Influence of Tidal Cycles on Embryonic Rotation, Hatching and Emergence of Mangrove Horseshoe Crab,Carcinoscorpius rotundicauda
KUANG Yang, TAN Kian Ann, FU Yijian, YANG Xin, XU Peng, ZHEN Wenquan,WANG Xueping, HUANG Xing, ZHU Junhua, WANG Chun-Chieh, and KWAN Kit Yue, *
Influence of Tidal Cycles on Embryonic Rotation, Hatching and Emergence of Mangrove Horseshoe Crab,
KUANG Yang1), #, TAN Kian Ann1), 2), #, FU Yijian1), YANG Xin1), XU Peng1), ZHEN Wenquan1),WANG Xueping1), HUANG Xing1), ZHU Junhua1), WANG Chun-Chieh3), and KWAN Kit Yue1), *
1),,,535011,2),,430070,3),,530007,
Horseshoe crabs are iconic and ecologically significant macroinvertebrates in coastal environments. The processes and mechanisms of larval hatching in Asian horseshoe crabs that occurs beneath the sand are largely unknown. The spawning and deve- lopmental ecology ofandare assumed to be similar to their Atlantic counterpart. However,has been cited as an exception owing to their frequent sightings in muddy mangrove areas even during low tides. To reveal the larval hatching mechanisms, in this study, we examined varying hatching responses ofembryos within the sediment to the environmental conditions under continuous tidal cycles. During the eight-week experiment, the count of hatched larvae ranged 4%–30% per week, while the cumulative emergence rate from the sediment was 0–47%. Embryos were observed to have the highest active rotation activity in the first two weeks after incubation. The inundation of tidal water significantly enhanced the occurrence of hatching, in which hydration, osmotic shock and possibly agitation had triggered or facilitated the eclosion. The larvae were found to remain in the sediment for approximately 2–6 weeks before emergence. In general,was found to share a similar hatching mechanism with. Our findings provide insight into the developmental ecology of Asian horseshoe crabs exposed to varying tidal conditions, and are helpful to the management and protection of their spawning habitats.
environmental cue; hydration; osmotic pressure; tidal inundation; egg; trilobite larva
1 Introduction
Horseshoe crabs are an ancient group of invertebrates that have survived for more than 450 million years (Rud- kin and Young, 2009). There are three extant Asian spe- cies, namely mangrove horseshoe crab,; tri-spine horseshoe crab,;and coastal horseshoe crab,, which can be found along the west coast of the Pacific Ocean. They are ecologically significant in coastal and estuarine food webs as prey and predators, providing protein- and lipid-rich eggs to shorebird populations, and reflecting the general health of coastal ecosystems (Botton, 2009; Kwan., 2018, 2021). Despite the IUCN Red List status ofwas currently ‘data deficient’, the status is un- der reassessment in view of recent reports describing the apparent population declines in some Asian regions (Wang., 2020). The extensive loss of mangrove habitats and the widespread event of fishing bycatches in Asia may haveaffected the existence ofpopulations (John., 2018; Supadminingsih., 2019).is legally protected in China, Bangladesh, Indonesia and India, despite the limited baseline data of their population status and habitat requirements (Wang., 2020).
The reproduction ecology of Atlantic horseshoe crab,has been studied extensively (Smith., 2017). The spawning pairs in amplexus migrate to sandy estuarine beaches, lay thousands of eggs in the mid to upper-intertidal zones, and deposit them at 15cm or deep- er beneath the sediment (Weber and Carter, 2009; Botton., 2010) to minimize the adverse impacts from wave action, extreme temperature and osmotic fluctuation (Rud- loe, 1979; Botton., 1994; Penn and Brockmann, 1994). Fertilized eggs undergo embryonic development within the sediment, followed by the hatching of trilobite larvae (first instars) after 2–4 weeks.
The processes and mechanisms of larval hatching of horseshoe crabs, which occurs beneath the sand remainlargely unknown. For, the larval abundance peaks at high spring tides (Botton and Loveland, 2003; Ehlinger., 2003). Although hatching can occur with- out external stimuli (Jegla, 1979; Ehlinger and Tankersley, 2003), it appears that environmental cues relevant to tidal inundation, such as hydration, osmotic shock and mecha- nical agitation, can stimulate or facilitate eclosion, which help the dispersal of larvae away from the nesting loca- tions (Ehlinger and Tankersley, 2003; Botton., 2010). A previous laboratory study observed the 2–5 times high- er hatching levels ofstage-21 embryos when exposed to 24-h submergence at high tide compared to controls incubated using moist paper towels (Ehlinger and Tankersley, 2003).
