Indiscriminate Dietary Compositions of Two Asian Horseshoe Crabs, Tachypleus tridentatus and Carcinoscorpius rotundicauda:Evidence from Hemolymph Stable Isotopes
LI Yuhong, FENG Jianxiang, KWAN Kit Yue, TAN Yiran, WENG Bosen,and JIA Ruiling
Indiscriminate Dietary Compositions of Two Asian Horseshoe Crabs,and:Evidence from Hemolymph Stable Isotopes
LI Yuhong1), *, FENG Jianxiang2), KWAN Kit Yue3), TAN Yiran1), WENG Bosen1),and JIA Ruiling1)
1),,361021,2),,519082,3),,535011,
Knowledge of dietary compositions is essential to the recovery of the dwindling populations of horseshoe crabs in China. The feeding habitsofandof Pearl Bay in southwesternChinawere studied by stable isotope analysis. Hemolymph samples of the two species were obtained from two age groups living in different habitats (inner, central, and outer Bay). In addition, their potential food source samples were collected in May 2019. Results showed that the mean values ofδ13C and δ15N inindividuals ranged from −19.01‰ to −16.47‰ and from 10.49‰ to 13.5‰, respectively, and those ofranged from−19.12‰ to −14.96‰ and from 8.78‰ to 13.48‰, respectively. These values indicated that the horseshoe crabs have a wide variety of food sources and therefore are highly omnivorous. No remarkably correlations were found between δ13C and δ15N values and individual widths in the two species. Thirteen potential food sources were selected, in which,,andcontributed largely to the dietary compositions of the two species of horse- shoe crabs. No significant correlation was found between the feeding habits and habitat geochemical characteristics of the horseshoe crabs. All these results possibly indicate the high diversity of their food sources and the indiscriminate dietary compositions of the twoAsian horseshoe crabs.
;; stable isotope; hemolymph; growth stage
1 Introduction
Horseshoe crabs, known as classic living fossils, have survived on earth for over 450 million years (Rudkin and Young, 2009; Van Roy, 2010). In the past few decades, the population of all four extant species of horseshoe crabs, namely,,,,, has declined rapidly (Razali, 2020; Wang, 2020). In particular,andarefound along the coast of southern China, and more than 95% of their populations are distributed in Beibu Gulf sur- rounded by the provinces of Hainan and Guangdong and the region of Guangxi (Liao and Li, 2001). Current populations of horseshoe crabs in China have drastically de-clined due to various threats, including overharvest, habitat loss, and anthropogenic pollution. In March 2019,was formally added to the category of ‘Endangered Species’ on the International Union for Conservation of Nature’s Red List(Laurie, 2019), followed by its classification as National Grade II protected wild animal in China in February 2021. Horseshoe crab conser- vation is vital because of its importance to the coastal eco- system as a major component of the food web and its great contribution to studies on evolutionary biology and local coastal culture and to the biomedical industry. Despite be- ing currently under the strict protection of state laws, these animals are still seriously threatened by habitat loss and destruction, including lack of suitable foods. Understanding the feeding habitats of horseshoe crabs, including their food source, is important in restoring their populations be- cause the variety, distribution, and quality of food sources may determine the survival rate, distribution, and move- ment patterns of naturalpopulations (Kwan, 2015, 2016, 2021).
Tidal flats in estuarine and coastal bays are critical nur- sery grounds forandjuveniles (Shin., 2009; Morton and Lee, 2010; Hu.,2011; Chen.,2015; Xie, 2019). These areas are also subjected to severe human disturbance due to their increasing economic development in offshore areas. A diet comprising a combination of algae, seagrass, bivalves, and other plant and animal sources is important in the food chains of Asian horseshoe crabs (Kwan, 2015, 2021; Fan, 2017). Hence, their omnivorous feeding beha- vior can help them adapt to harsh intertidal habitats. Feed- ing ecology, such as the type, quantity, timing, and mode of feeding,is important in understanding the basic survi- val food requirements of marine organisms and the food web structure of the ecosystem where they live. It is also important to learn their predatory or competitive relationships with other organisms. These data can provide an ecological basis for modeling the flow of materials and energy in the coastal ecosystem. For horseshoe crabs, understanding the contributions of these different potential food sources in their main habitats is important for their conservation (Chen, 2015).
Stable isotope analysis (SIA) has been applied to deter- mine the food sources of animals (Estrada, 2005; Gi- rard, 2012; Lin, 2013), track animal migrations (Ash- ley,2010), and construct food chains and webs in ecology (Zka,2010). The stable isotope composition of the food consumed by animals corresponds to that of their bodies (Fry,1988). In addition, the stable isotope ra- tios of carbon (13C/12C) and nitrogen (15N/14N) providetime-integrated information about food sources assimilatedby organisms. SIA is useful in inferring the relative con- tributions of given primary producers and reconstructing the relationship within the food web in aquatic ecosystems(Pasquaud,2007). Abundant data on nutritional struc-ture and food sources were successfully obtained using stable isotope technology (Lin, 2013; Feng, 2015; Jie, 2018). Previous diet studies on the Atlantic horse- shoe crabshowed that the early-instar ju- veniles (., 2nd and 3rd instars) derive food from ben- thic and suspended particulate organic matter, late-instar ju- veniles (5th to 11th instars) consume polychaetes and crus- taceans, and adults prey mainly on bivalves and gastro- pods (Gaines,2002; Carmichael, 2009).
