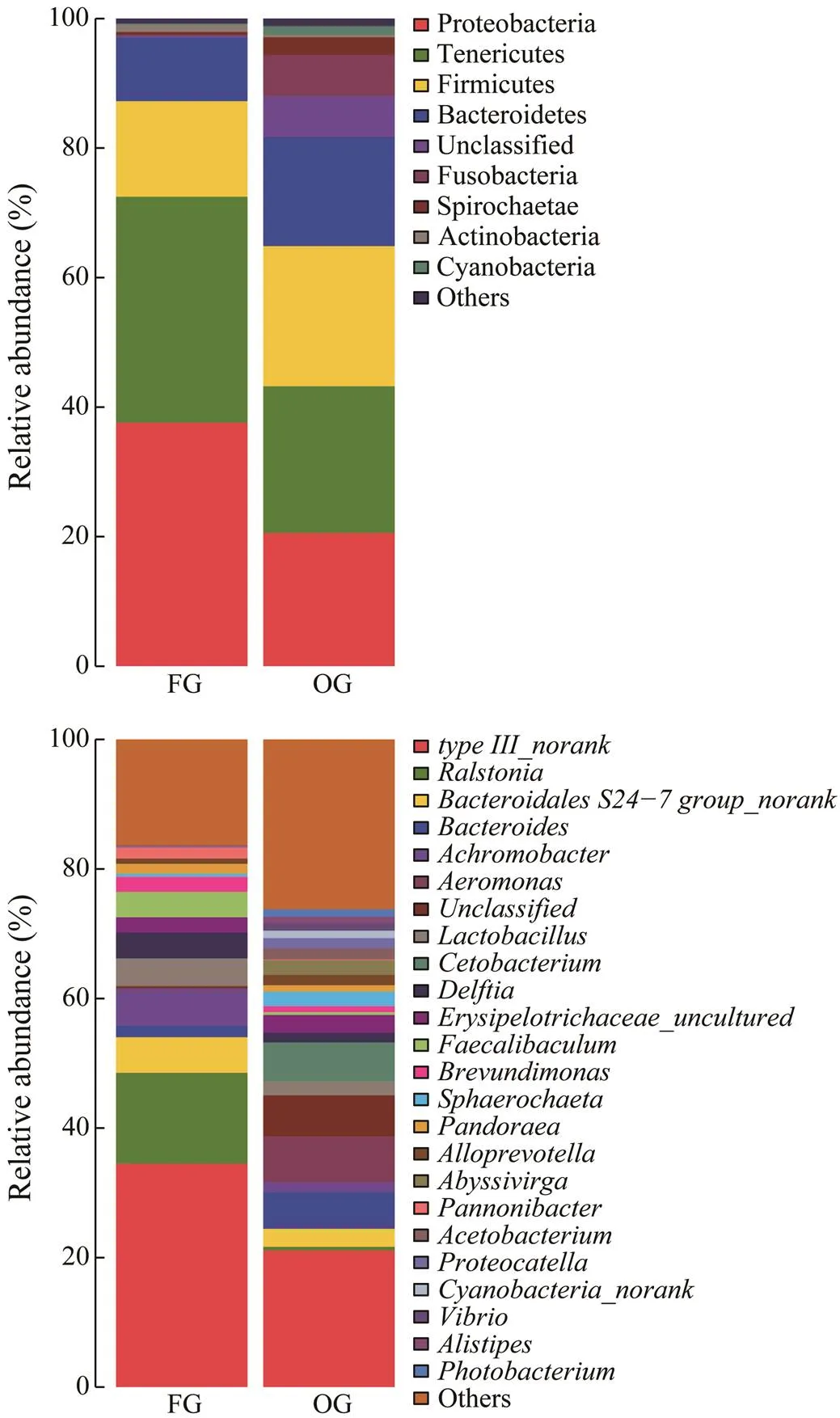
Fig.3 Dominant phyla (A) and genera (B) of intestinal florain the different dietary groups. FG, frozen fish group; OG, oyster group.
3.3.3 Species differences among the intestinal flora groups and taurine content in two diets
The results of the LEfSe analysis showed that Fuso- bacteria was a phylum-level biomarker and,,,, andwere genus-level biomarkers between FG and OG (Fig.4).