The developmental ecology study of Asian horseshoe crabs is limited compared to their Atlantic counterpart,. The existing data for Asian species are largely descriptive and assumed to be the same as those of. Horseshoe crab egg clusters are usually found on estuarine beaches with well-oxygenated sand to maximize egg survival (Nelson., 2015; Mohamad,2019), while the spawning adults ofare frequently sighted in the vicinity of muddy man- grove swamps even during low tides (Cartwright-Taylor, 2015; Fairuz-Fozi, 2018; Liao., 2019). There-fore,larval hatching and dispersal mecha- nisms might be different.
The aim of this study was to examine the hatching re- sponse ofembryos within the ‘nests’ un- der continuous simulated tidal cycles. We did not attempt to investigate the effects of environmental parameters (cues) and their interactions in a complete factorial design. Our primary focus was to understand embryonic responses to varying tidal conditions, along with the changes in environ- mental parameters, to replicate the tidal processes at the spawning habitats. These findings can enhance our under- standing of the developmental ecology of Asian horseshoe crabs, which will be helpful for the management and con- servation of Asian horseshoe crabs.
2 Materials and Methods
2.1 Experimental Setup
Five replicates of experimental setups were prepared. Each experimental setup consisted of a tall acrylic tank (hereafter ‘column’; 130cm×30cm×30cm), a rectangular acrylic box (hereafter ‘experimental box’, 15cm×15cm×15cm) and a reservoir for water storage (Fig.1). Each ex- perimental box contained a 9-cm sediment layer obtained from the spawning habitats ofin the nor- thern Beibu Gulf. There were small holes on three lateral sides of the box, except for the side where embryos were placed (Fig.1). The box was located at the bottom of the column throughout the experiment. An air pump and a wa- ter pump were placed within the column. The water pump was connected to the reservoir to simulate the tidal cycles. A sun lamp was positioned on the top of the column to re- plicate the natural daylight (Fig.1).

Fig.1 The experimental setups maintained under simulat- ed tidal conditions.
The northern Beibu Gulf has a diurnal tidal cycle which typically experiences one high and one low tide every lu- nar day.eggs were mostly found within the high tide zones with elevated, mildly slop- ing substratum (Fairuz-Fozi., 2018; Zauki., 2019). During high tides, preliminary studies demonstrated that the average water level inundating the high-density spawn- ing area along the northern Beibu Gulf shore was appro- ximately 120cm. In the present experiment, filtered artifi- cial seawater was pumped gradually from the reservoir in-to the column until reaching the maximum level at 120cmafter 30min at simulated high tides. Similarly, during simu- lated falling tides, the seawater was completely withdrawn from the columns within the same duration of time. Simu- lated rising and falling tides were manipulated once every day in the morning (08:00) and late evening (20:00), re- spectively. The light:dark cycle of the system was set to 12h:12h.
2.2 Experimental Animals
eggs were collected from Jiaodong and Yuzhouping beaches in the northern Beibu Gulf, China during summer 2020.eggs were distinguished fromby their discernably smaller size (diameter 1.9–2.2mm3.0–3.3mm for) with greenish-yellow color (Fig.2a). The eggs were cultured in the laboratory until reaching the late-stage (stage 20) embryos (Fig.2b). A stage- 20 embryo was characterized by the following criteria: 1) opaque chorion has split, 2) there is an active rotation with-in the clear inner egg membrane, 3) prosomatic appendages are fully segmented, and 4) its size is about double of the fertilized egg diameter.
