Application of Principal Component Analysis (PCA) to the Evaluation and Screening of Multiactivity Fungi
YANG Zonglin, SHI Yaqi, LI Pinglin, PAN Kanghong, LI Guoqiang,LI Xianguo, YAO Shuo, *, and ZHANG Dahai, *
Application of Principal Component Analysis (PCA) to the Evaluation and Screening of Multiactivity Fungi
YANG Zonglin1), 2), SHI Yaqi1), 2), LI Pinglin3), 4), PAN Kanghong1), 2), LI Guoqiang3), 4),LI Xianguo1), 2), YAO Shuo1), 2), *, and ZHANG Dahai1), 2), *
1)Key Laboratory of Marine Chemistry Theory and Technology (Ocean University of China), Ministry of Education, Qingdao 266100, China 2) College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao266100, China 3)Key Laboratory of Marine Drugs, Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266100, China 4)Laboratory of Marine Drugs and Biological Products, National Laboratory for Marine Science and Technology, Qingdao 266100, China
Continued innovation in screening methodologies remains important for the discovery of high-quality multiactive fungi, which have been of great significance to the development of new drugs. Mangrove-derived fungi, which are well recognized as prolific sources of natural products, are worth sustained attention and further study. In this study, 118 fungi, which mainly includedspp. (34.62%) andspp. (15.38%), were isolated from the mangrove ecosystem of the Maowei Sea, and 83.1% of the cultured fungi showed at least one bioactivity in four antibacterial and three antioxidant assays. To accurately evaluate the fungal bioactivities, the fungi with multiple bioactivities were successfully evaluated and screened by principal component analysis (PCA), and this analysis provided a dataset for comparing and selecting multibioactive fungi. Among the 118 mangrove-derived fungi tested in this study,spp. showed the best comprehensive activity. Fungi such as,and, which exhibited high comprehensive bioactivity as determined by the PCA, have great potential in the exploitation of natural products and the development of new drugs. This study demonstrated the first use of PCA as a time-saving, scientific method with a strong ability to evaluate and screen multiactive fungi, which indicated that this method can affect the discovery and development of new drugs.
principal component analysis; biological activity; fungi; mangrove ecosystem; activity evaluation
1 Introduction
Fungi, as important compartments of microbial communities in mangroves, constitute the second largest part of marine fungi (Bhimba., 2012). Mangrove-derived fungi show unique metabolic pathways, reproductive systems, and sensory and defense mechanisms because they have adapted to extreme environments with high concentrations of salt and moisture, frequent low and high tides and muddy and anaerobic conditions(Cheng., 2009; Behera., 2017; Guo., 2018). Hence, mangrove-derived fungi are widely recognized as prolific sources of natural products with unique structures and high bioactivity. These natural productshave been of great significance to the development of new drugs (Cui., 2017). Sun. 2018 reported that borrelidin H isolated fromSCSIO ZJ89, which originated from mangrove-derived sediment samples,has a therapeutic window superior to that of borrelidin A whichhas great potential as a new anticancer drugand could inhibit the migration of cancer cells. Bibi. (2020) concluded the articles published from 1990 to 2019, and observed that antimicrobial (48.9%) and antioxidant (12.2%) assays were identified as the two preferred assays for testing mangrove-derived fungi.
The identification of new fungi and compounds through increasing studies has become increasingly difficult (Wei., 2017). To overcome this issue, the one- strain-many-compounds (OSMAC) strategy (Bode., 2002; Scherlach., 2010), co-culture (Bao., 2017), precursor addition (Wang., 2011) and genomics-based approaches (He., 2018) are rapidly developing based on known active fungi for new natural product discovery. Using the OSMAC strategy, Meng. (2015, 2017) isolated twelve novel compounds with antimicrobial activity from the mangroveendophyteMA-231, which has been confirmed to be a bioactive fungus. Zhang. (2017) isolated five new compounds with α-glucosidase inhibitory activity together with twelve known compounds through the co-cultivation of the mangrove endophytic fungussp. 307 with the aquatic pathogenic bacteriumB2. Oakley. (2017) isolated two novel compounds and an antibiotic not previously reported to be produced byby deleting thegene, which was generally overexpressed in. Although these approaches could be used to find more natural products produced by fungi, they were established based on known high-activity fungi. Hence, continued innovation in sampling and screening methodologies remains important for the discovery of high-activity fungi (Luo., 2014).
It is obvious that high-quality multiactive fungi are crucial to the discovery of new natural products. Compared with the technologies developed for isolating compounds, the methodologies for activity screening are relatively weak. Most researchers have discovered fungi by evaluating their activity using only one indicator (Saito., 2018), and this analysis could be considered incompletely. Abdalla. (2020) advanced this methodology to test fungi with potential antimicrobial, extracellular enzymatic and phosphate-solubilizing activities. However, few studies have performed a comprehensive evaluation of the activities of these fungi. The evaluation and comparison of the comprehensive activity of many multiactive fungi at the same time would be more difficult.
To resolve this issue, our study constitutes the firstly use of principal component analysis (PCA) for the screening of fungi with the highest comprehensive activity in scientific and expedient manner. PCA is a simple and effective statistical tool that is widely used in dimensionality reduce- tion and factorial analysis of high-dimension datasets (Asante-Okyere., 2020).Datasets with several corre- lated variables are decomposed into a smaller number of linearly independent variables by PCA (Mahmoudi., 2021).Hence, we used PCA to evaluate multiactive fungi and compare their antibacterial and antioxidant activities with the aim of identifying the fungus with the highest comprehensive activity.
2 Materials and Methods
2.1 Isolates and Specimens
Mangrove ecosystem specimens were collected from Maowei Sea, Qinzhou City, Guangxi Province, P. R. China, and these samples included sediment, seawater, dead leaves and plant roots. The specimens were placed in sterilized zip-lock plastic bags on which the collection details were noted and transported to the laboratory under freezing conditions. The isolation media, potato dextrose agar (PDA) (Hernandez-Restrepo., 2017) and malt extract agar (MEA; Merck, Germany), are well suited (Chomnunti., 2014) for the purification of cultured fungi after sterilization at 121℃ for 20min (Er., 2015). Each specimen was placed on the surface of the media with a sterilized loop in triplicate and incubated in an incubator at 29℃. The mixture of cultured fungi was then transferred to new PDA or MEA plates for purification by picking up single colonies using a sterilized needle. All the strains were maintained at the Key Laboratory of Marine Chemistry Theory and Technology of Ocean University of China(Qingdao, Shandong, China).