The taurine content of two diets was significantly dif- ferent (<0.05). Taurine content in oysters was 362.19±13.46mg(100g)−1, whilewas 236.78±19.75mg(100g)−1.
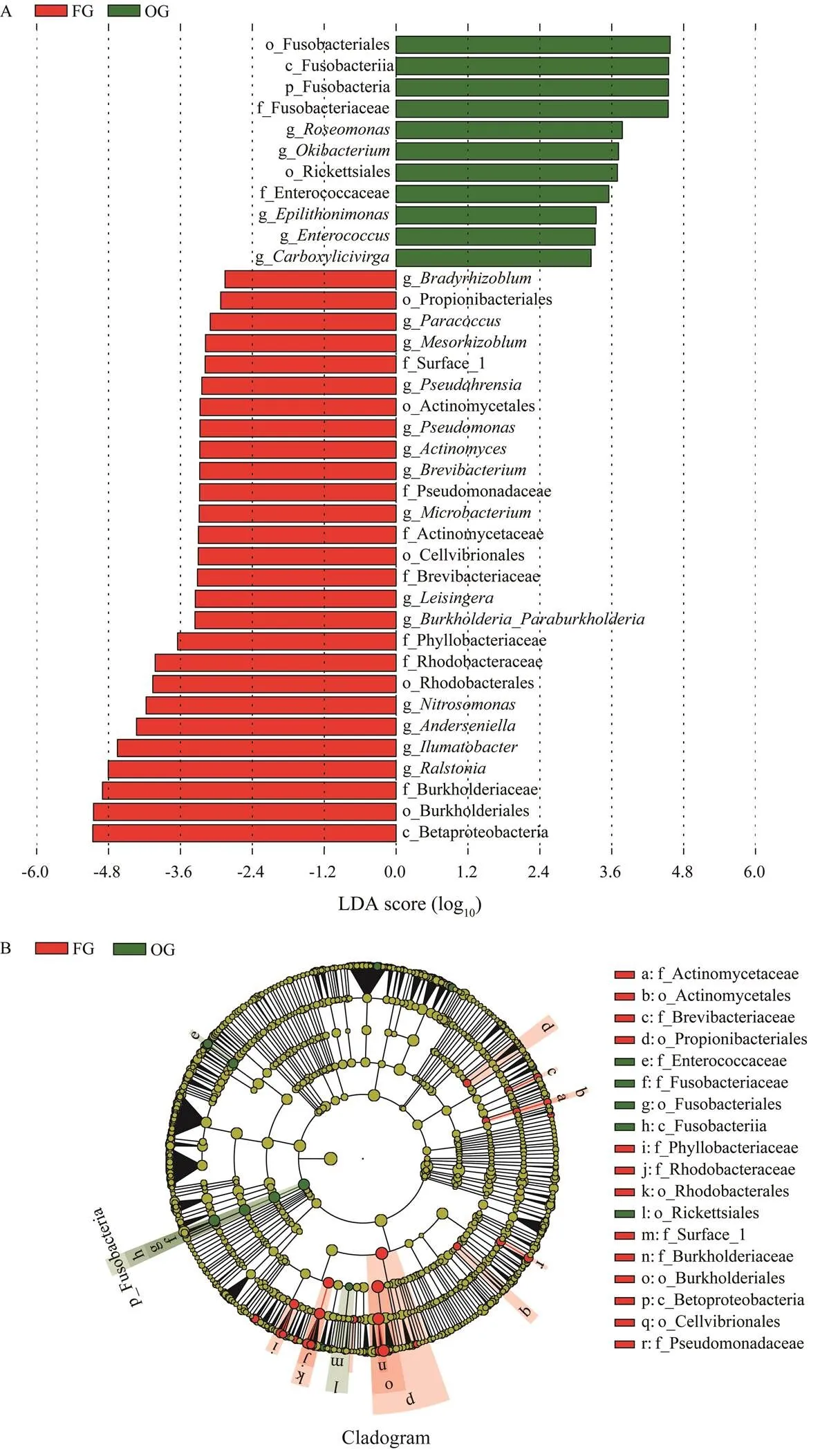
Fig.4 LEfSe results of intestinal microbial composition between the frozen fish group (FG) and oyster group (OG). A, LDAscores of the bacterial clades identified by the LEfSe analysis; B, Phylogenetic relationships of the bacterial clades revealed by LEfSe. The single characters before the underlines are abbreviations: p, phylum; c, class; o, order; f, family; g, genus.