The feeding compositions ofandhave been previously studied using the stable isotope compositions of muscle samples (Fan, 2017; Kwan, 2021) and fecal samples (Lee, 2021). However, blood samples have never been used for related experiments. The blood (usually called hemolymph) of ahorseshoe crab circulates around the organs within the bodycavity where it makes direct contact with all internal tissues and organs. Small changes in the internal and external en- vironments of this animal can stimulate its hemolymph andprovide necessary feedback to maintain the stability of theinternal environment. The hemolymph accepts and respondsto changes in environmental signals earlier and more sen- sitively than muscles and feces. Therefore, hemolymph samples might be useful in reflecting the recent and avail- able food sources (Caut, 2009; Therrien, 2011). In this study, the hemolymph samples of two Asian horseshoe crab species,and, and their potential food sources were collected from the nor- thernBeibu Gulf and subjected to carbon and nitrogen SIAs to provide information on their food choice at a short time scale and improve their conservation.
2 Materials and Methods
2.1 Study Area
The study sites were located at Pearl Bayon the west coast of northern Beibu Gulf, Guangxi Region, southwes- tern China. The bayoccupies a total area of 94.2km2, and the coastline is approximately 46km long. The bay opens into Beibu Gulf at its southern end (Fig.1). The area is cha- racterized by a subtropical oceanic monsoonal climate with a mean annual temperature of 22.5℃and 2221mm of annual precipitation. Tides are regular semidiurnal with an average tidal range of 2.24m and a spring tidal range of about 5m (Qiu, 2013). The tidal sand- and mud-flats in the intertidal zone in the bay are large and gener- ally extend 2–3km when tides recede, thus providing goodbreeding habitats for horse shoe crabs. Beds of seagrassand other speciesare distributed in ti- dal zones along the seaward edges of the mangrove for- ests. In this study, samples were obtained from Jintan (mouth of the bay, JT), Jiaodong (middle of the bay, JD), and Feng- huang (top of the bay, FH) in Pearl Bay during low tide in April 2019.
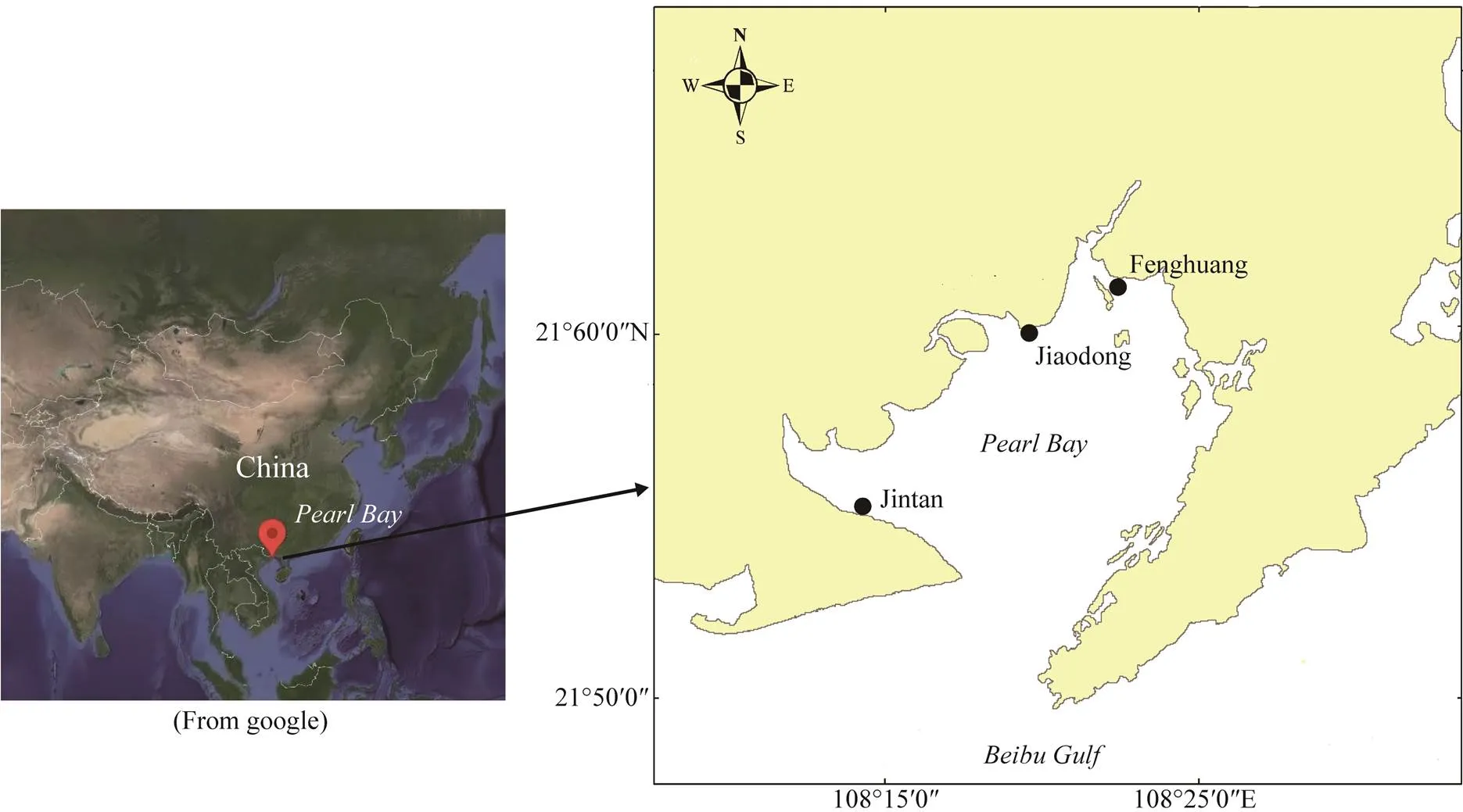
Fig.1 Sampling location map in Pearl Bay, northern Beibu Gulf,Guangxi Region, China
2.2 Field Sampling
2.2.1 Horseshoe crab sampling
Horseshoe crab distribution was investigated using the systematic quadrat method of Kwan(2016) and Xie(2019) with modifications and aided by the ‘TwoStep Path’ APP to pinpoint the coordinates of the sampling location points. Four horizontal transects were set parallel to the coastline and equally spaced between mean high and low water marks. The lengths of and spacing between the four transects were similar at each study site but differed between study sites with a length range of 0.88–2.89km and a spacing range of 45–300m. Quadrats with 8m×8m dimension (64m2area) were placed on each tran- sect at regular intervals of 100–140m in the pre-defined study area. The age classes of horseshoe crabs were classifiedaccording to prosomal widths (PWs).individuals with a PW range of 50.50–63.40mm are the older juveniles, and those with 90.40–145.20mm are the adults.individuals with PW of 49.85–55.50mm are the older juveniles, and those with 60.30–73.25mm are the adults. All the older juvenile and adult samples were collected from the survey fields.