Thirty developing embryos were randomly selected andplaced in each experimental box at the depth of 1.5cm within the sediment following the actual depth ofnests in the natural field (1.5–5.0cm; Fairuz- Fozi., 2018). The use of developing embryos in this study was to eliminate the potential data interference caused by the unfertilized or dead undeveloped eggs, which were difficult to be visually distinguished even under microsco- pic inspection (Vasquez., 2015). The embryos were arranged linearly in front of a lateral side of the experi- mental box (Fig.1), so that the hatching process could be clearly observed. The number of experimental embryos wassimilar to the actual range of the average eggs per nest (20–72) in their spawning habitats (Khan, 2003). The de- veloping larvae were incubated under the following envi- ronmental conditions during high tides similar to their spawning habitats along the Beibu Gulf shores: water tem- perature 30℃, salinity 20, dissolved oxygen DO 6mgL−1and pH 7.7. To depict the variations of environmental pa- rameters during the simulated rising and falling tides, tem- perature, DO and volumetric water content were measured in the environment near the embryos. Water temperature and DO were determined using a handheld YSI DO meter (ProODO, OH, USA), whereas volumetric water content was obtained with a portable soil analyzer (PRSENS, Shan-dong, China). The measurements were repeated three times and the average value was considered as the final value.

Fig.2 (A) Developing eggs and (B) stage-20 embryos of C. rotundicauda. C. rotundicauda eggs are distinguished from T. tridentatus by their discernably smaller size with greenish-yellow color, while the stage-20 embryos have apparently lar- ger size with active rotation activity in the clear inner egg membrane.
2.3 Video-Based Measurements
The experiment lasted for eight weeks, which encom- passed the entire incubation period for most embryos to complete the development and survive to be the trilobite larvae (2–4 weeks; Botton., 2010). The numbers of 1) embryos with active rotation within the clear inner eggmembrane (hereafter ‘rotating embryos’), 2) hatched trilo-bite larvae that remained within the ‘nests’ (hereafter ‘hatch- ed larvae’), and 3) emerged larvae from the sediment sur- face (hereafter ‘emerged larvae’), were recorded during si- mulated rising/falling tides through videos. Since the ex- perimental embryos/larvae could not be tagged individual- ly, the counting was based on the number of observations. The cumulative survival rate was calculated by deducting the count of embryos with apparent signs of decay that remained within the sediment at the end of the experiment. The entire video recordings lasted for 50min for each si- mulated rising and falling tidal process, respectively (10min prior to the simulated rising/falling tides, 30min for tidal processes with gradual changes in water levels, and 10min after reaching the maximum/minimum water le- vels). The total counts of embryonic/larval activities dur- ing the simulated rising/falling tides were categorized as follows: water levels 0cm, 1–40cm, 41–80cm, 81–119cm and 120cm, and.
2.4 Statistical Analysis
All data were examined for normality and homogeneity of variance prior to the analysis. Regardless of any possi- ble arithmetic transformations, the abundance data did not meet the normality requirements. Thus, non-parametric Scheirer-Ray-Hare extension of Kruskal-Wallis tests was employed to determine the differences among experimen- tal weeks and water levels during the simulated rising and falling tides, respectively. Once a significant difference was found, Kruskal-Wallis tests were used to examine the dif- ferences among experimental weeks or water levels, follow- ed by multiple comparisons between experimental groups with Bonferroni’s correction. The above statistical analy- ses were performed in SPSS Statistics version 26 (IBM, NY, USA), and Scheirer-Ray-Hare extension of the Krus- kal-Wallis test was conducted following the modified SPSS protocol of Shen. (2013).
3 Results
3.1 Embryonic Rotation Activities
Cumulative survival rates forembryos at the end of the eight-week experiment ranged from 40% to 95%. The number of ‘rotating embryos’ (., embryos with the active rotation within clear inner egg membrane) was significantly different among the experimental weeks, but not across water levels during both simulated rising and falling tides (Table 1, Fig.3). The count of rotating em- bryos was significantly higher during the first two weeks of the experiment, regardless of the simulated tidal condi- tions (Fig.3). It is also important to note a 60%–77% in-crease in the number of rotating embryos at 1–120cm wa- ter levels compared to that at 0cm, however, the difference was insignificant (Fig.3A).

Table 1 Statistical analysis results of observation counts of embryonic/larval activities among experimental weeks, water levels and their interactions during simulated rising and falling tides using Scheirer-Ray-Hare extension of Kruskal-Wallis test
Note: Thevalues with significance (<0.05) are in bold.

Fig.3 Number of embryos (mean±standard error) with ac- tive rotation within the clear inner egg membrane (‘rotat- ing embryos’) among varying experimental weeks and wa- ter levels during the simulated (A) rising and (B) falling tides (n=5). The data were analyzed by the post-hoc Krus- kal-Wallis tests followed by multiple Mann-Whitney U tests with the statistical difference among weeks (P<0.05) represented by different lowercase letters.