4 Discussion
Our data show that adultfed frozen fish (FG) did not spawn during the experiment (120d), while animals fed oysters (OG) laid eggs, suggesting that the nu- trients in oysters were more suitable for adultgrowth and development than frozen fish. Thefro- zen fish ()had a high fat content (Zeng, 2012). The different particle sizes and hardness of the diets may also be a reason for the results. Oysters are highly palatable with a soft meat quality, which are conducive to feeding horseshoe crabs. Frozen fish are difficult to chew and swallow. In addition, the width and thickness of the frozen fish pieces may have affected feeding ofA previous study showed that the feed intake of ju- veniledecreases with increasing particle size of the diet (Gao, 2003). Considering these dietary factors, both diets were cut into small size pieces before feeding. Oyster soft tissues are rich in amino acids, with taurine levels accounting for almost half of all free amino acids (Fuentes., 2010). Taurine levels in oysters from the southwestern South China Sea are 16.81–19.83mgg−1(Gao., 2013), which are 5–30 times higher than those of other marine fishes, such as, Chelido- nichthys kumu,and(Tan., 2000). Previous studies reported that taurine improves growth andreproduction (Xu., 2020; Yu., 2021). Oysters also contain highly unsaturated fatty acids (eicosapentaenoic acid and docosahexaenoic acid) that promote vitellogenesis and gonadal and embryonic development in aquatic ani- mals and are key nutrients affecting the reproductive performance of adult fish (Bell and Sargent, 2003; Watanabeand Vassalloagius, 2003; Callan., 2014). Similarly, these molecules enhance the reproductive performance of aqua- tic animals, including(Xu., 2016),(Røjbek., 2014), and(Luo., 2015).
Pepsin activity was significantly higher in the OG than in the FG, but no significant difference was observed in in- testinal α-amylase activity. This may be because oysters are rich in protein, glycogen, and taurine (Wang., 2011). Taurine increases protease activity and the feeding rate (Li., 2017; Wang., 2018). Similar to our data, Yu(2021) reported that the protease activity ofwas significantly higher in a taurine-treated group than that in a control group, whereas α-amy- lase activity was not significantly different. He(2017) showed that feed containing 1.3% taurine enhances pro- tease activity in the stomach, liver, and intestine of. Other studies have reported that the in- testinal protease activities of(Liang, 2018),(Wang., 2017), and juvenile(Dong., 2017) in- crease with dietary protein level. Thus, taurine could be supplemented infeed to observe its specific effect on the digestive enzyme activity ofPepsin activity in the OG was significantly higher com-pared to the FG. Therefore, adultof OG group had stronger ability to digest and absorb protein. Among the total fat of oyster, omega-3 highly unsaturated fatty acids (-3 HUFAs) such as eicosapentaenoic acid (EPA) and do-cosahexaenoic acid (DHA) accounted for 28% (Wang., 2003). Ma. (2005) found that turbotfed high-protein and high-3 HUFAs diet had highest spawning-stock biomass. This finding is in agree- ment with the results found in our study.
Bacteria have a strong capacity to metabolize taurine, which can be directly broken down into carbon, nitrogen, and sulfur for growth (Cook., 2006). Ma(2021) reported that taurine levels in feed affect the structure, rich- ness, and diversity of the intestinal flora. Our data show that Proteobacteria, Tenericutes, Firmicutes, and Bacteroi- detes were the common dominant phyla in the horseshoe crab groups, and Fusobacteria was the unique dominant phy-lum in the OG. Proteobacteria are Gram-positive bacilli that produce spores in harsh environments. Bacteroidetes are the largest Gram-negative bacilli group in the animal in- testine and are involved in metabolic processes, such as di- gestion of nutrients and absorption (Francois., 2011). Fusobacteria are Gram-negative bacilli with a higher de- tection rate in human colorectal tumors than surrounding normal tissues (Kelly., 2018). Studies have shown that high-protein diets enrichFlavobacteria and Fusobac- teriain male,and a low-protein diet benefits the growth of Bacteroidetes, Rikenellaceae, and Tannerellaceae(Wang., 2021).