2.2.2 Sample preparation
The species of horseshoe crabs were quickly identified within each quadrat, and their sizes were measured. The body size of horseshoe crabs was determined by measuring the PW to the nearest 0.1mm with vernier calipers. Af- ter all the sample sites were preliminarily surveyed, a re- presentative was selected at each location near the inner, middle, and outer bays of the Beilun River Estuary accord- ing to the distribution ofand.
The horseshoe crab body was divided into three major parts from anterior to posterior: prosoma, opisthosoma, and telson. The heart is located along the dorsal midline, just beneath the carapace of prosoma and opisthosoma (Arm- strong and Conrad, 2008). The carapace of horseshoe crabs was cleaned and disinfected before hemolymph was drawn. A 0.5–1mL disposable syringe was used to collect hemo- lymphsamples bycardiac puncture. The needle was not touched with fingers to avoid any potential contamination. The cannula with a needle was safely positioned, and the exposed base of the needle was elevated to avoid contact with any surface to maintain its sterility. Given that horse- shoe crab juveniles have a limited amount of hemolymph, only those with PW greater than 50mm were selected for hemolymph collection. The samples were stored in small 1.5mL centrifuge tubes and frozen immediately after sam- pling. Six horseshoe crab hemolymph (three juvenile and three adult samples) were collected for each species from each study site.
Potential food samples were collected from the same ar- eas as the horseshoe crabs. Food samples, including different plant and animal species that the horseshoe crab may consume, were collected and then stored frozen.,,,,,,,,,,, andwere collected. The plant samples were repeatedly rinsed in dis-tilled water until clean, and were packed, weighed, record- ed. Then they were dried in a oven at 70–80℃ until the weight was constant. Then they were crushed in a grinder, fully ground to a powder in a mortar, and finally passed through a 150µm sieve. The animal samples were repeatedly rinsed in distilled water and unshelled. Their muscle tissues were extracted, placed in glass petri dishes, and then freeze-dried in a LABCONCO freeze dryer at −25℃ to −65℃ for 72h. The muscle samples were then chopped, thoroughly ground to a powder, and passed through a sieve of 250µm. Freeze-drying was also used for thehemolymph samples.
Sediment and water samples were collected from each study site to determine the environmental conditions, in- cluding seawater salinity, pH, and sediment particle. The Malvern 2000 laser particle size analyzer was used for par- ticle size determination. Portable devices were employed for the immediate on-site measurement of the salinity and pH of surficial waters at each study site during low tides.
2.2 Sample Analysis and Isotope Mixing Model
The carbon isotope ratio (δ13C) and nitrogen isotope ra- tio (δ15N) of dried hemolymph and food source samples were determined using a Delta V Advantage isotope ratio mass spectrometer coupled with an HT2000-EA elemental analyzer (Thero Fisher Corp., USA). The previsions for δ13C and δ15N analysis were 0.1‰ and 0.2‰, respectively, based on the repeated analysis of a laboratory work standard PRO.
One-way ANOVA was conducted to examine differences in δ13C and δ15N values between different potential food sources. Prior to analysis, raw data were analyzed for va- riance homogeneity and were log-transformed (log10) to meet the ANOVA assumption. The data were counted and collated using SPSS 22.0 and analyzed for significant dif- ferences using a one-way ANOVA;<0.05 was consider- ed significant. Significant correlations between data were analyzed using Pearson correlation analysis, and<0.05 was considered significant (Feng., 2014).
On the basis of the δ13C and δ15N values, the relative importance of different potential food sources to the diet of these investigated horseshoe crabs was calculated using the MixSIR model developed within the Bayesian mo- deling framework (Moore and Semmens, 2008). Multiple sources of uncertainty, such as food source isotope values and fractionation, were integrated into the estimation by this model. Data on the relative contribution ratios of potential food sources ofandfrom different age groups were calculated separately byMixSIR 1.0.4 software. δ13C and δ15N distribution maps and food source contribution ratio distribution maps were then visualized with Origin 2017 software to study the po- tential food sources of horseshoe crabs.
3 Results
3.1 δ13C and δ15N Values of Horseshoe Crabs with Different Body Sizes
Soil and water samples were collected from the research sites. Some related environmental factors were measured,and the representative results are shown in Table 1. Ana- lysis showed a large difference in the soil grain size of the horseshoe crab habitats in the three sample sites from the outer bay to the inner bay. The soils in Jiaodong and Feng- huangtou sites were mostly muddy sediments, and those in the Jintan site were mostly fine sand. Meanwhile, the seawater salinity and pH were similar among the three sam-ple sites of horseshoe crab habitats. However, the food ha- bits of horseshoe crabs in these study areas did not show any significant correlation with their habitat geochemical characteristics.