2.2 Sequencing and Identification of Strains
Genomic DNA was extracted from fungal mycelium growing on PDA or MEA using the FastDNA kit (MP Biomedicals, CA, USA) following the manufacturer’s protocols. Their identification was based on a molecular genetic analysis using the internal transcribed spacer (ITS) region (Al-Hindi., 2017). The primers were forward ITS1 (TCCGTAGGTGAACCTGCGG) and reverse ITS4 (TCCTCCGCTTATTGATATGC). The PCR mixture consisted of 5μL of Ex Taq buffer, 2 μL of dNTPs (2.5mmolL−1), 0.2μL of TaKaRa Ex Taq, 1μL of genomic DNA as the template, 1μL (10μmolL−1) of the forward primer, 1μL (10μmolL−1) of the reverse primer and ddH2O to obtain a final volume of 50μL. The conditions for the PCR of ITS genes constituted of an initial denaturation step for 5min at 94℃, 35 cycles of denaturation at 94℃ for 30s, annealing at 52℃ for 30s and extension at 72℃ for 1min, and a final extensionstep of 10min at 72℃. The amplified PCR products were first visually examined in a 1% agarose gel stained with FloroSafe DNA stain using a gel documentation system (AlphaImager HP, CA, USA). The PCR products were sequenced using an ABI Prism 3730xl DNA Analyzer (Applied Biosystems,CA, USA) according to the manufacturer’s instructions. The amplified DNA sequences were used to retrieve consensus sequences from GenBank, and the fungal species were confirmed after a BLAST search (https://blast.ncbi.nlm.nih. gov/Blast.cgi).
2.3 Metabolite Extraction
Each strain was cultured in 500-mL Erlenmeyer flasks containing 200mL of liquid medium which consisted of 2g of peptone, 2 g of malt extract, 10g of glucose, 4g of glucose and 1L of distilled water. After fermentation for 21 days at 29℃, the broth and mycelia were separated by filtration using cotton gauze (Sheik and Chandrashekar, 2018). The fermentation broth was extracted twice with 100mL of ethyl acetate. The ethyl acetate was then eva- porated under reduced pressure to obtain a crude extract. A stock solution of the extract was prepared at a concentration of 1mgmL−1in methanol for antioxidant and antibacterial assays. After lyophilization, mycelia were extracted twice with25mL of acetone:methanol (V:V = 1:1). The rest of the extraction process was the same as that used for the broth.
2.4 Antioxidant Assays
2,2-Diphenyl-2-picrylhydrazyl radical scavenging (DP PHRS) (Pripdeevech and Machan, 2011; Shekhar and Anju, 2014), hydroxide radical scavenging (HRS) (Bruins, 2002) and cupric reducing antioxidant capacity (CUP RAC) (Apak., 2004) assays were used to evaluate the antioxidant activity of the crude extracts. All reactions were then performed in triplicate in 96-well microtiter plates and measured using a microplate reader (Multiskan FC, Thermo Scientific, China).Ascorbic acid (Vc) and methanol were used as the standard and blank, respectively.
2.4.1 DPPH radical scavenging assay
Extract stock solution (160μL) and 40μL of a DPPH solution (0.15molL−1) were added to a 96-well plate.The mixture was shaken vigorously and incubated at room temperature for 30min. The absorbance of the mixture was then measured at 517nm (Sinurat., 2018). The percent (%) radical scavenging capacity (RSC) was calculated using the following equation (Atiphasaworn., 2017):

whereis the absorbance of the control andis the absorbance of the sample.
2.4.2 HRS assay
The HRS assay was conducted as follows: 50μL of FeSO4(3mmolL−1) was reacted with 50μL of H2O2(2.5mmolL−1) to produce hydroxide radicals. Ten minutes later, 50μL of the sample extract was added to the mixture. To prepare the blank, the sample was replaced by methanol. After 10min, 50μL of salicylic acid (3mmolL−1) was added to detect the residual hydroxide radicals. The mixture was incubated at room temperature for 30min, and the absorbance was measured at 520nm. The HRS capacity was then calculated using the same method for determining the DPPH radical scavenging capacity.
2.4.3 CUPRAC assay
The CUPRAC assay was based on the reduction of Cu2+to Cu+by the combined action of antioxidants (Ya- mashita., 1998). Neocuproine (Nc, 2,9-dimethyl-1, 10-phenanthroline hemihydrate) is a chromogenic and selective complex with Cu+(maximum absorbance at 455 nm.) (Roohparvar., 2018). The CUPRAC assay was conducted using the crude extracts with the method described by Apak. (2004). First, 50μLof ammonium acetate (1molL−1) was added to the reaction system as a buffer to maintain a neutral pH, and then, 50μL of CuCl2(2mmolL−1) and 50μL of stock solution were successively added. After 10min, 50μL of Nc- methanol solution (1.5mmolL−1) was added to the system, and 30min later, the solution absorbance was measured at 455nm. The absorbance was then converted to units of Vc using a standard curve (Fig.1), and the results are expressed as equivalents to the concentration of Vc.

Fig.1 The typical standard curve in the CUPRAC assay.
2.5 Antibacterial Assay
Four human pathogenic bacteria, namely, gram-negative(CMCC 44102),(CMCC(B) 26003),(CMCC (B) 28001) and(CMCC(B) 50094), were used for bioassays based on the paper disk diffusion method (Mar and Pripdeevech, 2014). These bacteria were pre-cultured in sterile beef extract peptone broth (beef extract, 0.3g; peptone, 1g; NaCl, 0.5g; distilled water, 100mL; pH 7.4–7.6) at 37℃ for 48h. Sterilized 6-mm paper disks (WhatmanTM, USA) were soaked in the extract solution for 24h. Air-dried paper disks were placed in triplicate on beef extract peptone agar plates containing the bacteria. Paper disks saturated with me- thanol and kanamycin were used as negative controls and reference controls, respectively. To confirm the antibacterial activity, the diameter of the clear zone was measured after 24h of incubation at 37℃.