Oysters are high in protein and low in fat, and our re- sults were consistent with previous studies. The quantity ofwas higher in the OG (5.99%) than in the FG (5.49%). These bacteria produce butyric acid, a short-chain fatty acid that can be produced by intestinal bacteria from cellulose fermentation (Tang., 2018). Butyrate-producing probiotics can reduce non- alcoholic fatty liver disease (NAFLD) progression in rats (Endo., 2013). Zhou. (2017) also confirmed that sodium butyrate attenuates high-fat diet (HFD)-induced steatohepatitis in mice by improving the gut microbiota and gastrointestinal battier. Short-chain fatty acids maintain ho- meostasis in the intestinal environment and regulate the im- mune response (Serino, 2019).is a pathogen that causes bacteriemia and infections in wounds, the uri- nary tract, and other body regions (Rihs., 1993). Pa- thogens were detected in the intestines of the OG, which may have been due to remnant, undigested oysters.
Horseshoe crabs have a wide variety of food sources in the natural environment, where nutrients are highly abun- dant, whereas a single diet is usually fed to animals under artificial culture conditions. The differences in intestinal flo-ra between wildand artificially cultured ani- malsmust be comprehensively characterized in terms of differences in nutrient digestion and absorption to deve- lop compound feeds that promotegrowth, development, and reproductive performance.
5 Conclusions
Adultfed oysters had higher weight gain rate (WGR), specific growth rate (SGR), spawning-stock biomass. These results showed that oysters were more suit-able for enhancing the growth and reproduction perfor- mance of adultthan frozen fish,which is of great significance for the recovery of there- source. However, a high-protein diet can also provide nu- trients for pathogens to breed while promoting animal growth and reproduction. Thus, it is advisable to change the culture water frequently to remove the residual food and prevent replication of pathogens.
Acknowledgements
This research was supported by the fund of the Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (No. GML2019ZD0605), the Guangdong Pro- vincial Key Laboratory of Fishery Ecology and Environ- ment (No. FEEL-2020-2), the Science and Technology Plan- ning Project of Guangdong Province (No. 2019B12120 1001), the Special Fund for Basic Scientific Research of Central Public Research Institutes of South China Sea Fisheries Research Institute, the Chinese Academy ofFishery (No. 2019TS21), and the National Natural Science Foundation of China (No. 42067038).
Bell, J. G., and Sargent, J. R., 2003. Arachidonic acid in aqua- culture feeds: Current status and future opportunities., 218 (1-4): 491-499, DOI: 10.1016/S0044-8486(02)00 370-8.
Cai, L., Chen, X., Fu, S., Yang, D., and Zhao, X., 2021. Popula- tion dynamics and benthic environment ofin the intertidal zone of Eyu Islet in Xiamen., 17 (1): 14-18, DOI: 10.3969/j.issn. 1673-3290.2021.01.03(in Chinese with English abstract).
Callan, C. K., Laidley, C. W., Kling, L. J., Breen, N. E., and Rhyne, A. L., 2014. The effects of dietary HUFA level on flame angelfish () spawning, egg quality and early larval characterstics., 45 (7): 1176-1186, DOI: 10.1111/are.12063.
Chen, H., Yan, H., Zhao, Y., Xiao, H., and Chen, S., 2018. Metagenomics and its advances in research of fish gut microbiome., 37 (5): 699-706, DOI: 10.16378/j. cnki.1003-1111.2018.05.020 (in Chinese with English abstract).
Cook, A. M., and Denger, K., 2006. Metabolism of taurine in microorganisms: A primer in molecular biodiversity., 583: 3-13, DOI: 10. 1007/978-0-387-33504-9_1.