3.2 Larval Hatching and Emergence
The percentage of embryos hatched and developed into the trilobite stage varied from 3.8% to 29.5% per week. The results demonstrated that the water level, but not the experimental time, during the stimulated rising tides has a significant effect on the larvae hatching (Table 1, Fig.4). A significantly higher number of hatched larvae was obser- ved when the water level was between 1cm and 120cm than those with no water level change (., water level 0cm, Fig.4). However, the count of hatched larvae that was retained within the sediment during the simulated falling tides was statistically similar at varying water levels across the experimental weeks (Table 1).
The cumulative emergence rates of trilobite larvae from the sediment ranged 0–47%. The number of emerged lar- vae was statistically indistinguishable at different water le- vels and among the experimental time (Table 1). The du- ration between larval hatching and emergence was appro- ximately 2–6 weeks.
3.3 Changes in Environmental Conditions During Simulated Tidal Cycles
Water temperature within the sediment remained rela- tively stable between 26℃ and 29℃ during the simulated rising tides (Fig.5). Dissolved oxygen level declined gra- dually from around 6mgL−1to 4mgL−1when the water le-vel reached 120cm. Volumetric water content increased dramatically from 11% before the water rising to 60% after the water level achieved 40cm, and maintained at appro- ximately 63% throughout the water rising process.
During the simulated falling tides, water temperature in the environment near the embryos within the sediment was 27–28℃. Dissolved oxygen concentration experienced a nearly double increase from 4.0–4.3mgL−1during the wa- ter falling process to 8.4mgL−1when the water was com- pletely drained from the experimental setups (Fig.5). Vo- lumetric water content rose steadily from 57% to 61%, ex- cept for a drop at the water level of 1–40cm.
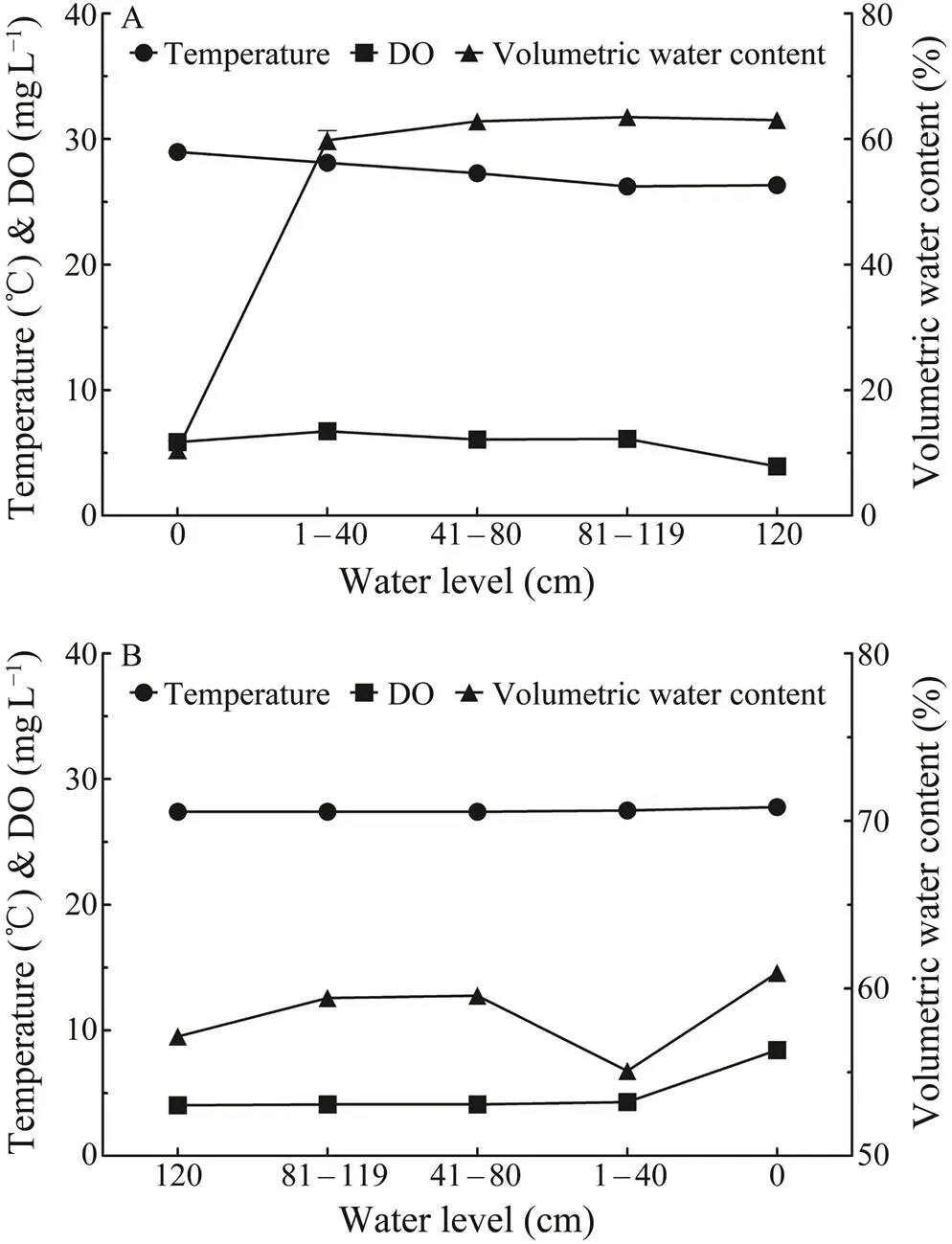
Fig.5 Primary environmental parameters, including water temperature, dissolved oxygen (DO) and volumetric water content within sediments at different water levels during the simulated (A) rising and (B) falling tides.
4 Discussion
Larval hatching is a sophisticated process involving phy- siological and ecological transitions throughout the deve- lopmental stages within the life cycle (Warkentin, 2011). The hatching process occurs only after the completion of morphological and physiological functional trait develop- ment, which allows their survival, growth and reproduc- tion in an open environment (Warkentin, 2011). Previous