2.6 Statistical Analysis
Each experiment was performed in triplicate. The data are expressed as the means ± standard deviations. The comprehensive activity was evaluated by combining the antioxidant activity of the mycelial and broth extracts and the antibacterial activity of the mycelia. PCA was performed using SPSS.v23 (https://www.ibm.com/analytics/ spss-statistics-software). The dependency matrix was analyzed using the Kaiser-Meyer-Olkin (KMO) test and Bartlett’s method of sphericity. Data extraction was performed based on a normalized PCA using the quartimax rotation method with varimax rotation and Z-score normalization (Vandermarken., 2018). A composite score () was used to evaluate the comprehensive activity of the fungi and was calculated with the following equation:

wherepis the score of a principal component (PC) andis the variance in the corresponding PC.
3 Results
3.1 Fungal Diversity
A total of118 different fungal species identified by colony morphology and molecular biology were separated, purified and successfully cultured. These belonged to 30 genera, 22 families, 11 orders, six classes and three phyla. The phyla Basidiomycota, Zygomycota and Ascomycota accounted for 2.54%, 9.32% and 88.14%, respectively (Fig.2). In the Basidiomycota phylum, only three fungal species were listed as belonging to one class, two orders, two families and two genera. The Zygomycota phylum included 11 species that belonged to one class, one order, six families (one incertae sedis) and six genera (one incertae sedis). The Ascomycota phylum, which exhibited the maximum count and highest diversity, included four classes, eight orders, 14 families, 22 genera and 104 species.Thegenus recorded the highest count (34.62%), followed by thegenus (15.38%),genus (9.62%),genus (9.62%) andgenus (5.77%). The genera,andconstituted the largest class of Eurotiomycetes among all fungi isolated from mangrove ecosystems.
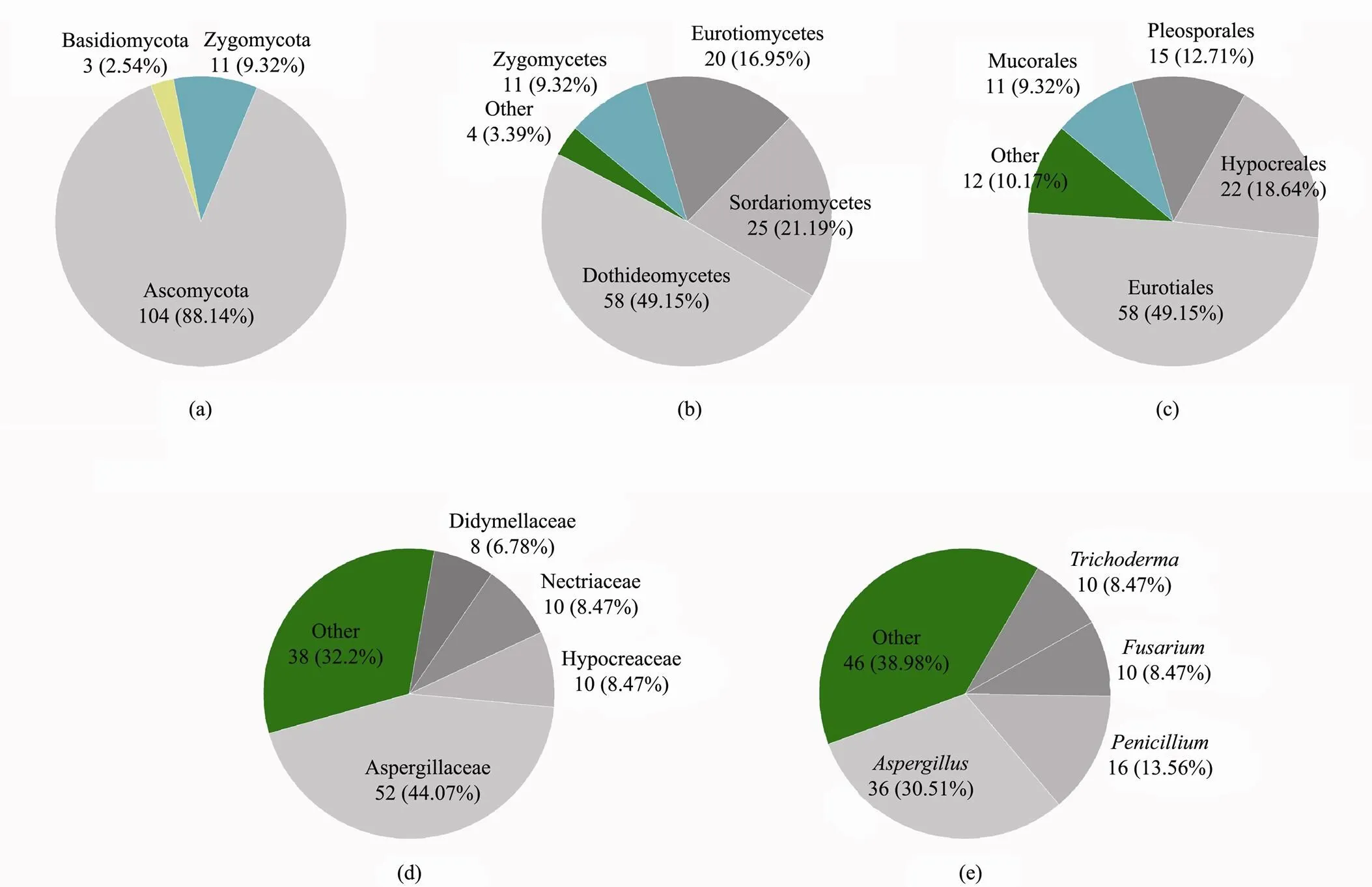
Fig.2 Numbers of dominant fungi at the phylum (a), class (b), order (c), family (d) and genus (e) levels.
3.2 Statistical Analysis of Activity
Seven activity bioassays, namely, DPPHRS (A1), HRS (A2), CUPRAC (A3) assays and inhibition assays against(A4),(A5),(A6) and(A7), were selected to test the activities of crude broth and mycelial extracts from each fungus. Among the crude broth extracts, 69.5% and 26.3% extracts showed DPPH and hydroxide RSCs higher than 90%, respectively (Fig.3). Eight and 11 crude broth extracts could completely scavenge DPPH and hydroxide radicals, respectively, and 34.7% of fungal crude broth extracts (1.00mgmL−1), which showed better antioxidant capacity than the positive control, showed values equal to or higher than those of 1.00mgmL−1Vc.The number of crude mycelial extracts with antioxidant activity, particularly HRS activity, was less than that of the crude broth extracts (Fig.3). Only three fungal crude mycelial extracts could scavenge hydroxide radicals, with values reaching 90%, and none could completely scavenge hydroxide radicals. In addition, 10.2% of the fungal crude mycelial extracts could scavenge DPPH radicals, with values reaching 90%. Only the crude mycelial extract fromcould completely scavenge DPPH radicals. In addition, 7.6% of the fungal crude mycelial extracts (1.00mgmL−1) showed better antioxidant capacity than Vc. The antibacterial activity results showed that 29.6% of the crude broth extracts were resistant to at least one of the tested bacteria, and no crude mycelial extracts showed antibacterial activity. The crude broth extracts of,,andshowed broad-spectrum antibacterial activity against all four human pathogenic bacteria.

