Activity Recording of Free-Ranging Tri-spine Horseshoe Crabs in the Southeastern Coast of Sabah, Borneo
WATANABE ShinichiNELSON Bryan Raveen2MANCA Azwarfarid3,and MOHAMAD Faridah4
Activity Recording of Free-Ranging Tri-spine Horseshoe Crabs in the Southeastern Coast of Sabah, Borneo
WATANABE Shinichi1),*, NELSON Bryan Raveen2), MANCA Azwarfarid3),and MOHAMAD Faridah4)
1).,1130021,2),,3),4),,,21030,
In Inderasabah (southeast Sabah),tri-spine horseshoe crabwas observed for their locomotion activity using data loggers from September to November in 2015. A female with acceleration and depth-temperature loggers and five males with acceleration loggers were recaptured between 10 and 49 days after their release. From the record of 194 activity days that involve all six, four horseshoe crabs, including the female, were active throughout the 24.0h cycle, whereas the activity of the remaining two males was consistent with the 12.4h cycle. Using the 40-day recording, three horseshoe crabs, including the female, were primarily active around the new moon and full moon, but they were dormant around the first and third quarter moon days. The female spent much time in shallow shores (depth<0.3m) during the new moon and full moon. This result indicated that the female attempted to spawn in a minimum of three spring tide periods while lingering in the vicinity. Meanwhile, after spawning, the female spent time foraging in shallow water (depth 0.3–18m). As for the two male individuals, their activity was consistent with semi-lunar periodicity. Therefore, both ofthem were in amplexus. In addition, a solitary male individual was active only during the first and third quarter moon days. Through activity recording, all thein Inderasabah was active during daytime and night- time. This result was contrary toactivity cycles in western Japan, where the species was found to be primarily nocturnal.Perhaps, the regional differences in activity cycles forwere related to their population adaptation toward water temperature, depth, and prey-searching periods.
acceleration logger; activity cycle; depth-temperature logger; foraging behavior; spawning behavior; tropical region;
1 Introduction
Organisms living in coastal environments, which are ex- posed to environmental changes in day to night (24.0h) and tidal (12.4h) cycles, could adapt to complex environmen- tal changes in comparison with terrestrial organisms (for reviews, see Palmer, 1976; Naylor, 2010). Although acti- vity cycles of various terrestrial organisms have been in- vestigated, information on organisms living in coastal en- vironments is limited. In recent decades, physiological and biochemical experiments have been conducted to investi- gate the presence of biological clocks for intertidal arthro- pods(Naylor, 2010; Chabot and Watson, 2014). As a well-documented model species, American horseshoe crab Li- mulus polyphemus has been used to understand the horse- shoe crab biology in a global view. In brief, horseshoe crabs (class Merostomata) comprise an ancient marine ar- thropod group that only evolved minutely in their external morphology since the Cretaceous period (Botton et al., 1996). The four surviving species are assigned to three genera and two families, in which L. polyphemus is the only extant species of family Limulidae. This arthropod lin- gers in the intertidal zone along the eastern coast of North America (Sekiguchi and Shuster Jr., 2009). Earlier, artificial light/dark (24.0h) and tidal (12.4h) cycles were experimen- tally tested on L. polyphemus (Chabot et al., 2004, 2007, 2008). Considering that locomotion activities synchronize with the amplitude of artificial tidal cycles, L. polyphemus could only react toward an endogenous tidal (12.4h) clock. Based on these field observations, L. polyphemus possess a daily pattern of activity, which is being active either du- ring the daytime or nighttime (Chabot et al., 2004, 2007). Later experiments have revealed that temperature and pho-toperiod trigger the locomotion activity of L. polyphemus(Chabot and Watson, 2010). By identifying two daily bouts in the L. polyphemus activity, researchers believe that lo- comotion is driven by two separates, where independent circatidal rhythms for each (circalunidian) clock are ac- tive for a period of 24.8h (Chabot and Watson, 2010, 2014).
The variety of activity patterns expressed by each L. po- lyphemus population is due to genetic variations after be- ing exposed to tidal conditions in different regions (Tho- mas et al., 2020). Thus, evolution and adaptation are re- sponsible for the activity patterns (locomotion and reac- tion)of horseshoe crabs. The locomotion of L. polyphemus is studied using boxes and running wheels, whereas horse-shoe crab is restrained in water tanks (normal depth is less than 0.5m) in laboratory experiments (Chabot et al., 2004, 2007, 2008). However, the sea surface in natural environ- ments does not have consistent heights because tidal heightsdepend on lunar cycles. In addition, the capacity of experi- mental devices could only accommodate small (male) horse-shoe crabs. Thus, the spawning activity expressed by thefemales cannot be investigated in experimental conditions.In some field works, the activity of adult L. polyphemus was monitored by acoustic transmitters and fixed recei- vers in natural habitats. Adult horseshoe crabs have strong bimodal rhythms of activity. Therefore, the arthropod is significantly active during high tide (Watson and Chabot, 2010; Watson et al., 2016).