Dong, L., Tong, T., Zhang, Q., Xu, M., Su, Q., Nie, Z.,., 2017. Effect of dietary protein level on growth performance, body composition, and digestive enzyme activities in green mud crab () juveniles., 24 (3): 524-532, DOI: 10.3724/SP.J. 1118.2017.16289 (in Chinese with English abstract).
Doxa, C. K., Divanach, P., and Kentouri, M., 2013. Consumption rates and digestibility of four food items by the marine gastropod(Aradas & Benoit, 1870)., 446: 10-16, DOI: 10.1016/j.jembe.2013.04.019.
Endo, H., Niioka, M., Kobayashi, N., Tanaka, M., and Watanabe, T., 2013. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the pro- biotics for the gut-liver axis., 8 (5): e63388, DOI: 10.1371/journal.pone.0063388.
Espinosa, E. P., and Allam, B., 2006. Comparative growth and survival of juvenile hard clams,, fed commercially available diets., 25 (6): 513-525, DOI: 10.1002/zoo.20113.
Francois, T., Jan-Hendrik, H., Etienne, R., Mirjam, C., and Gur- van, M., 2011. Environmental and gut bacteroidetes: The food connection., 2: 93, DOI: 10.3389/ FMICB.2011.00093.
Fuentes, A., Fernández-Segovia, I., Serra, J. A., and Barat, J. M., 2010. Comparison of wild and cultured sea bass () quality., 119 (4): 1514-1518, DOI: 10.1016/j.foodchem.2009.09.036.
Gao, F., Liao, Y., and Ye, F., 2003. Feed study of thejuvenile., 22 (4): 92-96 (in Chinese with English abstract).
Gao, J., Zhang, C., Qiu, W., Cao, W., and Qin, X., 2013. Deter- mination of taurine infrom different parts of the South China Sea., 34 (10): 164-168, DOI: 10.7506/spkx1002-6630-201310035 (in Chinese with English abstract).
Guo, Q., Gu, Y., Bao, Y., Li, Y., Zhou, C., and Xie, X., 2021. Dietary composition and trophic position of, 17 (4): 35-40, DOI: 10.12131/20200234 (in Chinese with English abstract).
Halim, A. S. A., Mohamad, F., Ahmad, F., Ismail, N., Chilek, T. Z. T., Ahmad, A. R.,., 2021. Possible predation on com- mercial bivalves by: An assessment of horse- shoe crab reintroduction in Setiu Lagoon of Terengganu, Malaysia., 848 (4): 841-855, DOI: 10.1007/s10750- 020-04493-7.
He, M., Liu, L., Qu, H., Zhang, X., and Gao, J., 2017. Effects of dietary taurine on growth performance and digestive enzyme activity of., 26 (2): 227-234, DOI: 10.12024/jsou.20151201609 (in Chinese with English abstract).
Hong, S., 2011.. Xiamen University Press, Xiamen, 77-79.
Hu, M., Wang, Y., Cheung, S. G., and Shin, P. K. S., 2011. Com- parison of different frozen natural foods on survival and growth of juvenile Chinese horseshoe crab, (Leach, 1819): Implications on laboratory culture., 44 (4): 567-573, DOI: 10.1111/j.1365-2109.2011. 03059.x.
Kelly, D., Yang, L., and Pei, Z., 2018. Gut microbiota,, and colorectal cancer., 6 (4): 1-10, DOI: 10. 3390/diseases6040109.
Kwan, B. K. Y., Chan, A. K. Y., Cheung, S. G., and Shin, P. K. S., 2014. Hemolymph quality as indicator of health status in ju- venile Chinese horseshoe crab(Xipho- sura) under laboratory culture., 457: 135-142, DOI: 10.1016/j.jembe. 2014.04.011.
Kwan, K. Y., Virginia, K. Y., Cheung, S. G., and Paul, K. S., 2018.Horseshoe crabs as potential sentinel species for coastal health: Juvenile haemolymph quality and relationship to habitat con- ditions., 69 (6): 894-905, DOI: 10.1071/MF17210.
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H. L., John, A.,, 2019.(Errata Version Pub- lished in 2019). e.T21309A149768986. IUCN, Gland, Switzerland, 60pp, DOI: 10.2305/IUCN.UK.2019-1.RLTS.T2130 9A149768986.en.