Fig.3 Number of fungi with bioactivity observed in the broth extracts (a) and mycelium extracts (b). A1–A3 represent the antioxidant activity analyses based on the DPPHRS, HRS and CUPRAC assays, respectively. The A1 and A2 results are represented as the percent (%) radical scavenging capacity (RSC). The A3 results are expressed as the concentration of Vc equivalent to 1.00mgmL−1 extract. Vc (A1=97%, A2=95%, A3=1.00mgmL−1) and methanol (A1=0%, A2=0%, A3=0mgmL−1) were used as the standard and blank, respectively. A4–A7 represent the analyses of the antibacterial activities against Salmonella paratyphi B, Escherichia coli, Staphylococcus aureus and Micrococcus luteus.Paper disks saturated with methanol (A4=0mm, A5=0mm, A6=0mm, A7=0mm) and kanamycin (A4=27.8mm, A5=15.2mm, A6=25.0mm, A7=18.9mm) were used as negative and reference controls, respectively.
3.3 Principal Component Analysis
The dataset with ten variables (three variables for broth antioxidant activity, four variables for broth antibacterial activity and three variables for mycelium antioxidant activity) was decomposed into a smaller number of linearly independent variables by PCA (Fig.4). The first three PCs named PC1, PC2 and PC3 were selected as significant be- cause their eigenvalues were larger than 1 (Kaiser, 1960). The dependency matrix was tested by the Kaiser-Meyer- Olkin test (KMO>0.7) and Bartlett’s method of sphericity (<<0.05). The results showed that 66.46% of the dataset variance was explained by PC1, PC2 and PC3 (Table 1). The PC scores were calculated as the product of the input data matrix and the eigenvector matrix, which represents the strength of each PC for each of the observed points (Martini., 2017). The comprehensive activity of a fungal strain was evaluated and represented by, which was calculated using the following equations:
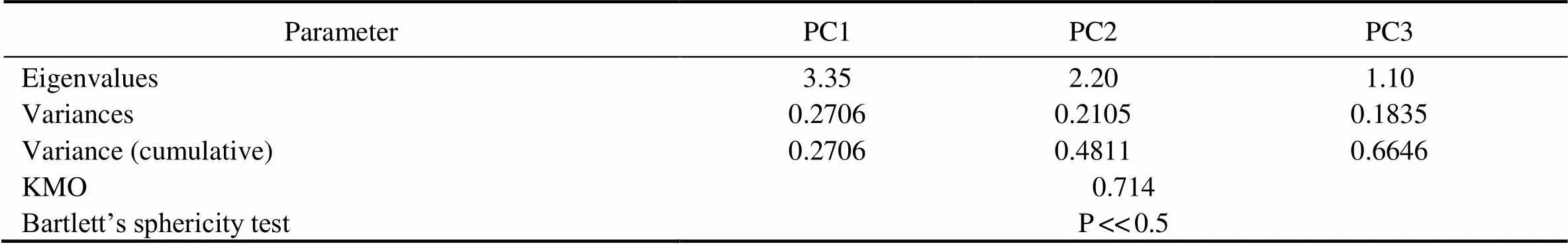
Table 1 Statistical results of the PCA and information of the first three PCs
Notes: The available PCA results were statistically tested using the Kaiser-Meyer-Olkin (KMO) test (>0.7) and Bartlett’s sphere- city test (<<0.5).
=0.27061+0.21052+0.18353, (3)
1=−0.012b1+0.201b2+0.140b3+0.750b4+0.866b5+0.836b6+0.787b7+0.081m1−0.042m2+0.091m3, (4)
2=0.737b1+0.531b2+0.782b3− 0.070b4+0.004b5+0.180b6+0.261b7+0.506m1– 0.034m2+0.552m3, (5)
3=−0.121b1+0.272b2+0.131b3+0.201b4− 0.110b5+0.020b6−0.057b7+0.728m1+0.865m2+0.628m3, (6)
where1,2and3represent the scores of PC1, PC2 and PC3, respectively,b1tob7represent the normalized results of the A1–A7 analyses of the crude broth extracts, andm1tom3represent the normalized results of the A1–A3 analyses of the crude mycelial extracts.
4 Discussion
This study demonstrated that mangrove-derived fungi could be recognized as prolific sources of bioactive natural products and involved the first application of PCA to evaluate multiactive fungi and identify the fungi with the best comprehensive bioactivity.The screened fungi with bioactivity could be used for the isolation of natural products with potential antioxidant and antibacterial activities. These fungi had great potential in the development of new drugs.