In comparison with, the activity cycles of the other three Asiatic Merostomata (,, and) are poor- ly studied. The difference between Asiatic and American lineages may occur during the Jurassic period and resultin at least 100 million years of behavioral isolation between the two groups of horseshoe crabs (Botton., 1996). Recently, the activity cycle of tri-spine horseshoe crab ()has been investigated using animal-borne data loggers in natural conditions of the Seto Inland Sea (Wa- tanabe., 2022). The use of animal-borne data loggers (biologging) to study the physiology and behavior of various species has increased over the past decades,thereby providing new insights into the behavior ofthese animals in their natural habitats (for reviews, see Cooke, 2008; Ropert-Coudert., 2009). In the Seto Inland Sea (West Japan), the activity cycle ofis affect- ed by day to night and tidal cycles. These species are pri- marily nocturnal and more active during the nighttime tide rise. The activity ofoccurs during daytime and nighttime during the spawning season in July and Au- gust (Watanabe., 2022).
broadly ranges in distribution from North (34˚N in Japan) to South (7˚S in Indonesia) Asia (see the distribution map in the IUCN Red List, Laurie., 2019). Although the center of distribution is in the tropical region of Southeast Asia, the activity cycles of this species has not been reported in Sabah. Moreover,in Kudat (north) and Kunak (east), which areisolated and less studied, have wide carapace measurements, whereas crabs from Semporna (southeast) are relatively smaller in size (Mohamad., 2021).
In this study, the activity cycles ofin the southeastern coast of Sabah (4˚N), Malaysian Borneo wereinvestigated from late September to early November in 2015. Then, the locomotion activity and surrounding environ- ment of free-ranging adultwere recorded us-ing biologging devices (acceleration and depth-tempera- ture loggers). This study aimed i) to provide information for the activity cycles ofin Sabah, a tropical region; ii) to compare the results with that in western Ja- pan, a temperate region; and iii) to present a preliminary un-derstanding of the ecological importance and evolution- ary significance of the activity cycles of.
2 Materials and Methods
2.1 Field Sampling
This study was conducted in a fishing village located in Inderasabah (4.30˚N, 118.18˚E), southeast Sabah (Malay- sian Borneo), facing the Celebes Sea (Fig.1). The bathy- metric data used the General Bathymetric Chart of the Oceans (GEBCO 2021), and contour lines with 20m depths were produced using QGIS ver.3 (https://qgis.org). In the map, a shallow sandy and muddy bottom area (depth<20m) located around 5–15km offshore from the coast falls within the vicinity of the village where approximately 3000 residents inhabit, and most of them are fishermen.