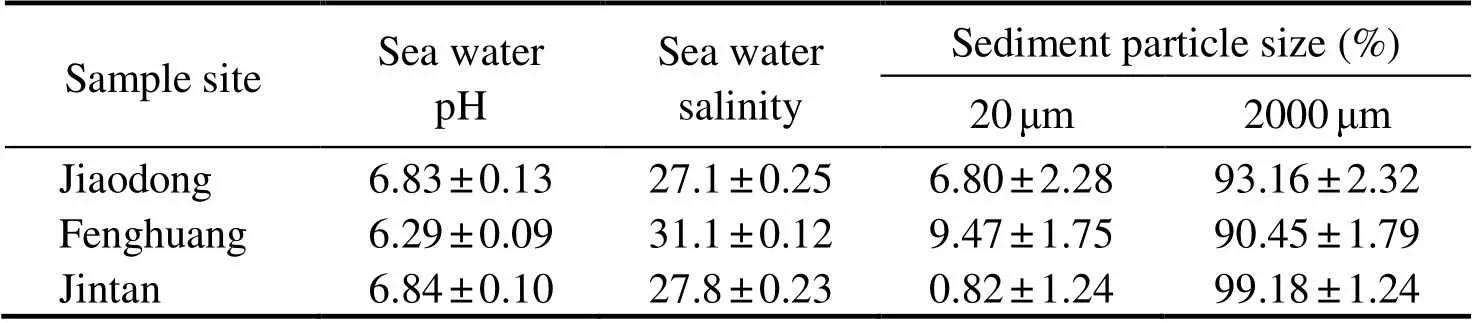
Table 1 Geochemical characteristics of horseshoe crab habitats among three study sites of the intertidal zone in Pearl Bayduring low tides
Note: Data are averaged from sea water/sediment samples taken from each sampling quadrat and expressed as mean±standard deviation (=3–5).
The δ13C and δ15N values ofandfrom different age groups from the three study sites along the northern Beibu Gulf are shown in Table 2. The PW of the investigatedspecimensranged from 50.50mm to 142.45mm, and that ofranged from 49.85mm to 73.25mm.The δ13C and δ15N mean values ofindividuals ranged from −19.01‰ to −16.47‰ and from 10.49‰ to 13.5‰, res- pectively, and those ofranged from −19.12‰ to −14.96‰ and from 8.78‰ to 13.48‰, respectively.
For both species, the δ13C value tended to decrease with increasing PW, whereas the δ15N valueseemed to increase with increasing body size. However, the Pearson correlation analysis showed that these relationships were not sig- nificant (>0.10,=11 and 18 forand, respectively).

Table 2 Carbon (δ13C) and nitrogen (δ15N) stable isotope ratios of hemolymph from two Asian horseshoe crab species,T. tridentatus and C. rotundicauda in different size groups sampled along the northern Beibu Gulf shore
Notes: Data are expressed as mean±standard deviation (=3, except foradults at JT where=2). PW, prosomal width; JT, Jintan; JD, Jiaodong; FH, Fenghuang.
3.2 δ13C and δ15N Values of Food Sources for Horseshoe Crabs
Fig.2 shows that among the 13 possible potential food items for horseshoe crabs, sediment had the lowest mean δ13C value of −22.31‰±3.16‰, andhad the highest mean δ13C value of −13.36‰±0.12‰.(−13.49‰) and(−15.16‰) had relatively high- er mean δ13C values. The lowest δ15N value was found forat 3.67‰±3.91‰, followed by sediment. Thehighest mean δ15N value was found forat 11.28‰±3.80‰.(10.71‰) and(10.66‰) had relatively higher mean δ13N values. In general, the mean
δ15N values of all potential food items were lower than those of horseshoe crabs, except for those of,, andthat were slightly higher than those for juvenile. In the horseshoe crab hemo- lymph samples, the highest and lowest δ13C mean values were obtained from the juvenileand the adult, respectively. Meanwhile, the highest and lowest δ15N average values were found in the adultand the juvenile, respectively.
3.3 Relative Contributions of Food Sources
The feasible contributions of each food source to the two species of horseshoe crabs were calculated using the MixSIR model as shown in Fig.3. The contribution of each potential food source in the juvenile(PW ranged from 50.50mm to 63.40mm) was in the following order:>>>>>>>>>>>>sedi-ment. The contribution of individual potential food sources to the adults was consistent with those to the juveniles, with only slight differences observed in contribution rates.