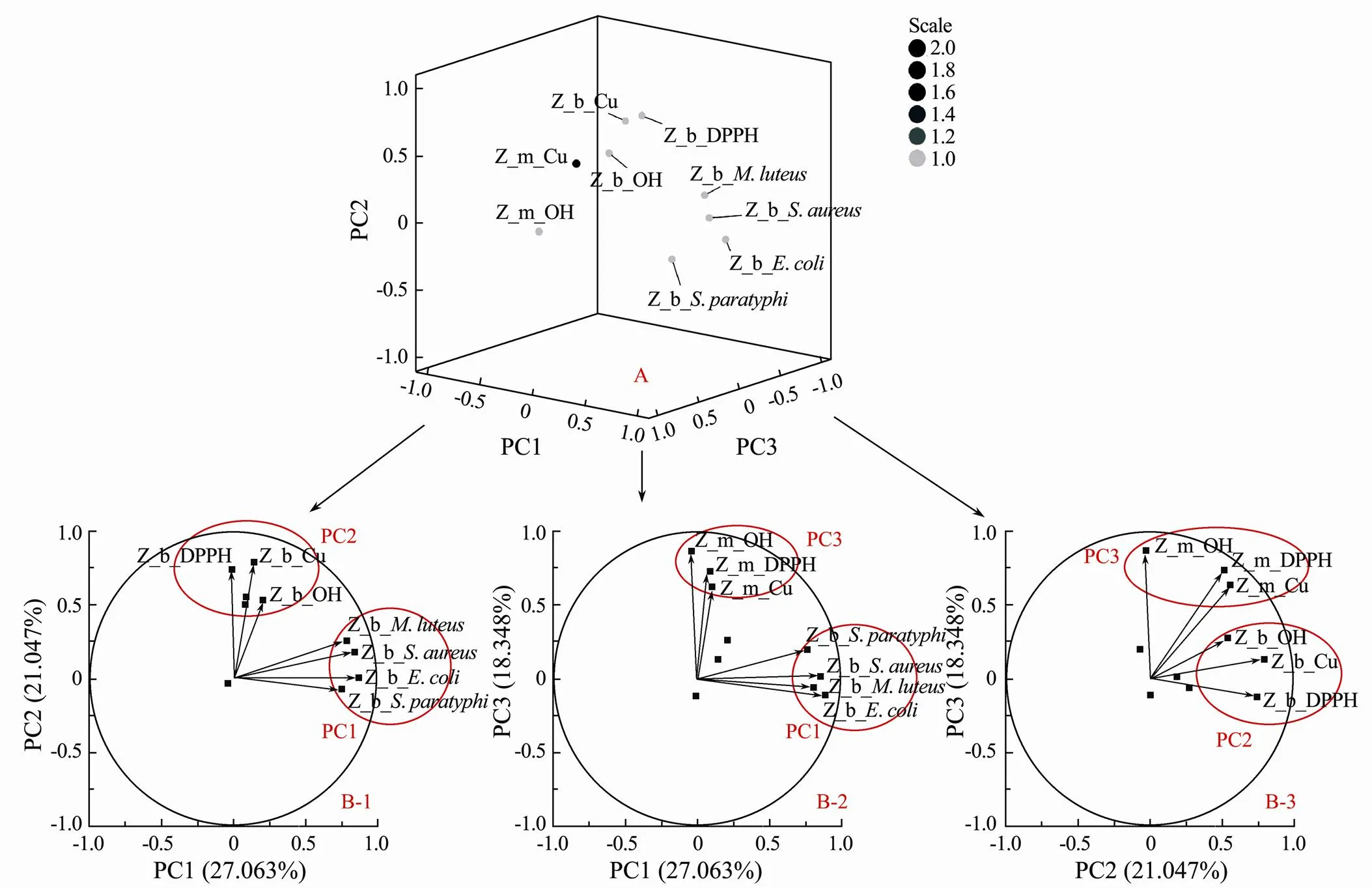
Fig.4 PCA loading plots obtained after varimax rotation of the whole dataset. The factor loadings represent the correlations between the original variables and the principal components. A shows the correlations in three dimensions. B-1, B-2 and B-3 show PC1 vs. PC2, PC1 vs. PC3 and PC2 vs. PC3 in two dimensions, respectively. The prefix Z represents the data normalized by the Z-score. The prefixes b and m represent the activities of the broth and mycelium, respectively. The further away a variable lies from the origin, the stronger its influence on the PCs. For example, the antibacterial activities of broth extracts against S. paratyphi, E. coli, S. aureus and M. luteus were strongly correlated with PC1. Therefore, PC1 could represent the antibacterial activities of broth extracts. Similarly, PC2 and PC3 represent the antioxidant activity of broth and mycelial extracts, respectively.
Most of the cultured mangrove-derived fungi showed high antioxidant and antibacterial activities and are worth further study. Ascomycota, which included the most abundant species, accounted for 88.14% of the 118 cultured fungal species isolated from mangrove ecosystems. Jones. (2009) tested 530 marine-derived fungi and reported that Ascomycotais themost dominant phylum in marine fungi. Monika and Rohit have also demonstrated that Ascomycota is the most dominant phylum and is found in various environments (Sharma and Sharma, 2016). Among the 118 fungi, thespp. (30.51%) was the most common, followed by thespp. (13.56%).genusandgenus have been widely reported as the two major bioactive fungal genera in mangrove ecosystems (Kumaresan and Suryanarayanan, 2001; Ananda and Sridhar, 2002; Liu., 2007).spp. have been widely reported to accumulate high amounts of organic acids and utilize a number of carbon sources in the natural environment (Brown., 2013; Yang., 2016). In addition, lots of bioactive secondary metabolites produced bywere used in industrial applications such as food additives, pharmaceuticals and detergents (Yang., 2017). Inspp. study, there was a well know antibiotics group compounds of penicillin (Reschke and Schügerl, 1984). 29.6% of the crude broth extracts were found to exhibit resistance to at least one of the test- ed bacteria. However, no crude mycelia extracts showed antibacterial activity due torejection (Huband., 2015). Fungi can secrete secondary metabolites to face complicated environments and protect themselves from infection. Hence, the bioactivity of broth extracts was usually better than that of mycelium extracts (Fig.3).Among the 118 cultured fungi, only 20 fungal species did not show any bioactivity (the RSCs of DPPH and hydroxide were less than 90%, the crude extracts at a concentration of 1.00mgmL−1were equivalent to Vc at a concentration lower than 1.00mgmL−1, and the diameter of the clear zone was 0). In addition, 83.1% of the mangrove-derived fungi showed at least one bioactivity in the A1–A7 analyses. Rahmawati. (2019) confirmed that mangrove-derived fungi produce secondary metabolites with bioactivities similar to those of the original plants or even relatively high activities. Mangrove-derived fungi could be recognized as prolific sources of bioactive natural products.