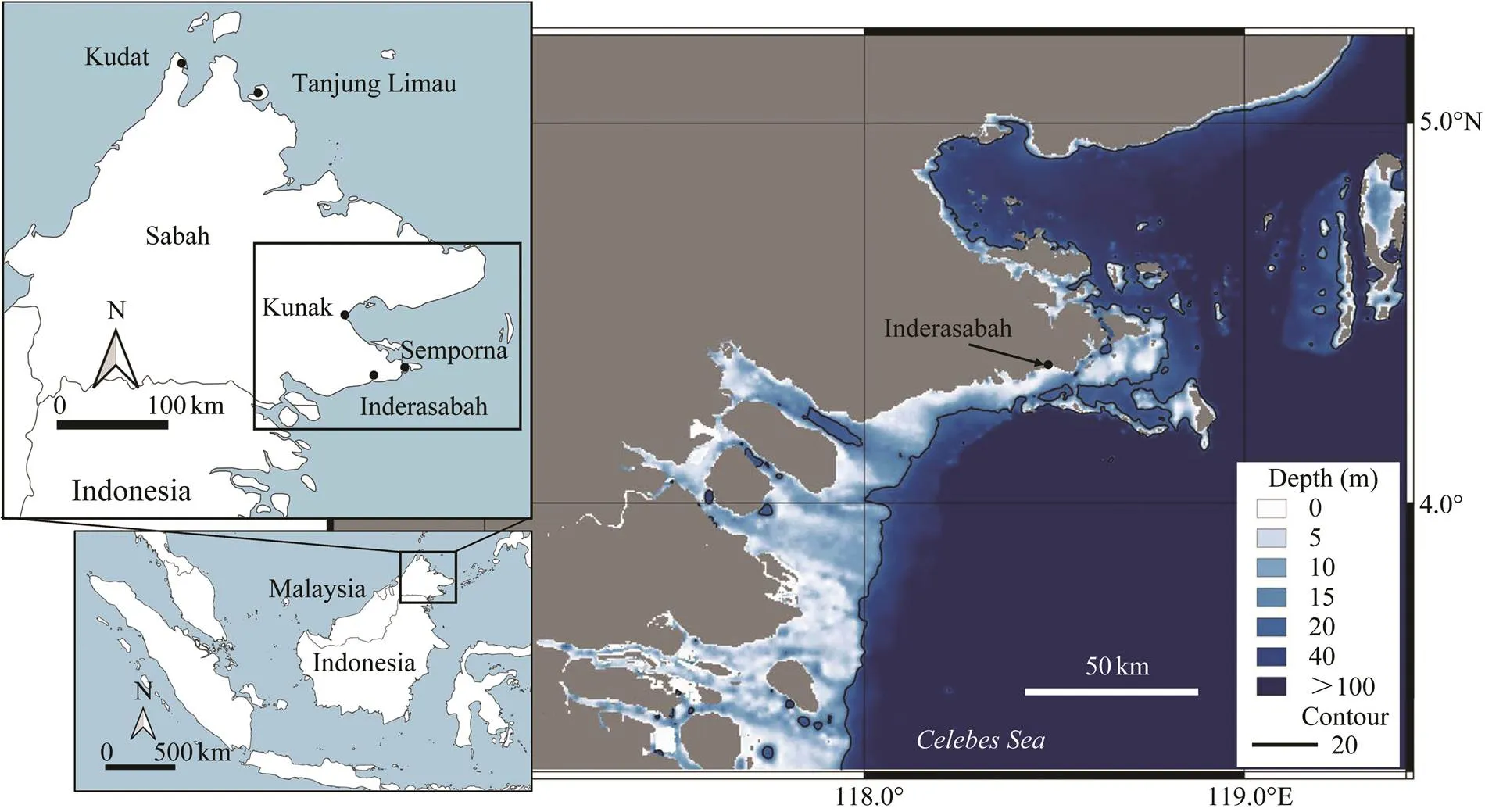
Fig.1 Map showing the location of study site, Inderasabah in Southeast Sabah, Malaysian Borneo. Thecontour lines with 20-m depth are shown on the bathymetric chart of the Celebes Sea.
This study site was used to estimate the population size ofusing a standard mark-recapture method (Manca., 2017). A total of 18 adult(8 females and 10 males) were collected from September 16 to 18, 2015, and used for this study. Horseshoe crabs were captured using 4-inch gill nets deployed into the sea down to 5–10m depth, approximately 1–3km off-shore. Then, they were marked with a white button tag on the left side of the prosoma, which had a unique individual number and researcher’s phone number (Fig.2). The detailed informa- tion of sampling is described by Manca. (2017). With this method, 15 mark-recapture samples werecollectedbe- tween 2014 and 2015, from which 182 to 1095 sexually ac-tive adultwere retrieved from this water (Manca., 2017). Our study was conducted at the end of the survey, from which sexually mature horse-shoe crabs were presented in amplexus and sexed by observing the presence of rounded (males) or elongated (females)pin- cers in the first and second appendage.

Fig.2 A female horseshoe crab Tachypleus tridentatus with (a) an acceleration logger: HOBO Pendant Glogger, (b) a depth-temperature logger: DST milli, and (c) a white button tag with the ID number and theresearcher’s phone number.
2.2 Biologging Devices
Two types of biologging devices were used for this study (Fig.2). An acceleration logger (HOBO Pendant G logger, 58mm×33mm×23mm, 18 gram in air, Onset Corporation, Pocasset, MA) was used to record the locomotion activity of a horseshoe crab. The acceleration logger was set to re- cord acceleration along the longitudinal axis at 1min in- tervals, which had a memory capacity of 64000 data points (Watanabe.,2022). A depth-temperature logger (DST milli, 13mm×39mm, 5g in water, Star-Oddi Co., Reykja- vik, Iceland) was used to record the surrounding environ- mental conditions. The depth-temperature logger had a me- mory capacity of 698800 depth and temperature data points with accuracies of 0.27m and 0.1℃, respectively. The depth-temperature logger was set to record depths and tempera- ture at 3 and 15min intervals for 3 years. All devices were set to start recording at 0:00 on September 20, approximate-ly 2–4d after horseshoe crabs were released (Table 1).