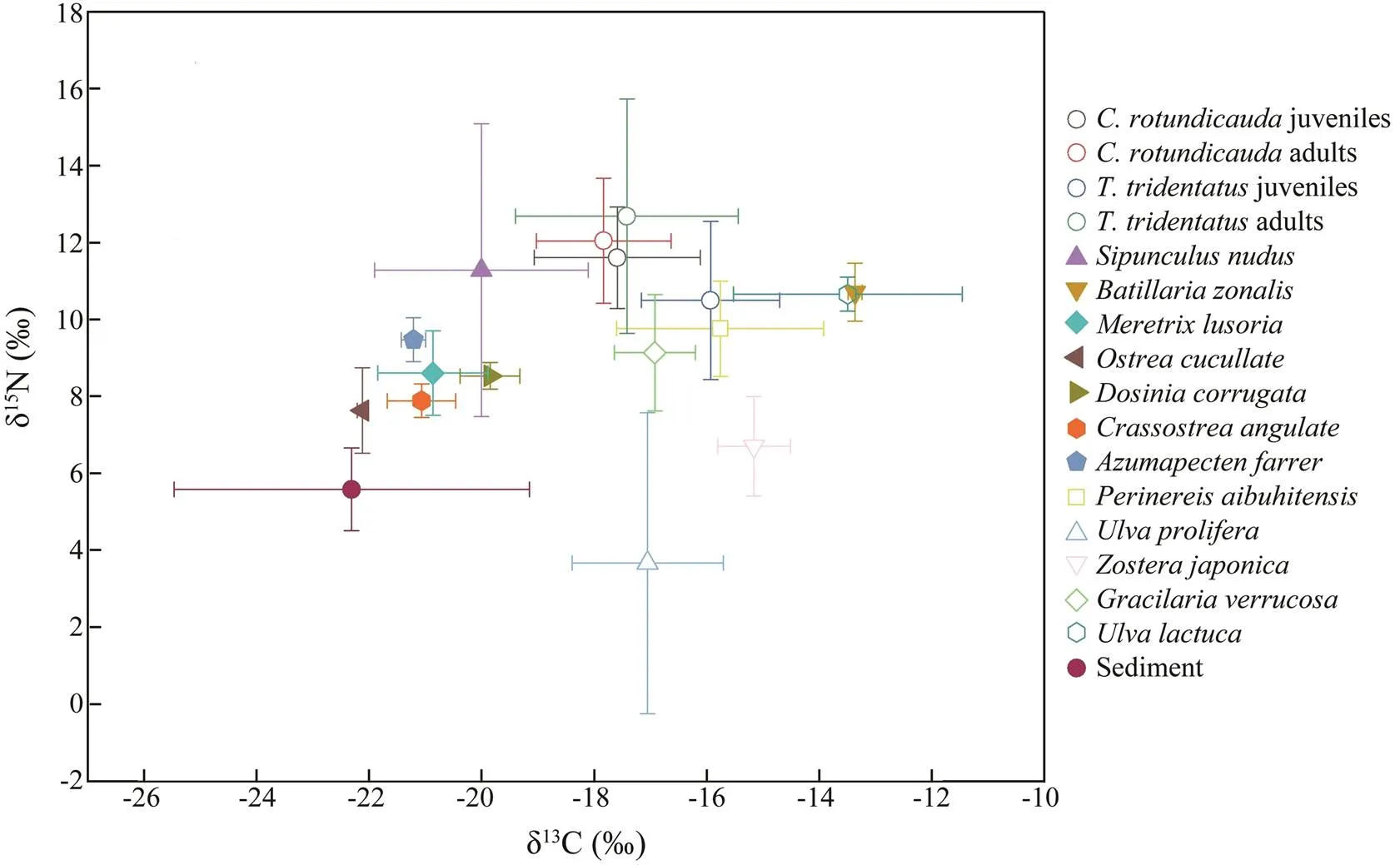
Fig.2 Stable isotope biplot for the hemolymphfrom T. tridentatus and C. rotundicauda and their potential food sourcesin Pearl Bay(mean±standard deviation).
,, andwere the main food sources for. The most dominant food source for the juveniles was, with an average contribution of 19.80%. For the adults, the most important food source was, with an average contribution of 34.20%.and sediment are the food sources ofwith the lowest contributions.
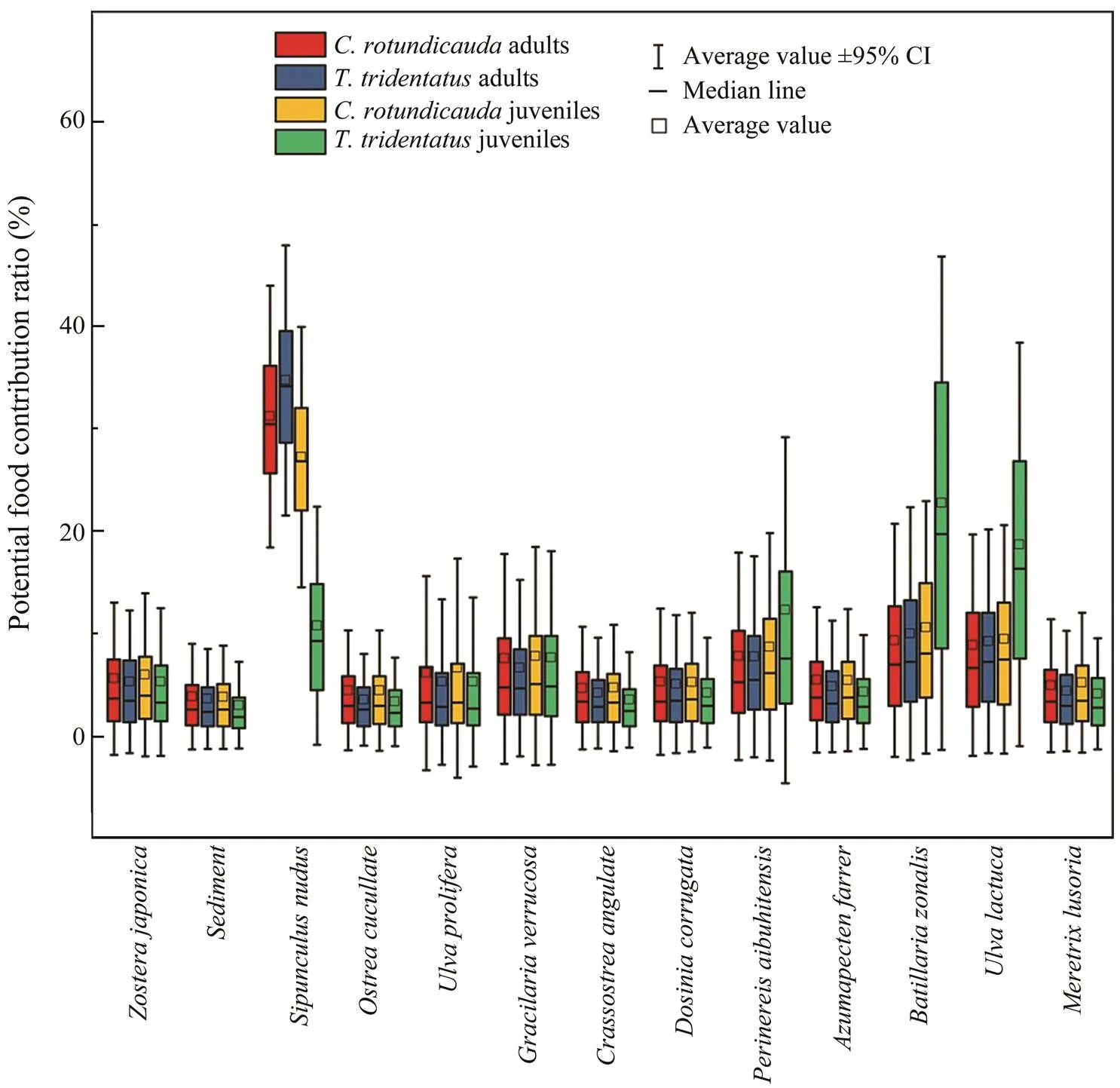
Fig.3 Relative contribution (%) of potential food sources to the diets of T. tridentatus and C. rotundicaudain Pearl Bay at different growth stages (boxes were interquartile range: low=25th percentile and upper=75th percentile).