However, the evaluation and screening of fungi with the best comprehensive bioactivity were difficult. The extractsofwithout anyantioxidant activity showed high antibacterial activity against,andwith clear zone diameters of (19.7±0.2)mm, (19.3±0.1)mm and (36.3±0.1)mm, respectively.showed specific antibacterial activity againstwith a clear zone diameter of (23.1±0.8)mm.showed specific antibacterial activity againstwith a clear zone diameter of (15.8±0.2)mm.Broth extract ofcould completely scavenge DPPH radicals but did not show any other bioactivity.andyielded the same results as. Althoughshowed better DPPHRS activity (95±1%) and improved CUPRAC (equivalent to (3.13±0.19)mgmL−1Vc) than(DPP HRS: 86%±1%, CUPRAC: equivalent to (0.63±0.03)mgmL−1Vc),showed better HRS activity (100±0%) than(HRS: 29%±7%). Furthermore, some fungi, such as,,and, only exhibited antioxidant activity in the broth extracts, whereas only the mycelial extracts of other fungi, such asand, showed antioxidant activity. Zhou. (2018) and Chi. (2019) also reported that most mangrove-derived fungi show antioxidant and antibacterial activities but did not perform any comparisons or evaluations of fungal bioactivity. In short, the evaluation fungal bioactivity using only one activity indicator was imprecise. All activity indicators should be taken into consideration for the evaluation of fungal bioactivity and screening fungi with the best comprehensive bioactivity.
PCA, which has been used in dimensionality reduction and factorial analysis of high-dimension datasets, was found to be suitable for the evaluation of the comprehensive bioactivity of fungi and allow the comparison and ranking of fungal activity. The new distribution axes, which called principal components (PCs), preserved most of the information of the sample variance by summarizing the features of the original dataset (Ringnér, 2008; Cor- radi., 2020). The PCA loading plots (Fig.4) display- ed the relationship between the original variables and the principal components, and the orthogonal vectors in the loading plots indicated the degree of correlations among variables. Hence, PC1, PC2 and PC3 were used in this study as new activity indicators to individually evaluate the antibacterial activities of the broth extracts, the antioxidant activities of the broth extracts and the antioxidant activities of the mycelial extracts. The PC scores andwere successfully calculated to individually evaluate the corresponding bioactivities and the comprehensive bioactivity.The rankings of the fungi based on their activities were extremely clear according to the PC scores andvalues, which allowed the comparison of multiple fungal activities.
The activity evaluation indexes1,2,and3were used to evaluate the antibacterial activities of broth extracts, antioxidant activities of both extracts and antioxidant activities of mycelial extracts. According to the PC1 score,spp. showed the highest antibacterial activity. Among the top 20 genera ranked in terms of antibacterial activity, 15 belonged to thegenus, and the others were(ranked 5th),(ranked 8th),(ranked 9th),(ranked 10th) and(ranked 17th).showed the highest antibacterial activity against,,and,with clear zone diameters of (27.0±0.6)mm, (26.6±0.7)mm, (28.5±0.2)mm and (32.9±0.4)mm, respectively. With respect to antioxidant activity, each phylum had a representative strain among the top 20 genera. The broth extract with the highest ranking was obtained from, which could completely scavenge DPPH under the experimental conditions. With respect to Cu2+reduction, the extract at a concentration of 1.00mgmL−1was found to be equivalent to (2.83±0.03)mgmL−1Vc. The mycelial extract with the highest antioxidant activity was obtained from,and this extract exhibited strong abilities to scavenge DPPH (91%±1%) and hydroxyl groups (97%±6%) and a moderate ability to reduce Cu2+. The PC1, PC2 and PC3 scores were successfully used to evaluate the corresponding activities.
The comprehensive activity of a fungal strain was eva- luated and represented by. Among the 118 mangrove- derived fungi,spp. showed the best comprehensive activity. The analysis of thevalues showed that 16 fungi among the top 20 strains in terms of comprehensive activity werespp., and the other four strains were(ranked 9th),(ranked 12th),(ranked 13th) and(ranked 16th).exhibited the highest comprehensive activity, followed byand. The comprehensive bioactivity of fungi was expediently and accurately evaluated by(Eq. (1)), which considers all activity indicators.
The fungi with high comprehensive bioactivity identified based onvalues were worth further study.hasoften been isolated and reported as an endophytic fungus with high antimicrobial activity, and Huang reported its activity against the mycelial growth of two phytopathogens,and, with inhibition rates of 98.16% and 79.34%, respectively (Huang., 2010). Mishra showed thatexhibits strong antibacterial activity against Bacillus subtilis, Micrococcus luteus and Staphylococcus aureus with minimum inhibitory concentrations (MICs) of 0.078, 0.156 and 0.312mgmL−1, respectively (Mishra., 2017).Twenty-eight volatile compounds and known antibiotics, such as ampicillin, streptomycin, chloramphenicol, rifampicin, miconazole, ketokonazole and fluconazole, have been detected in the secondary me- tabolite pool produced by(Mishra., 2017).Mishra concluded thatcould be exploited not only in the development of biocontrol agents for crop disease management but also as a sustainable and alternative resource for the discovery of potent antimicrobial metabolitesbased on the detection of beta-ketosynthase domains in polyketide synthase gene clusters and adenylation domains in nonribosomal peptide synthase gene clusters. In this study,not only showed strong antibacterial activity but also effectively scavenged DPPH and hydroxyl radicals with scavenging rates of 95%±1% and 88%±3%, respectively. In Cu2+reduction, 1.00mgmL−1broth extract ofwas equivalent to (1.77±0.09)mgmL−1Vc. The fungus.have been well known for producing an abundance of metabolites, such as pectinase (Martí- nez-Trujillo., 2011), butyrolactones (Nagia., 2012), aspochalasin (Kwon., 2012) and L-me- thioninase (El-Sayed, 2011), which endowed the fungus with antioxidant and antimicrobial activities (Guo and Wang, 2017; Si., 2018). Guo and Wang employed a Plackett–Burman design and a Box-Behnken design to optimize the fermentation conditions ofand reported the optimized production of substances with antibacterial activity against aquatic pathogenicfromwith potential for aquatic applications (Guo and Wang, 2017). Furthermore, Si. (2018) used the OSMAC strategy to isolate cytotoxic cytochalasans, which could be potential antitumor drug candidates, fromand found that these exhibited significant cytotoxic activities against three human cancer cell lines (THP1, HL-60 and PC3) with IC50values ranging from 3.00 to 15.10μmolL−1.without any reported bioactivity was first identified and named by Guinea. (2015). In our study,show- ed strong antioxidant activity and moderate antibacterial activity. Its broth extract showed a high RSC for DPPH and hydroxyl radicals, with values of 95±3% and 96±3%, respectively, and at a concentration of 1.00mgmL−1, this extract was equivalent to (3.57±0.07)mgmL−1Vc. This broth extract also showed antibacterial activity againstand,as demonstrated by their formation of inhibition zones with diameters of (21.2±1)mm and (12.0±1.3)mm, respectively. The fungi identified based on, which showed higher multibioactivity, showed great potential in the exploitation of natural products and the development of new drugs.