Both types of data loggers were fixed on a meshed PVC sheet (4cm×6cm) using two plastic cable ties and attach- ed to horseshoe crabs using two-component epoxy glue. The acceleration loggers were attached on the dorsal cara- pace of eight female and 10 male. Meanwhile, the depth-temperature loggers were attached on the telson of five female horseshoe crabs. After the glue was cured,all animals were released into the water at the captured point.
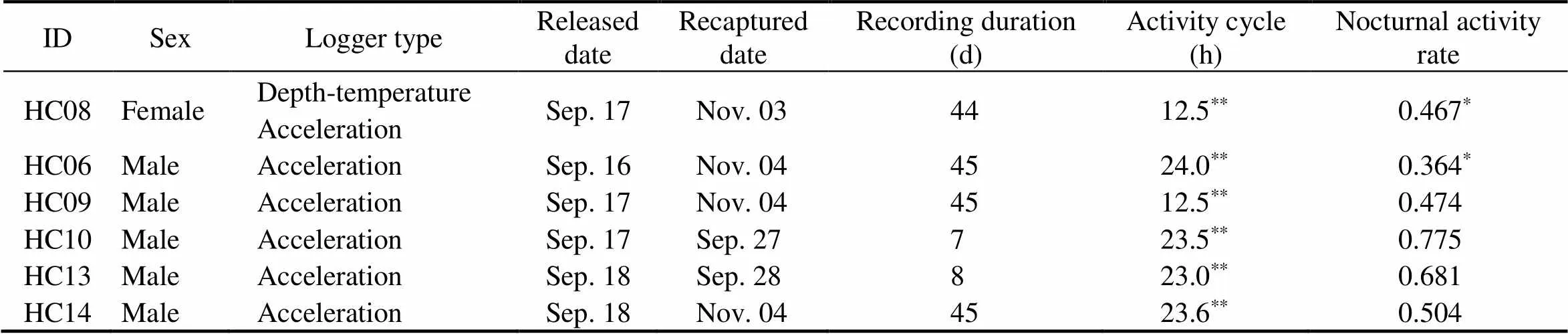
Table 1 Summary of logger data recorded on free-ranging tri-spine horseshoe crabs in the southeastern coast of Sabah, Borneo
Notes: All devices were set to start recording on September 20, 2015.*Wilcoxon signed-rank test,<0.05;**maximal value of the pri- mary component of periodicity,<0.01 in Lomb-Scargle periodogram analysis; activity cycle refers to movement detected by an accelerometer; nocturnal activity rate is the ratio of night time over the overall movement time detected for each horseshoe crab.
The tagged horseshoe crabs were recapture (as bycatch) passively through local fishing activities. We advertised the fishermen and other local people in Inderasabah to check the ID number on the white button tag and retrieve the data loggers. Then, compensation (postage fees) was provided upon receiving the loggers.
2.3 Data Analysis
Data from retrieved loggers were read out by using HOBO ware Pro for Pendant G logger (Onset Corporation, Pocasset, MA) or SeaStar for DST milli (Star-Oddi Co., Reykjavik, Iceland), and saved in ASCII format for further processing. Data processing was performed using custom-build macro programs in IGOR Pro ver. 6.0 (Wave Me- trics, Inc., Lake Oswego, OR). Based on the accelerationdata, we determined whether the animal was ‘active’ or ‘inactive’ by the minute. Acceleration signals were reflect- ed by accelerations related to changes in the movements of animals, that is, dynamic accelerations and gravitationalacceleration, resulting from changes in body posture (Yoda., 2001). The resolution of the acceleration sensor was 0.025g (0.245ms−2),which was sufficient to determine whether an animal was active or inactive. This practice was adopted from Watanabe. (2022). Changes between consecutive data points and longitudinal acceleration (deltaXG) were calculated. 0.1G (0.98ms−2) was used as an‘active’ or ‘inactive’criterion as the bimodal distribution with high values (>0.1G) corresponded to walking or dig- ging on the seafloor. Active point numerals were summed into 10min bins for the construction of double-plotted ac- tograms that represented rhythmicity (activity patterns are described in Fig.3).

Fig.3 Examples of activity cycles with a peak in the 24.0-h (a: HC14) and in the12.4-h (b: HC08)expressed by free-rang- ing adult horseshoe crabs Tachypleus tridentatus in Sabah, Malaysian Borneo. Upperleft panel: actograms double-plotted, black/white bars at the top indicate daytime (06:00–18:00) andnighttime (18:00–06:00). Upper right panel: cumulated number of activity points during each of daytimeand nighttime. Lower panel: Lomb-Scargle periodogram analyses of the actogram; vertical scale is relative power. Largest peak value above horizontal line of significance (P<0.01) indicated by numerical value (tau).