had a slightly higher median contribution to the juveniles and adults of(at 26.90% and 30.50%, respectively) and the juveniles of(34.20%)than most of the above food sources. The contribution of each potential food source in the small age-class group ofwas in the following descend- ing order:>>The lowest-con- tributing potential food source was sediment, followed by,, and. The contribution of potential foods was similar for all groups of horseshoe crabs, except for the potential food items that contributed more to the juvenilethan to the other groups.
4 Discussion
4.1 Comparison of the Potential Food Source Composition of Horseshoe Crabs
Results showed that the δ13C and δ15N values ofandin the Beilun River Estuary varied over a wide range, indicating their wide feedingarray. These obtained value ranges were similar to those previously reported (Hu,2015). The mean δ13C values of both species were within the mean δ13C values of the potential food sources in the present study. In addition, the mean δ15N values of horseshoe crabs were higher than those of the potential food source species, suggesting that all selected food items are potential food sources forand
According to previous studies on the feeding habits of horseshoe crabs, the juveniles ofandfeed on a wide range of crustaceans, bivalves, polychaetes, and gastropods (Chatterji, 1992; Carmi- chael, 2009). The results presented here also show- ed the large contribution ofand, with polychaetes and gastropods being the main sources of the horseshoe crabs, probably due to the convenience of feeding. Another reason is the high abundance and wide distribution ofin the three sample sites compared with other potential food source species.
This study reported the intermediate contribution ratios of seagrass. By contrast, a previous study using muscle samples indicated that the two Asian horseshoe crab species,and, primarily rely on seagrass-derived nutrients from local vegetated estuarine and coastal habitats in the Beilun Estuary throughout their life stages (Fan, 2017). This difference might be at- tributed to the sample sites being heavily explored for sea- food harvesting. Frequent anthropogenic disturbance in thelocal environment may have a significant effect on the die- tary characteristics ofand, leading to great changes in food web structure and qualities in the research sites. As a result, the food source com- positions of the local horseshoe crabs have been altered. In another light, this finding may also display the differ- ence between hemolymph and muscle samples in the experiments.
The dietary compositions of the juveniles of these two species were assessed using their fecal samples in Hong Kong (Lee, 2021), and the results suggested that oli- gochaetes are the major prey items for(41.6%) and(32.4%), followed by bivalves and crustaceans for(8.6% and 8.4%, re- spectively). The difference in these results might be related to the variation of food resource availability and ex- perimental materials (muscle, feces, and hemolymph).
4.2 Relationship Between the Feeding Habits and Growth of Horseshoe Crabs
Studies based on isotope technology by Carmichael(2003) and Gaines(2002) showed that the food chain of horseshoe crab juveniles changes with their age. With the growth of juvenile horseshoe crabs, the nutritional po- sition in the food web structure changes significantly. The food of juvenile horseshoe crabs is mainly organic matter particles on the sediment surface in the environment. Horse-shoe crabs of age-class 5 begin to feed on polychaetes and crustaceans, and larger gastropods and bivalves are fed by adult horseshoe crab.
No significant difference was observed between the feed- ing habits and age classes of horseshoe crabs. One reason might be that the horseshoe crab juveniles are older juveniles from ten instar with PW not less than 50mm (Liu, 2014; Fan, 2017), and they are older than those in previous research. The horseshoe crab juveniles at ten in- star already have hard mouthparts and mature organs si- milar to mature individuals.
4.3 Influence of Environmental Factors
Similar basic environmental factors revealed similar feed-ing habitats betweenandfrom different sites. Previous studies found a significant correlation between the growth of horseshoe crabs and the en- vironmental conditions of their habitat.andhave different habitat preferences:prefers sandy habitats, andprefers a muddy environment (Liu, 2014). In Fenghuang in- tertidal zone, the sediments are muddy, and the population of horseshoe crabs is small. Meanwhile, a large number ofindividuals were foundat the fine sandy sediments of the Jintan intertidal zone, and this find- ing is consistent with previous studies. Horseshoe crabs span their whole life history across diverse habitat types from beaches near the high-tide line through intertidal mud- flats and coastal waters within 30m in bathymetry as their nesting, nursery, and feeding grounds, respectively (Chen., 2015). Given that the habitats of horseshoe crabs from different age classes are distributed in different coas- tal areas, the environmental factors of the habitats might affect the feeding characteristics of these animals. How- ever, no significant correlation was found between the feed- ing habits of horseshoe crabs and habitat geochemistry. These findings might also be attributed to the complex food sources for horseshoe crabs and the relatively less difference among individuals from different sites. The indiscri- minate dietary compositions ofandsuggested that they might compete vigorously for the food sources that have become limited due to human disturbances in coastal regions.