Li, H., Huang, X., Wang, X., Yan, M., and Zheng, X., 2017. Ef- fect of dietary taurine supplementation on the growth, body composition, digestive enzyme activity and anti-stress ability ofin freshwater culture., 26 (5): 706-715, DOI: 10.12024/ jsou.20170101950 (in Chinese with English abstract).
Li, X. D., Peng, J. X., Wu, H. Y., Zheng, G. C., Guo, M. M., Zhao, X. N.,., 2021. Review on nutrition, taste and functional components in oysters., DOI: 10. 16378/j.cnki.1003-1111.20245.
Liang, P., Qin, Z., Lin, J., Wu, G., Zhu, Q., and Qiu, M., 2018. Effect of feed protein levels on growth performance and digestive enzyme activity of juvenile., 34 (2): 136-140 (in Chinese with English abstract).
Liu, N., and Peng, Z., 2021. Analysis of intestinal microorganismsin(Teleostei, Cypriniformes)., 45 (1): 118-124, DOI: 10.7541/2020.2019. 169 (in Chinese with English abstract).
Luo, L., Ai, L., Li, T., Xue, M., Wang, J., Li, W.,., 2015. The impact of dietary DHA/EPA ratio on spawning perfor-mance, egg and offspring quality in Siberian sturgeon ()., 437 (4): 140-145, DOI: 10.1016/ j.aquaculture.2014.11.036.
Ma, A., Chen, C., Lei, J., Chen, S., and Zhuang, Z., 2005. The effect of protein and n-3 HUFA on the reproduction of turbot ()., 26 (1): 7-12 (in Chinese with English abstract).
Ma, Q., Guo, L., Liu, B., Liu, B., Zhu, K., Guo, H.,., 2021. Effect of taurine on intestinal microbes and immune function in golden pompano ()., 17 (2): 87-96, DOI: 10.12131/202000193 (in Chinese with English abstract).
Miao, F., Zhao, Z., Li, Q., Song, J., Wang, Y., and Hu, M., 2020. Impact of initial feeding and molting ongut microbiota., 77: 2847-2858, DOI: 10.1007/s00284-020-02108-x.
Millamena, O. M., 2002. Replacement of fish meal by animal by- product meals in a practical diet for grow-out culture of grouper, 204 (1-2): 75-84, DOI: 10. 1016/S0044-8486(01)00629-9.
Pan, Q., Tian, X., Ye, J., Chen, J., Zhang, J., Wang, Y.,., 2009.Effect of diets on growth and fecundity of., 33 (6): 1005-1010, DOI: 10.3724/SP.J.0000.2009.61005 (in Chinese with English abstract).
Rihs, J. D., Brenner, D. J., Weaver, R. E., Steigerwalt, A. G., Hol- lis, D. G., and Yu, V. L., 1993., a new genus as- sociated with bacteremia and other human infections., 31 (12): 3275-3283, DOI: 10.1128/ jcm.31.12.3275-3283.1993.
Røjbek, M. C., Støttrup, J. G., and Jacobsen, C., 2014. Effects of dietary fatty acids on the production and quality of eggs and larvae of Atlantic cod (L.)., 20 (6): 654-666, DOI: 10.1111/anu.12124.
Serino, M., 2019. SCFAs–The thin microbial metabolic line be- tween good and bad., 15: 318- 319, DOI: 10.1038/s41574-019-0205-7.
Tacon, A. G. J., Cody, J. J., Conquest, L. D., Divakaran, S., For- ster, I. P., and Decamp, O. E., 2002. Effect of culture system on the nutrition and growth performance of Pacific white shrimp(Boone) fed different diets., 8 (2): 121-137, DOI: 10.1046/j.1365-2095.2002. 00199.x.
Tan, L., Zhang, C., Xue, C., and Lin, H., 2000. Bioactivity of tau- rine and its distribution in marine organisms., 20 (3): 75-79 (in Chinesewith Eng- lish abstract).
Tang, W., Yao, X., Xia, F., Yang, M., Chen, Z., Zhou, B.,., 2018. Modulation of the gut microbiota in rats by Hugan Qingzhi tablets during the treatment of high-fat-diet-induced nonalcoholic fatty liver disease., 2018 (4): 1-14, DOI: 10.1155/2018/7261619.