studies demonstrated that the larval hatching in marine organisms can be triggered by chemical/biochemical sub- stances (Tankersley., 2002; Ziegler and Forward, 2007),physical disturbances (Griem and Martin, 2000; Martin., 2011), and sudden changes in physiochemical con- ditions such as hydration and hypoxia (Ehlinger and Tan- kersley, 2003; Polymeropoulos., 2016). These cues can help larval hatching and emergence during suitable en- vironmental conditions that often maximize survival and growth rates (Botton., 2010; Vasquez., 2015).
The hatching process of the embryos ofand other Asian horseshoe crabsis virtually unknown. For their Atlantic counterpart, major releases of larvae were coincided with high spring tides, particu- larly when localized storms with strong onshore winds oc- curred, and the water reached the level of the nests (Rud- loe, 1979). Similarly, previous laboratory studies demon- strated that hatching ofembryos was sti- mulated by the environmental cues relevant to high water conditions such as hydration, hypoosmotic shock and agi- tation (Ehlinger and Tankersley, 2003; Botton., 2010). However, it is unknown whether and how the tidal cycles of submersed and dry/moisture conditions, and the asso- ciated changes in temperature, salinity and other environ- mental parameters, affect the hatching process. The pre- sent results found that the number oflar- vae was significantly higher during the water rising pro- cess when the embryos were inundated by tidal water. Theembryonic rotation within the transparent inner membrane is a characteristic behavior during the latter stage of de- velopment prior to hatching. In this study, there was also a 60%–77% increase in the number of rotating embryos at 1–120cm water levels compared to that before the wa- ter rising (0cm) in the first week (Fig.3A). This difference was not significant, probably due to the greater variation of responses among experimental replicates (Fig.3). The findings were consistent with that of, reaf- firming the important role of hydration in triggering the hatching in horseshoe crab embryos. A possible explana- tion is that hydration can induce a hypoosmotic shock and cause the embryos to swell and eventually rupture to re- lease the developed larvae (Hayakawa., 1985; Eh- linger and Tankersley, 2003). The secretion of osmotically active solutes, such as hexose and uronic acid, into the pe- rivitelline fluid can maintain the slightly hyperosmotic os- molarity of embryos at around 750mmolkg−1, which cor- responded to an approximate salinity of 30 (Sugita, 1988; Ehlinger and Tankersley, 2003).