To extend the method, the PCs and-values of the 118 fungi will be used as a database for comparing normalized activity data reported in the future as long as the same activity assays will be used in the analyses. Values of PCs andgreater than 0indicate that the corresponding fungal activity is better than average level, and values greater than 1 indicate that the corresponding fungi exhibit high activity performance and are worth further research. Furthermore, the activity bioassays can be replaced by other analyses to establish a database for scre- ening fungi with desired activity. Hence, PCA is a time- saving, scientific method with a strong ability to evaluate and screen multiactive fungi.
5 Conclusions
Mangrove-derived fungi were diverse and bioactive. In the mangrove ecosystem of the Maowei Sea,Ascomycota was the most dominantphylum, andandwere the two major genera. For accurate evaluation of fungal bioactivity, PCA was successfully employed to evaluate and screen high-quality fungi with bioactivity. The results showed that PCA allowed the scientific comparison of multiactive fungi. Among the 118 tested mangrove-derived fungi,spp. showed the best comprehensive activity. Fungi such as,and, which exhibited high comprehensive bioactivity as demonstrated by PCA, showed great potential in the exploitation of natural products and the development of new drugs. Furthermore, a database that included information on the activities of 118 fungi was provided such that other researchers could conveniently compare fungal activities in the future work. The application of PCA provides a scientific basis for evaluating and screening fungi with multiple activities, and this method thus affects the discovery and development of new drugs. Mangrove-derived fungi recognized as prolific sources of natural products are worth sustained attention and further study.
Acknowledgements
We gratefully acknowledge the members of the Key Laboratory of Marine Chemistry Theory and Technology for useful discussions. This work was financially supported by the Key R&D Program of Shandong Province (No. 2020CXGC010703) and the Key Project of the Natural Science Foundation of Shandong Province (No. ZR2020KB021).
Abdalla, M. A., Aro, A. O., Gado, D., Passari, A. K., Mishra, V. K., Singh, B. P.,., 2020. Isolation of endophytic fungi from South African plants, and screening for their antimicrobial and extracellular enzymatic activities and presence of type I polyketide synthases., 134: 336-342.
Al-Hindi, R. R., Aly, O. E., Hathout, A. S., Alharbi, M. G., Al- Masaudi, S., Al-Jaouni, S. K.,., 2017. Isolation and molecular characterization of mycotoxigenic fungi in agarwood., 25: 1781-1787.
Ananda, K., and Sridhar, K. R., 2002. Diversity of endophytic fungi in the roots of mangrove species on the west coast of India., 48: 871-878.
Apak, R., Güçlü, K., Özyürek, M., and Karademir, S. E., 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUP RAC method., 52: 7970-7981.
Asante-Okyere, S., Shen, C., Ziggah, Y. Y., Rulegeya, M. M., and Zhu, X., 2020. Principal component analysis (PCA) based hybrid models for the accurate estimation of reservoir water saturation., 145: 104555- 104555.
Atiphasaworn, P., Monggoot, S., Gentekaki, E., Brooks, S., and Pripdeevech, P., 2017. Antibacterial and antioxidant constituents of extracts of endophytic fungi isolated fromvar.Leaves., 74: 1185-1193.
Bao, J., Wang, J., Zhang, X. Y., Nong, X. H., and Qi, S. H., 2017. New furanone derivatives and alkaloids from the co- culture of marine-derived fungiand., 14: e1600327- 1600327.
Behera, B. C., Sethi, B. K., Mishra, R. R., Dutta, S. K., and Thatoi, H. N., 2017. Microbial cellulases–Diversity & biotechnology with reference to mangrove environment: A review., 15: 197- 210.
Bhimba, B. V., Franco, D. A. A. D., Mathew, J. M., Jose, G. M., Joel, E. L., and Thangaraj, M., 2012. Anticancer and antimicrobial activity of mangrove derived fungiVB1., 10: 77-80.
Bibi, S. N., Gokhan, Z., Rajesh, J., and Mahomoodally, M. F., 2020. Fungal endophytes associated with mangroves–Chem- istry and biopharmaceutical potential., 134: 187-212.
Bode, H. B., Bethe, B., Höfs, R., and Zeeck, A., 2002. Big effects from small changes: Possible ways to explore nature’s chemical diversity., 3: 619-627.
Brown, S. H., Bashkirova, L., Berka, R., Chandler, T., Doty, T., McCall, K.,., 2013. Metabolic engineering ofNRRL 3488 for increased production of l-malic acid., 97: 8903-8912.
Bruins, A. P., 2002. Electrochemically assisted Fenton reaction: Reaction of hydroxyl radicals with xenobiotics followed by on-line analysis with high-performance liquid chromatography/tandem mass spectrometry., 16: 1934-1940.
Cheng, Z. S., Pan, J. H., Tang, W. C., Chen, Q. J., and Lin, Y. C., 2009. Biodiversity and biotechnological potential of mangrove-associated fungi., 20: 63- 72.
Chi, W. C., Pang, K. L., Chen, W. L., Wang, G. J., and Lee, T. H., 2019. Antimicrobial and iNOS inhibitory activities of the endophytic fungi isolated from the mangrove plantvar.., 60: 1-8.
Chomnunti, P., Hongsanan, S., Aguirre-Hudson, B., Tian, Q., Peršoh, D., Dhami, M. K.,., 2014. The sooty moulds., 66: 1-36.
Corradi, E., Agostini, M., Greco, G., Massidda, D., Santi, M., Calderisi, M.,., 2020. An objective, principal-compo- nent-analysis (PCA) based, method which improves the quartz-crystal-microbalance (QCM) sensing performance.:, 315: 112323-112323.
Cui, H., Lin, Y., Luo, M., Lu, Y., Huang, X., and She, Z., 2017. Diaporisoindoles A-C: Three isoprenylisoindole alkaloid derivatives from the mangrove endophytic fungussp. SYSU-HQ3., 19: 5621-5624.
El-Sayed, A. S. A., 2011. Purification and characterization of a new L-methioninase from solid cultures of., 49: 130-140.
Er, C. M., Sunar, N. M., Leman, A. M., and Othman, N., 2015. Direct growth inhibition assay of total airborne fungi with application of biocide-treated malt extract agar., 2: 340-344.