5 Conclusions
The δ13C and δ15N values ofandof Pearl Bay in southwesternChinavaried over a wide range.In addition, their food sources vary widely, implying their omnivorousness. No significant correlations were found between the δ13C and δ15N values and indi- vidual widths in the two species.,,andare the dominant food resources of horseshoe crabs from the inner, middle, and outer bays of the Pearl Bay in northern Beibu Gulf.No significant correlation wasobserved between the feeding habits of horseshoe crabs and habitat geochemical characteristics.All these results might indicate the highly diverse food sources and indiscriminate dietary compositions of the two Asian horseshoecrabs.
Acknowledgements
This work was supported by the Scientific ResearchProject of Huaqiao University (No. 605-50X18005), Guang-xi Bagui Youth Scholars Programme, Guangxi Recruitment Program of 100 Global Experts.
Armstrong, P., and Conrad, M., 2008. Blood collection from the American horseshoe crab,., 20: e958, DOI: 10.3791/958.
Ashley, P., Hobson, K. A., Wilgenburg, S. L. V., North, N., and Petrie, S. A., 2010. Linking Canadian harvested juvenile Ame- rican black ducks to their natal areas using stable isotope (δD, δ13C, and δ15N) methods., 5 (2): 7, DOI: 110.5751/ACE-00397-050207.
Balasse, M., 2010. Reconstructing dietary and environmental his- tory from enamel isotopic analysis: Time resolution of intra- tooth sequential sampling., 12 (3): 155-165, DOI: 10.1002/oa.601.
Carmichael, R. H., and Brush, E., 2012. Three decades of horseshoe crab rearing: A review of conditions for captive growth and survival., 4 (1): 32-43, DOI: 10. 1111/j.1753-5131.2012.01059.x.
Carmichael, R. H., Gaines, E., Sheller, Z., Tong, A., Clapp, A., and Valiela, I., 2009. Diet composition of juvenile horseshoe crabs: Implications for growth and survival of natural and cul- tured stocks. In:. Tanacredi, J.,., eds., Springer, Boston, 521-534, DOI: 10. 1007/978-0-387-89959-6_33.
Carmichael, R. H., Rutecki, D., and Valiela, I., 2003. Abundance and population structure of the Atlantic horseshoe crabin Pleasant Bay, Cape Cod., 246: 225-239, DOI: 10.1007/BF00005621.
Caut, S., Angulo, E., and Courchamp, F., 2009. Variation in dis- crimination factors (Δ15N and Δ13C): The effect of diet isoto- pic values and applications for diet reconstruction., 46 (2): 443-453, DOI: 10.1111/j.1365-2664. 2009.01620.x.
Chatterji, A., Mishra, J. K., and Parulekar, A. H., 1992. Feeding behaviour and food selection in the horseshoe crab,(Müller)., 246 (1): 41-48, DOI: 10. 1007/BF00005621.
Chen, C. P., Yang, M. C., Fan, L. F., Qiu, G., Liao, Y. Y., and Hsieh, H. L., 2015. Co-occurrence of juvenile horseshoe crabsandin an estuarine bay, southwestern China.Biology,24: 117- 126, DOI: 10.3354/ab00641.
Estrada, J. A., Lutcavage, M., and Thorrold, S. R., 2005. Diet and trophic position of Atlantic bluefin tuna () inferred from stable carbon and nitrogen isotope analysis., 147 (1): 37-45, DOI: 10.1007/s00227-004-1541-1.
Fan, L. F., Chen, C. P., Yang, M. C., Qiu, G. L., Liao, Y. Y., and Hsieh, H. L., 2017. Ontogenetic changes in dietary carbon sources and trophic position of two co-occurring horseshoe crab species in southwestern China.,26: 15-26, DOI: 10.3354/ab00670.
Feng, J., Huang, Q., Qi, F., Guo, J., and Lin, G., 2015. Utilization of exoticby fish community in the mangrove ecosystem of Zhangjiang Estuary: Evidence from stable isotope analyses., 17 (7): 2113-2121, DOI: 10.1007/s10530-015-0864-9.
Fry, B., 1988. Food web structure on Georges Bank from stable C, N, and S isotopic compositions., 33 (5): 1182-1190, DOI: 10.4319/lo.1988.33.5.1182.
Gaines, E. F., Carmichael, R. H., and Valiela, G. I., 2002. Stable iotopic evidence for changing nutritional sources of juvenile horseshoe crabs., 203 (2): 228-230, DOI: 10.2307/1543412.
Girard, J., Baril, A., and Mineau, P., 2012. Foraging habitat and diet of Song Sparrows () nesting in farmland: A stable isotope approach., 90 (11): 1339-1350, DOI: 10.1139/z2012-103.
Hu, M., Kwan, B. K., Wang, Y., Cheung, S. G., and Shin, P. K., 2015. Population structure and growth of juvenile horseshoe crabsand(Xiphosura) in southern China. In:. Carmichael, R. H.,., eds., Springer International Publishing, Cham, 167-180, DOI: 10.1007/978-3-319-19542-1_8.