In addition to hydration and osmotic shock, other en- vironmental triggers such as external agitation induced by wave action and hypoxic condition may also play roles in the hatching of horseshoe crab embryos. Ehlinger and Tan- kersley (2003) observed thatstage-21 em- bryos exhibited a higher hatching rate when exposed to simulated agitation using a flat tray shaker at a speed of 60rmin−1for 30min with the addition of hydration and sand provision. In the present study, external agitation was likely to be present when the large amount of water enter- ing and outflowing during simulated tidal cycles, even though the magnitude of agitation was not quantified dur- ing the experiment. Hypoxia was also frequently cited as one of the important triggers for egg hatching in myriad assortments of fish, amphibians and reptiles (Warkentin, 2002; Doody, 2011; Polymeropoulos., 2016). Hypo- xic conditions (., O2<2mgL−1) were not observed dur- ing the present experiment, despite an apparent reduction in DO level to around 4mgL−1at the maximum water le- vel (120cm) during the simulated rising tides. In the ex- periment by Ehlinger and Tankersley (2003), hypoxia was not observed to facilitate the hatching ofembryos. Instead of being an environmental trigger, hy- poxia might be an unfavorable incubation condition foreggs because the clusters are naturally de- posited at the shallow depths of 2–5cm within the sedi- ment, where the anaerobic conditions in muddy mangrove areas can be avoided (Fairuz-Fozi, 2018). A labora- tory study also pointed out that the three-day exposures at DO 2 and 4mgL−1decreased feeding rate, respiration rate and scope for growth of the early instars of(Shin., 2014). The current findings also supported the hypothesis that hatched larvae would remain in nests for several more weeks (circa 2–6 weeks in this study) be- fore being transported from the breeding grounds (Rudloe, 1979; Botton., 2010; Srijaya., 2014).
Overall, the present results enhance the holistic under- standing of hatching process ofembryos under continuous tidal cycles. The transport of emerged lar-vae was not explored in this study, but warrants further investigation. Trilobite larvae are weak swimmers; how- ever, high densities of juvenile Asian horseshoe crab po- pulations are found several kilometers away from the near- by nesting sites (Seino., 2000; authors’ personal ob- servations), and crowded along the outer edges of man-grove forests (Xie., 2020). We postulate that the new- ly hatched larvae are passively transported by tidal creeks among mangrove patches. The settlement process may also be aided by chemical cues from submerged coastal vege- tations (Butler and Tankersley, 2020). It is also suggested that the larvae are able to swim vertically to take advan- tage of selective tidal stream transport in the appropriate direction (Botton, 2010).
5 Conclusions
This study provides novel insight into larval hatching response ofto environmental conditions during continuous tidal cycles. The number of embryos with active rotation activity was significantly higher dur- ing the first two weeks of the experiment, regardless of the tidal conditions. On the other hand, water level during the rising tides was found to influence the hatching, inwhich a statistically higher count of hatched larvae oc-curred after the beginning of rising tides. Larval emergence rates did not vary among different experimental time and water levels. The findings are consistent with the avail- able data from both previous laboratory studies and field observations ofthat high water conditions, including hydration, osmotic shock and possibly agitation, can stimulate or facilitate the hatching of horseshoe crabs. The results can deepen the understanding of Asian horse- shoe crab developmental ecology and identify their criti- cal habitat requirements, which will facilitate the manage- ment strategies.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 32060129), Guangxi BaGui Youth Scholars Programme, and Guangxi Recruitment Program of 100 Global Experts. The assistance from Dr. Justin Bopp of Michigan State University, U.S. for proof reading this article is highly appreciated.
Botton, M. L., 2009. The ecological importance of horseshoe crabs in estuarine and coastal communities: A review and spe-culative summary. In:. Tanacredi, J. T.,., eds., Springer, Massachusetts, Boston, 45-63.
Botton, M. L., and Loveland, R. E., 2003. Abundance and dispersal potential of horseshoe crab () larvae in the Delaware estuary.,26: 1472-1479.
Botton, M. L., Colon, C. P., Sclafani, M., Loveland, R. E., Elbin, S., and Parkins, K., 2021. The relationships between spawning horseshoe crabs and egg densities: Recommendations for the assessment of populations and habitat suitability., 31: 1570- 1583.
Botton, M. L., Loveland, R. E., and Jacobsen, T. R., 1994. Site selection by migratory shorebirds in Delaware Bay, and its relationship to beach characteristics and abundance of horseshoe crabeggs., 111: 605-616.