Van Roy, P., Orr, P. J., Botting, J. P., Muir, L. A., Vinther, J., Le- febvre, B. E. L.,., 2010. Ordovician faunas of Burgess Shale type., 465: 215-218, DOI: 10.1038/NATURE 09038.
Wang, A. R., Ran, C., Ringø, E., and Zhou, Z. G., 2018. Progress in fish gastrointestinal microbiota research., 10: 626-640, DOI: 10.1111/raq.12191.
Wang, C., Hu, G., Sun, P., Gu, W., Wang, B., and Xu, Q., 2017. Effects of dietary protein an lipid levels on growth perfor- mance, digestive enzyme activities and serum indices ofbroodstock., 29 (2): 571-582, DOI: 10.3969/j.issn.1006-267x.2017.02.025 (in Chinesewith English abstract).
Wang, D., Zhao, Y., Zeng, M., Liu, Z., and Dong, S., 2011. Nu- tritional components and water-extraction process ofmeat., 36 (3): 209- 212, DOI: 10.13684/j.cnki.spkj.2011.03.029 (in Chinese with English abstract).
Wang, H., Yang, R., and Wang, Z., 2003. Nutritional components and proteolysis of oyster meat., 27 (2): 163-168 (in Chinese with English abstract).
Wang, Y., Tian, B., Zhang, B., Fan, J., Ge, F., and Wang, G., 2021. Effects of dietary protein levels on intestinal bacterial com- munities of., 41 (13):5495-5505, DOI: 10.5846/stxb202006221621 (in Chinese with English abstract).
Watanabe, T., and Vassalloagius, R., 2003. Broodstock nutrition research on marine finfish in Japan., 227 (1-4): 35-61, DOI: 10.1016/S0044-8486(03)00494-0.
Xie, X., Zhong, J., Guan, J., and Jia, X., 2021. The urgency of horseshoe crab protection in China from the perspective of Tachypleus Amebocyte Lysate industry., 39 (2): 109-116 (in Chinese with English abstract).
Xu, Y., Liu, X., Zheng, Y., Li, W., and Ding, Z., 2020. Effect and mechanism of taurine on the metabolism of aquatic animal., 41 (16): 35-40, DOI: 10.13302/j.cnki.fi.2020. 16.007(in Chinese with English abstract).
Xu, Y. Q., Li, W. F., and Ding, Z. K., 2016. Polyunsaturated fattyacid supplements could considerably promote the breeding per- formance of carp., 119 (5): 1-8, DOI: 10.1002/ejlt.201600183.
Yu, W., Yang, Y., Lin, H., Huang, X., Huang, Z., Li, T.,., 2021. Effects of taurine on growth performance, digestive en- zymes, antioxidant capacity and immune indices of., 17 (2): 78- 86, DOI: 10.12131/20200223 (in Chinese with English abstract).
Zeng, G., Lv, Y., Huang, P., Yu, J., and Yang, L., 2012. Analy- sis of flesh content and nutritional component in the muscle ofBasilewsky andGunther., 33 (5): 1-7, DOI: 10.3875/j.issn.1674-3563.2012.05.001 (in Chinese with English abstract).
Zhou, D., Pan, Q., Xin, F. Z., Zhang, R. N., He, C. X., Chen, G. Y.,., 2017. Sodium butyrate attenuates high-fat diet-in- duced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier., 23 (1): 60-75, DOI: 10.3748/wjg.v23.i1.60.
J. Ocean Univ. China(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5199-4
ISSN 1672-5182, 2022 21 (3): 541-548
#The two authors contributed equally to this work.
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
Corresponding author. E-mail: xyxie@scsfri.ac.cn
(September 17, 2021;
November 8, 2021;
January 11, 2022)
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe Crab Tachypleus tridentatusEggs
- Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
- Experimental Investigation on the Interactions Between Dam-Break Flow and a Floating Box
- Variational Solution of Coral Reef Stability Due to Horizontal Wave Loading
- High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
- Influence of Autonomous Sailboat Dual-Wing Sail Interaction on Lift Coefficients