Double-plotted actograms and Lomb-Scargle periodo- grams (Lomb, 1976) were created for each animal to ex- amine their individual activity cycles as described in Cha- bot. (2008). The maximum value of primary perio- dicity components (,<0.01) was determined by us- ing a periodogram withnearly equal to 24.0h (Fig.3a) or 12.4h (Fig.3b). We calculated a cumulative number of active points from each daytime (06:00–18:00) and night- time (18:00–6:00) period to determine whether each horse- shoe crab exhibited a diurnal or nocturnal activity. The proportion of active points during nighttime (nocturnal activity rate) was also calculated for each horseshoe crab as an index for the degree of nocturnality (Watanabe., 2022).
Based on the depth and temperature data, boxplots with 10, 25, 50, 75, and 90 percentiles were created in each daily bin and then compared with the daily total activity times.The daily total activity time in shallow water (<0.3m, the accuracy of depth sensor) was estimated for the spawning activity of. This approach promotedarrival into the water edge (shore) or other- wise, where it remained in slightly deeper waters within the intertidal zone. In addition, daytime and nighttime wa-ter depth (average values) and active points (0 meansin- active; >0 means active) in each hourly bin were compared. Then, Wilcoxon signed-rank test (Zar, 2010) was used to determine the statistical significance (<0.05) among the average values because all data were non-parametric.
3 Results
For 10–49 days after horseshoe crabs were released, five malewith acceleration loggers and one fe- male with acceleration and depth-temperature loggers wererecaptured as bycatch through local fishing activities in In- derasabah (recapture rate: 33.3%, Table 1). The loggers were active for 7–45days, from which an overall 194 ac- tivity days were achieved for the six horseshoe crabs (Ta- ble 1). Periodogram analyses indicated four individuals with significant activity peaks in the 24.0h range (23.0–24.0h,., Fig.3a) and two individuals with peaks in the 12.4h range (Fig.3b, Table 1). Nocturnal activity rates that range from 0.364 to 0.775 only indicated two(HC06 and 08) with different daytime and nighttime activities (Table 1). Nocturnal activity rates of 0.364 and 0.467 (<0.05) indicated that two horseshoe crabs exhibited day- time locomotion. However, based on the actogram, all sixwere active during the daytime and nighttime.
Four horseshoe crabs (HC06, 08, 09, and 14) with acti- vity records for more than 40 days revealed that one male (HC14) was active daily, whereas the other crabs were dor-mant around the first and third quarter moon (Fig.4). Mean- while, the active and dormant periods of female (HC08) crabs were consistent with the moon phases (Fig.5). For female, the marked movement was witness- ed at least 1–2 days before the new moon or full moon, and its locomotion continued to the next first or third quarter moon. However, episodes of dormancy were observed du-ring the active periods, which began from the first and third quarter moon and continued for more than 5 days.

Fig.4 Temporal changes of data recorded by biologging devices on free-ranging adult horseshoe crabs Tachypleus tri-dentatus in Sabah, Malaysian Borneo in September to November 2015. Water depth and temperature data was recorded on the female (HC08).

Fig.5 Daily changes of activity, water temperature and depth, recorded on an adult female horseshoe crab Tachypleus tri- dentatus (HC08).
During the new and full moon, the female spent all the active periods in deeper water for at least 1–2 days before the first and third quarter moon (October 3 to 4 and Oc- tober 18, respectively) and for 6 days after the second full moon (October 28 to November 2, Fig.5). The female moved in the range of 0–18m depth with bimodal peaks at 0–3m and 9–12m, respectively (Fig.6). During the active periods, the female moved in a wide range of depth from shallow to deep water (depth 0–18m, Fig.5). The female was inactive when approaching deeper water (=94313,<0.001) either during the daytime or the nighttime (=139574,=0.7372; Fig.6).

Fig.6 Comparisons of histograms of water depth between active and inactive time (a) andbetweendaytime and night- time (b)recorded on an adult female horseshoe crab Tachypleus tridentatus (HC08).
4 Discussion
4.1 Recovery of Biologging Devices
In this study, 6 to 18 (recapture rate: 33%) tagged horse- shoe crabs were recaptured within 49 days after being re- leased. In 15 mark-recapture samplings ofin Inderasabah, four were recaptured as bycatch (recapture rate: 1.5%, Manca., 2017). Low recapture rates by mark-recapture samplings were also reported onpopulations in the east coast of Peninsular Malaysia (6%, Mohamad., 2015) and onpopulations in the American coasts with negligible tides (7%, Swan,2005) and within a semi-closed bay (11%, James-Pirri, 2010). In the semi-closed bay, depth-temperature loggers were attached to 15 adult, from which only one female (7% of tagged crabs) was recovered as a car- cass on the beach (James-Pirri, 2010). Compared with these studies, the recapturing of tagged horseshoe crabs became more efficient with the use of local fisheries.