Botton, M. L., Tankersley, R. A., and Loveland, R. E., 2010. De- velopmental ecology of the American horseshoe crab.,56: 550-562.
Brockmann, H. J., 1990. Mating behavior of horseshoe crabs,., 114: 206-220.
Butler, C. B., and Tankersley, R. A., 2020. Smells like home: The use of chemically-mediated rheotaxes bylarvae., 525: 151323.
Cartwright-Taylor, L., 2015. Studies of horseshoe crabs around Singapore. In:. Carmichael, R. H.,., eds., Springer, Zug, Cham, 193-211.
Doody, J. S., 2011. Environmentally cued hatching in reptiles., 51(1): 49-61.
Ehlinger, G. S., and Tankersley, R. A., 2003. Larval hatching in the horseshoe crab,: Facilitation by environmental cues.,292(2): 199-212.
Ehlinger, G. S., Tankersley, R. A., and Bush, M. B., 2003. Spatial and temporal patterns of spawning and larval hatching by the horseshoe crab,, in a microtidal coas- tal lagoon., 26: 631-640.
Fairuz-Fozi, N., Satyanarayana, B., Zauki, N. A. M., Muslim, A. M., Husain, M. L., Ibrahim, S.,., 2018.(Latreille, 1802) population status and spawning behaviour at Pendas coast, Peninsular Malaysia., 15: e00422.
Griem, J. N., and Martin, K. L. M., 2000. Wave action: The en- vironmental trigger for hatching in the California grunion(Teleostei: Atherinopsidae)., 137: 177-181.
Hayakawa, M., Tanimoto, S., Kondoand, A., and Nakazawa, T., 1985. Changes in osmotic pressure and swelling in horseshoe crab embryos during development: (horseshoe crab embryo/swelling/water influx/perivitelline fluid/osmotic pressure).,27(1): 51-56.
Jegla, T. C., 1979. Thebioassay for ecdysteroids, 156: 103-114.
John, B. A., Nelson, B. R., Sheikh, H. I., Cheung, S. G., War- diatno, Y., Dash, B. P.,., 2018. A review on fisheries and conservation status of Asian horseshoe crabs., 27(14): 3573-3598.
Khan, R. A., 2003. Observations on some aspects of the biology of horseshoe crab,(Latreille) on mud flats of Sunderban estuarine region., 101: 1-23.
Kwan, B. K. Y., Un, V. K. Y., Cheung, S. G., and Shin, P. K. S., 2018. Horseshoe crabs as potential sentinel species for coastal health: Juvenile haemolymph quality and relationship to habi- tat conditions., 69: 894-905.
Kwan, K. Y., Bopp, J., Huang, S., Chen, Q., Wang, C. C., Wang, X.,., 2021. Ontogenetic resource use and trophic dynamics of endangered juvenileamong diversified nursery habitats in the northern Beibu Gulf, China., 16 (6): 908-928, DOI: 10.1111/1749-4877.12495.
Liao, Y., Hsieh, H. L., Xu, S., Zhong, Q., Lei, J., Liang, M.,, 2019. Wisdom of Crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China., 53(2): 222-229.
Martin, K., Bailey, K., Moravek, C., and Carlson, K., 2011. Ta- king the plunge: California grunion embryos emerge rapidly with environmentally cued hatching., 51 (1): 26-37.
Mohamad, F., Mohd Sofa, M. F. A., Manca, A., Ismail, N., Che Cob, Z., and Ahmad, A. B., 2019. Nests placements and spawn- ing in the endangered horseshoe crab(Leach, 1819) (Merostomata: Xiphosurida: Limulidae) in Sa- bah, Malaysia., 39: 695-702.
Nelson, B. R., Satyanarayana, B., Zhong, J. M. H., Shaharom, F., Sukumaran, M., and Chatterji, A., 2015. Episodic human ac- tivities and seasonal impacts on the(Müller, 1785) population at Tanjung Selangor in Peninsular Malaysia., 164: 313-323.
Penn, D., and Brockmann, H. J., 1994. Nest-site selection in the horseshoe crab,.,187(3): 373-384.
Polymeropoulos, E. T., Elliott, N. G., and Frappell, P. B., 2016. The maternal effect of differences in egg size influence me- tabolic rate and hypoxia induced hatching in Atlantic salmon eggs: Implications for respiratory gas exchange across the egg capsule., 73 (8): 1173-1181.