Kwan, B. K. Y., Hsieh, H. L., Cheung, S. G., and Shin, P. K. S., 2016. Present population and habitat status of potentially threat- ened Asian horseshoe crabsandin Hong Kong: A proposal for ma- rine protected areas., 25 (4): 673- 692, DOI: 10.1007/s10531-016-1084-z.
Kwan, B. K. Y., Shin, P. K. S., and Cheung, S. G., 2015. Prelimi- nary home range study of juvenile Chinese horseshoe crabs,(Xiphosura), using passive tracking me- thods. In:. Carmichael, R. H.,., eds., Springer International Publishing, Cham, 149-166, DOI: 10.1007/978-3-319-19542-1_7.
Kwan, K. Y., Bopp, J., Huang, S., Chen, Q., Wang, C. C., Wang, X.,., 2021. Ontogenetic resource use and trophic dyna-mics of endangered juvenileamong diversified nursery habitats in the northern Beibu Gulf, China., 16 (6): 908-928, DOI: 10.1111/1749-4877. 12495.
Liao, Y. Y., and Li, X. M., 2001. Present situation of horsecrab resources in the sea area of China and tactics of preservation., 23 (2): 53-57, DOI: 10.3321/j.issn:1007-7588. 2001.02.012.
Lin, G. H., 2013.. Higher Education Press, Beijing, 492pp (in Chinese).
Liu, W. R., 2014. Biologial study ofandin coastal areas of Beibu Gulf in Guangxi. Master thesis. Guanxi University, DOI: 10.7666/ d.D523850 (in Chinese).
Moore, J. W., and Semmens, B. X., 2008. Incorporating uncer- tainty and prior information into stable isotope mixing models.,11: 470-480, DOI: 10.1111/j.1461-0248.2008.01163.x.
Morton, B., and Lee, C., 2010. Spatial and temporal distributionsof juvenile horseshoe crabs (Arthropoda: Chelicerata) approach- ing extirpation along the northwestern shoreline of the New Territories of Hong Kong SAR, China.,45: 227-251, DOI: 10.1080/00222933.2010.522263.
Pasquaud, S., Lobry, J., and Elie, P., 2007. Facing the necessity of describing estuarine ecosystems: A review of food web eco-logy study techniques., 588: 159-172, DOI: 10. 1007/s10750-007-0660-3.
Prochazka, P., Reif, J., Horak, D., Klvana, P., Lee, R. W., and Yo- hannes, E., 2010. Using stable isotopes to trace resource ac- quisition and trophic position in four Afrotropical birds with dif-ferent diets., 81 (3): 273-275, DOI: 10.2989/00306525. 2010.519889.
Qiu, G. L., Fan, H. Q., Li, Z. S., Liu, G. H., Shi, Y. J., and Li, S., 2013. Population dynamics and seed banks of the threatened seagrassin Pearl Bay, Guangxi., 33 (19): 6163-6172, DOI: 10.5846/stxb20130 6091489 (in Chinese with English abstract).
Rudkin, D. M., and Young, G. A., 2009. Horseshoe crabs–An an- cient ancestry revealed. In:. Tanacredi, J. T.,., eds., Springer, New York, 25-44, DOI: 10.1007/978-0-387-89959-6_2.
Schreibman, M. P., and Zarnoch, C. B., 2009. Aquaculture me- thods and early growth of juvenile horseshoe crabs (s). In:. Tanacredi, J.,., eds., Springer, Boston, 501-511, DOI: 10. 1007/978-0-387-89959-6_31.
Sekiguchi, K., Seshimo, H., and Sugita, H., 1988. Post-embryo- nic development of the horseshoe crab., 174 (3): 337, DOI: 10.2307/1541959.
Therrien, J. F., Fitzgerald, G., Gauthier, G., and Bêty, J., 2011. Diet-tissue discrimination factors of carbon and nitrogen stable isotopes in blood of Snowy Owl ()., 89 (4): 343-347, DOI: 10.1139/z11- 008.
Van Roy, P., Orr, P. J., Botting, J. P., Muir, L. A., Vinther, J., Le- febvre, B.,., 2010. Ordovician faunas of Burgess Shale type., 465 (7295): 215-218, DOI: 10.1038/nature09038.
Wang, C. C., Kwan, K. Y., Shin, P. K. S., Cheung, S. G., Itaya, S., Iwasaki, Y.,., 2020. Future of Asian horseshoe crab conservation under explicit baseline gaps: A global perspective., 24:e01373, DOI: 10. 1016/j.gecco.2020.e01373.
Xie, X., Wu, Z., Wang, C. C., Fu, Y., Wang, X., Xu, P.,., 2019. Nursery habitat for Asian horseshoe crabs along the nor- thern Beibu Gulf, China: Implications for conservation ma- nagement under baseline gaps., 30 (2): 260-272, DOI: 10.1002/ aqc.3259.
Zhou, H., and Morton, B., 2004. The diets of juvenile horseshoe crabs,and(Xiphosura), from nursery beaches proposed for conservation in Hong Kong., 38 (15): 1915-1925, DOI: 10.1080/0022293031000155377.
J. Ocean Univ. China(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5227-4
ISSN 1672-5182, 2022 21 (3): 583-590
(October 11, 2021;
February 28, 2022;
April 5, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
Corresponding author. E-mail: liyuh@hqu.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe Crab Tachypleus tridentatusEggs
- Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
- Experimental Investigation on the Interactions Between Dam-Break Flow and a Floating Box
- Variational Solution of Coral Reef Stability Due to Horizontal Wave Loading
- High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
- Influence of Autonomous Sailboat Dual-Wing Sail Interaction on Lift Coefficients