4.2 Daily Periodicity of Activity
In this study, the majority (four of six, 67%) ofexhibited 24.0h (circadian) cycles for their activity. This finding is similar to the discovery in western Japan, where the activity of this arthropod coincides with natural photoperiods and tidal cycles (Watanabe., 2022). Inthe laboratory, artificial light or dark and tidal cycles re-vealed thatexhibited 12.4h activity cycles (Chabot., 2007). In addition, their activity was syn-chronized with rising tides during the light and dark periods.Therefore, after this experiment,was as- sociated with an endogenous circatidal (12.4h) clock that triggered its locomotion activity (Chabot., 2008). Un- like,possessed an endogenous circadian (24.0h) clock.
However, the daily activity patterns ofweredifferent in Sabah (Borneo) and the Seto Inland Sea (Japan). In this study, of the six individuals, only two showed sig- nificant differences by being more active during the day- time. None of the recapturedshowed noctur- nal activity. This activity pattern was similar to. In the experimental study on, only two of the eight individuals exhibited significantly more acti- vities during the light period than during the dark period. On the contrary, the remaining sixwere notsignificantly active under light or dark conditions (Chabot., 2008). More than half (57%) offrom western Japan exhibited nocturnal activity, whereas the othersshowed neither nocturnal nor diurnal activity (Wa- tanabe., 2022).
Considering thatwere fed on bivalves, polychaetes, crustaceans, and gastropods (Botton, 2009), we hypothesized thatalso predated on benthic polychaetes in western Japan. Considering thatwere mostly available during the nighttime, we could piece the prey searching usingnocturnal ac- tivity (Watanabe., 2022). In the present study, the female (HC08) spent all the active periods in deeper water after the spawning season, and the male (HC14) was ac- tive aside from the spawning ritual (Section 4.4). Thus,in Inderasabah were primarily active for forag- ing during the non-spawning season. In addition to day-time catching and size classifications (Mohamad.,2016; Manca., 2017; Mohamad., 2021), the food habits of adulthave not been reported in the tropical region. Thus, the regional chronotype differences inmay suggest that feeding habits during evo- lution have made temperate and tropical populations uni-que.
4.3 Dormant and Active Periods
In this study, prolonged inactive (dormant) periods for more than 5 days were found, which indicated that themoon or lunar phases were responsible for the arthropod’slocomotion into shallow water.in westernJapan behaves similarly under lunar periodicity. In the tem- perate environment of Japan,was active du- ring the spring tides after the new moon and full moon. This arthropod would become dormant for 2–3 days du- ring the neap tides after the first and third quarter moon (Watanabe., 2022). However, forin Sa- bah, their dormant periods were longer than those in west- ern Japan (Fig.4).
In Sabah, the spawning sites ofare found in Tanjung Limau, the northern part of Sabah facing the Sulu Sea (Fig.1). The inaccessible knee-deep muddy ter- rain of Inderasabah might be the nests in this vicinity (Mo- hamad., 2016). In Tanjung Limau, the number of am-plexus that relocate themselves into shallow water for theirspawning activity will remain high for at least 2–3 days after the new moon or full moon days. This finding coin- cides with our data and indicates that femalewill become active for at least several days prior to the spawning ritual.
On the contrary, the reduced activity ofmaybe related to the dormant periods. Low temperature is cru- cial for horseshoe crabs to conserve their energy before reproducing.For instance, we observed that the lowest tem- perature of the Seto Inland Sea is18℃ from November to May as previous report(Nishii, 1975). However, the aver- agetemperature of tropical waters around Inderasabah is29.7℃±0.81℃ (mean±SD, range 24.7–36.3℃).Given the contrasting exceed of the lower limit, the dormant be- havior inis unlikely triggered by tempera- ture dips. Hence, for populations in Inderasabah, the up-per limit of temperature tolerance of this speciesis unclear. Moreover, femaleremained active when the logger recorded a temperature higher than 35℃ (Fig.4). Thus, a water temperature of 29℃ indicates a completely sub-merged. Considering that temperature spikes (above 30℃) only occur during the daytime of new andfull moon periods (Fig.4),has reached water fringe and exposed the logger to the air. Given the depth- temperature logger on the female (HC08), its presence in shallow water (depth 0.1–0.3m) during lunar dayswould indicate its role in the spawning ritual (Section 4.4).