Rudkin, D. M., and Young, G. A., 2009. Horseshoe crabs–An an-cient ancestry revealed. In:. Tanacredi, J. T.,., eds., Springer, Massachusetts,Boston, 25-44.
Rudloe, A., 1979. Locomotor and light responses of larvae of the horseshoe crab,(L.)., 157: 494-505.
Seino, S., Uda, T., Tsuchiya, Y., Maeda, K., and Sannami, T., 2000. Field observation of geomorphological features of the spawn- ing site and dispersion of hatchlings of the horseshoe crab–Towards mitigation planning for the rare species., 3 (1): 7-19 (in Japa- nese with English abstract).
Shen, X., Qi, H., Liu, X., Ren, X., and Li, J., 2013. Two-way non-parametric ANOVA in SPSS., 30: 913-914 (in Chinese with English abstract).
Shin, P. K. S., Chan, C. S., and Cheung, S. G., 2014. Physiological energetics of the fourth instar of Chinese horseshoe crabs () in response to hypoxic stress and re- oxygenation.n, 85 (2): 522-525.
Smith, D. R., Brockmann, H. J., Beekey, M. A., King, T. L., Mil- lard, M. J., and Zaldivar-Rae, J., 2017. Conservation status of the American horseshoe crab, (): A regionalassessment., 27(1): 135-175.
Srijaya, T. C., Pradeep, P. J., Hassan, A., Chatterji, A., Shaharom, F., and Jeffs, A., 2014. Oxygen consumption in trilobite larvae of the mangrove horseshoe crab (; Latreille, 1802): Effect of temperature, salinity, pH, and light-dark cycle., 6(1): 1-15.
Sugita, H., 1988. Environmental adaptations of embryos.In:. Sekiguchi, K., ed., Science House Co., Ltd., Tokyo, 195-224.
Supadminingsih, F. N., Wahju, R. I., and Riyanto, M., 2019. Com- position of blue swimming craband horse-shoe crab Limulidae on the gillnet fishery in Mayangan Waters, Subang, West Java., 12 (1): 14-24.
Tankersley, R. A., Bullock, T. M., Forward Jr., R. B., and Ritt- schof, D., 2002. Larval release behaviors in the blue crab: Role of chemical cues., 273 (1): 1-14.
Vasquez, M. C., Johnson, S. L., Brockmann, H. J., and Julian, D., 2015. Nest site selection minimizes environmental stressor ex- posure in the American horseshoe crab,(L.)., 463: 105-14.
Wang, C. C., Kwan, K. Y., Shin, P. K. S., Cheung, S. G., Itaya, S., Iwasaki, Y.,., 2020. Future of Asian horseshoe crab con- servation under explicit baseline gaps: A global perspective., 24: e01373.
Warkentin, K. M., 2002. Hatching timing, oxygen availability, and external gill regression in the tree frog,., 75 (2): 155-164.
Warkentin, K. M., 2011. Environmentally cued hatching across taxa: Embryos respond to risk and opportunity., 51: 14-25.
Weber, R. G., and Carter, D. B., 2009. Distribution and develop- ment of Limulus egg clusters on intertidal beaches in Delaware Bay. In:. Tanacredi, J. T.,., eds., Springer, New York, USA, 249-266.
Zauki, N. A. M., Satyanarayana, B., Fairuz-Fozi, N., Nelson, B. R., Martin, M. B., Akbar-John, B.,., 2019. Citizen science frontiers horseshoe crab population regain at their spawning beach in East Peninsular Malaysia., 232: 1012-1020.
Ziegler, T. A., and Forward, R. B., 2007. Control of larval release in the Caribbean spiny lobster,: Role of che- mical cues., 152 (3): 589-597.
J. Ocean Univ. China(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5178-9
ISSN 1672-5182, 2022 21 (3): 557-563
(August 27, 2021;
October 16, 2021;
November 2, 2021)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
#The two authors contributed equally to this work.
Corresponding author. E-mail: kityuekwan@bbgu.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe Crab Tachypleus tridentatusEggs
- Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
- Experimental Investigation on the Interactions Between Dam-Break Flow and a Floating Box
- Variational Solution of Coral Reef Stability Due to Horizontal Wave Loading
- High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
- Influence of Autonomous Sailboat Dual-Wing Sail Interaction on Lift Coefficients