Femalewas constantly mobile in shallow water (depth<1m) during the daytime and nighttime and completely rested (inactive) in deeper water (depth9m) during several semi-lunar days (Fig.6). Based on water depth and temperature data, we found that HC08 avoided shal- low areas (depth<1m) during dormancy because water tem- peratures were exceeding 30℃, particularly during the day-time tide fall. Thus, femalemight opt to be- come dormant in deeper yet temperature-stable waters du- ring the non-breeding periods for energy conservation.
4.4 Lunar Periodicity of Activity
In general,spawns in shallow waters (Se- kiguchi, 1988; Laurie., 2019). It coincides with the movement of femalein such conditions (depth<0.3m) during the new moon and full moon periods. Con-sidering that moreemerge into shallow wa- ters as amplexus from April to October (Mohamad., 2016), November should be the late spawning season. In West Japan, the spawning activity ofis asso- ciated with diurnal and nocturnal high tides. The spawn- ing activity ofpeaks during lunar spring tides (Sekiguchi, 1988). Therefore, the female HC08, which was in shallow water during daytime and nighttime lunar tide rise, could exhibit 12.4h (circatidal) activity cycles.
During a spawning ritual, the male would latch onto the opisthosoma of the female by using its first and second modified pincers. This act does not obstruct the movementof the female (Sekiguchi, 1988). In this study, we found that two malehad their activity synchronized with the new moon and full moon days. These males were latching (in amplexus) onto their respective suitors. Hence, the semi-lunar periodicity in their activity is conclusive for spawning. However, the activity of one male (HC14) did not follow the moon phases. With more activities (perio- dicity in locomotion), the distance proximity and short read lengths within the acceleration graphs during the first and third lunar quarter moon indicate that this outlier (HC14) is solitary or alone. Although this study is based on the li- mited information obtained from one female and five male, we were able to develop a baseline for the behavioral ecology ofin southeast Sabah. Inaddition, later studies have good recapture efficiency, which indicates that the high percentage of biologging device re- trieval would rectify the knowledge gaps concerning the life history ofoutlined in this study.
Acknowledgements
This study was funded by the Sabah Biodiversity Cen- ter, SaBC (No. TJ 66917). The authors thank the villagers/fishermen in Inderasabah, especially Mr. Jeffri and his fa- mily, for their help during the sampling period and parti- cularly for the recoveries of biologging devices.
Botton, M. L., 2009. The ecological importance of horseshoe crabs in estuarine and coastal communities: A review and specula- tive summary. In:Tanacredi, J.,., eds., Springer, Heidelberg, 45-63.
Botton, M. L., Shuster Jr., C. N., Sekiguchi, K., and Sugita, H., 1996. Amplexus and mating behavior in the Japanese horse- shoe crab,., 13: 151-159, DOI: 10.2108/zsj.13.151.
Chabot, C. C., and Watson III, W.H., 2010. Circatidal rhythms of locomotion in the American horseshoe crab: Underlying mechanisms and cues that influence them., 56: 499-517, DOI: 10.1093/czoolo/56.5.499.
Chabot, C. C., and Watson III, W. H., 2014. Biological rhythms in intertidal animals. In:. Numa-ta, H., and Helm, B., eds., Springer, New York, 41-64.
Chabot, C. C., Betournay, S. H., Braley, N. R., and Watson III, W. H., 2007. Endogenous rhythms of locomotion in the Ameri- can horseshoe crab,., 345: 79-89, DOI: 10.1016/j.jembe.2007.01.009.
Chabot, C. C., Kent, J., and Watson III, W. H., 2004. Circatidal and circadian rhythms of locomotion in., 207: 72-75, DOI: 10.2307/1543630.
Chabot, C. C., Skinner, S. J., and Watson III, W. H., 2008. Rhy- thms of locomotion expressed by, the American horseshoe crab: I. synchronization by artificial tides., 215: 34-45, DOI: 10.2307/25470681.
Cooke, S. J., 2008. Biotelemetry and biologging in endangered species research and animal conser-vation: Relevance to regional, national, and IUCN Red List threat assessments., 4: 165-185, DOI:10.3354/esr00063.
General Bathymetric Chart of the Oceans, 2020. The gridded ba- thymetric data set (). Available at: https://www.gebco.net/data_and_products/gridded_bathymetry_data/. Accessed 5th September 2021.
James-Pirri, M. J., 2010. Seasonal movement of the American horseshoe crabin a semi-enclosed bay onCape Cod, Massachusetts (USA) as determined by acoustic te- lemetry., 56: 575-586, DOI: 10.1093/czoolo/56.5.575.
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H., John, A.,., 2019..: e.T21309A149768986, DOI: 10.2305/IUCN.UK.2019-1.RLTS.T21309A149768986.en.
Lomb, N. R., 1976. Least-squares frequency analysis of unequal-ly spaced data., 39: 447-462, DOI: 10.1007/BF00648343.
Manca, A., Mohamad, F., Ahmad, A., Sofa, A. M., and Ismail, N., 2017. Tri-spine horseshoe crab,(L.) in Sabah, Malaysia: The adult body sizes and population estimate., 10: 355-361, DOI: 10.1016/j.japb.2017.04.011.
Mohamad, F., Ismail, N., Ahmad, A. B., Manca, A., Rahman, M. Z. F. A., Bahri, M. F. S.,., 2015. The population size and movement of horseshoe crab (Müller) on the east coast of Peninsular Malaysia. In:Carmichael, R. H.,., eds., Springer, Heidelberg, 213-228.
Mohamad, F., Manca, A., Ahmad, A., Fawwaz, M., Sofa, A. M., Alia’m, A. A.,., 2016. Width-weight and length-weight relationships of the tri-spine horseshoe crab,(Leach 1819) from two populations in Sabah, Malay- sia: Implications for population management., 11: 1-13.
Mohamad, R., Paul, N. A., Isa, N. S., Damanhuri, J. H., Shahimi, S., Pati, S.,., 2021. Using applied statistics for accurate size classification of the endangeredhorseshoe crab.,22(4): 273-282, DOI: 10.12911/22998993/132432.
Naylor, E., 2010.. Cam- bridge University Press, Cambridge, 252pp.
Nishii, H., 1975.. Private publication, Kasaoka, 221pp (in Japanese).
Palmer, J. D., 1976.. Aca- demic Press, New York, 375pp.
Ropert-Coudert, Y., Beaulieu, M., Hanuise, N., and Kato, A., 2009. Diving into the world of biologging., 10: 21-27, DOI: 10.3354/esr00188.
Sekiguchi, K., 1988.. Science House Co., Tokyo, 428pp.
Sekiguchi, K., and Shuster Jr., C.N., 2009. Limits on the global distribution of horseshoe crabs (Limulacea): Lessons learned from two lifetimes of observations: Asia and America. Biol- ogy and conservation of horseshoe. In:Tanacredi, J.,., eds., Springer, Heidelberg, 5-24.
Swan, B. L., 2005. Migrations of adult horseshoe crabs,, in the middle Atlantic Bight: A 17-year tagging study., 28: 28-40, DOI: 10.1007/BF02732751.
Thomas, T. N., Watson III, W. H., and Chabot, C. C., 2020. The relative influence of nature. nurture on the expression of circatidal rhythms in the American horseshoe crab., 649: 83-96, DOI: 10.3354/meps13454.
Watanabe, S., Oyamada, S., Mizuta, K., Azumakawa, K., Mori- nobu, S., and Souji, N., 2022. Activity rhythm of the tri-spine horseshoe crabin the Seto Inland Sea, western Japan, monitored with acceleration data-loggers. In:–.Tanacredi, J. T.,., eds., Springer, Heidelberg (in press).
Watson, W. H., and Chabot, C. C., 2010. High resolution track- ing of adult horseshoe crabsin a New Hampshire Estuary using fixed array ultrasonic telemetry., 56: 599-610, DOI: 10.1093/czoolo/56.5.599.
Watson, W. H., Johnson, S. K., Whitworth, C. D., and Chabot, C. C., 2016. Rhythms of locomotion and seasonal changes in ac- tivity expressed by horseshoe crabs in their natural habitat., 542: 109-121, DOI: 10.3354/meps11556.ar.
Yoda, K., Naito, Y., Sato, K., Takahashi, A., Nishikawa, J., Ro- pert-Coudert, Y.,., 2001. A new technique for monitoring the behaviour of free-ranging Adélie penguins., 204: 685-690, DOI: 10.1242/jeb.204.4.685.
Zar, J. H., 2010..5th edition. Prentice Hall/Pearson, NJ, 960pp.
J. Ocean Univ. China(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5190-0
ISSN 1672-5182, 2022 21 (3): 549-556
(September 8, 2021;
October 11, 2021;
March 7, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
Corresponding author. E-mail: watanabe@l-leo.com
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe Crab Tachypleus tridentatusEggs
- Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
- Experimental Investigation on the Interactions Between Dam-Break Flow and a Floating Box
- Variational Solution of Coral Reef Stability Due to Horizontal Wave Loading
- High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
- Influence of Autonomous Sailboat Dual-Wing Sail Interaction on Lift Coefficients