Guinea, J., Sandoval-Denis, M., Escribano, P., Peláez, T., Guarro, J., and Bouza, E., 2015., a new species of section terrei isolated from samples of patients with nonhematological predisposing conditions., 53: 611-617.
Guo, L., and Wang, C., 2017. Optimized production and isolation of antibacterial agent from marineagainst. 3, 7: 383-392.
Guo, Z. K., Zhou, Y. Q., Han, H., Wang, W., Xiang, L., Deng, X. Z.,., 2018. New antibacterial phenone derivatives asperphenone a-c from mangrove-derived fungussp. YHZ-1., 16: 1-7.
He, Y., Wang, B., Chen, W., Cox, R. J., He, J., and Chen, F., 2018. Recent advances in reconstructing microbial secondary metabolites biosynthesis inspp., 36: 739-783.
Hernandez-Restrepo, M., Gene, J., Castaneda-Ruiz, R. F., Mena-Portales, J., Crous, P. W., and Guarro, J., 2017. Phylogeny of saprobic microfungi from southern Europe., 86: 53-97.
Huang, S. S., Xu, L. L., Huang, X., Zhang, R. C., Chen, L. M., Lei, F.,., 2010. Isolation and identification of 3 strains of marine fungi with antagonistic ability against litchi and rice pathogenic fungi., 29: 665- 672.
Huband, M. D., Bradford, P. A., Otterson, L. G., Basarab, G. S., Kutschke, A. C., Giacobbe, R. A.,., 2015.antibacterial activity of AZD0914, a new spiropyrimidinetrione DNA gyrase/topoisomerase inhibitor with potent activity against gram-positive, fastidious gram-negative, and atypical bacteria., 59: 467- 474.
Jones, E., Sakayaroj, J., Suetrong, S., Somrithipol, S., and Pang, L. K., 2009. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota., 35: 1-187.
Kaiser, H. F., 1960. The application of electronic computers to factor analysis., 20: 141-151.
Kumaresan, V., and Suryanarayanan, T. S., 2001. Occurrence and distribution of endophytic fungi in a mangrove community., 105: 1388-1391.
Kwon, Y. J., Sohn, M. J., Kim, C. J., Koshino, H., and Kim, W. G., 2012. Flavimycins A and B, dimeric 1,3-dihydroisobenzo- furans with peptide deformylase inhibitory activity from., 75: 271-274.
Liu, A. R., Wu, X. P., and Xu, T., 2007. Research advances in endophytic fungi of mangrove., 18: 912-918.
Luo, Y., Cobb, R. E., and Zhao, H., 2014. Recent advances in natural product discovery., 30: 230-237.
Mahmoudi, M. R., Heydari, M. H., Qasem, S. N., Mosavi, A., and Band, S. S., 2021. Principal component analysis to study the relations between the spread rates of COVID-19 in high risks countries., 60: 457- 464.
Mar, A., and Pripdeevech, P., 2014. Chemical composition and antibacterial activity of essential oil and extracts offlowers from Thailand., 9: 707-710.
Martínez-Trujillo, A., Arreguín-Rangel, L., García-Rivero, M., and Aguilar-Osorio, G., 2011. Use of fruit residues for pectinase production byFP-500 andFP-370., 53: 202- 209.
Martini, E., Wollschläger, U., Musolff, A., Werban, U., and Za- charias, S., 2017. Principal component analysis of the spatiotemporal pattern of soil moisture and apparent electrical conductivity., 16: 1-12.
Meng, L. H., Li, X. M., Liu, Y., and Wang, B. G., 2015. Polyoxygenated dihydropyrano[2,3-c]pyrrole-4,5-dione derivatives from the marine mangrove-derived endophytic fungusMA-231 and their antimicrobial activity., 26: 610-612.
Meng, L. H., Li, X. M., Liu, Y., Xu, G. M., and Wang, B. G., 2017. Antimicrobial alkaloids produced by the mangrove endophyteMA-231 using the OSMAC approach., 7: 55026-55033.
Mishra, V. K., Passari, A. K., Chandra, P., Leo, V. V., Kumar, B., Uthandi, S.,., 2017. Determination and production of antimicrobial compounds bystrain MJ31, an endophytic fungus fromL. using UPLC-ESI-MS/MS and TD-GC-MS analysis., 12: e0186234-0186234.
Nagia, M. M. S., El-Metwally, M. M., Shaaban, M., El-Zalabani, S. M., and Hanna, A. G., 2012. Four butyrolactones and diverse bioactive secondary metabolites from terrestrialMM2: Isolation and structure determination., 2: 1-8.
Oakley, C. E., Ahuja, M., Sun, W. W., Entwistle, R., Akashi, T., Yaegashi, J.,., 2017. Discovery of McrA, a master regulator ofsecondary metabolism., 103: 347-365.
Pripdeevech, P., and Machan, T., 2011. Fingerprint of volatile flavour constituents and antioxidant activities of teas from Thailand., 125: 797-802.
Rahmawati, S. I., Izzati, F. N., Hapsari, Y., Septiana, E., Rachman, F., and Bustanussalam, S. P., 2019. Endophytic microbes and antioxidant activities of secondary metabolites from mangrovesand., 278: 012065-012065.
Reschke, M., and Schügerl, K., 1984. Reactive extraction of penicillin I: Stability of penicillin G in the presence of carriers and relationships for distribution coefficients and degrees., 28: B1-B9.
Ringnér, M., 2008. What is principal component analysis?, 26: 303-304.
Roohparvar, R., Shamspur, T., and Mostafavi, A., 2018. Application of silica coated magnetite nanoparticles modified with Cu(I)-neocuproine as nanosorbent to simultaneous separation-preconcentration of trace amounts of nitrate and nitrite., 73: 9-14.
Saito, Y., Tsuchida, H., Matsumoto, T., Makita, Y., Kawashima, M., Kikuchi, J.,., 2018. Screening of fungi for decomposition of lignin-derived products from Japanese cedar., 126: 573-579.
Scherlach, K., Schuemann, J., Dahse, H. M., and Hertweck, C., 2010. Aspernidine A and B, prenylated isoindolinone alkaloids from the model fungus., 63: 375-377.
Sharma, M., and Sharma, R., 2016. Drugs and drug intermediates from fungi: Striving for greener processes., 42: 322-338.
Sheik, S., and Chandrashekar, K. R., 2018. Fungal endophytes of an endemic plantwall. of western Ghats (India) and their antimicrobial and DPPH radical scavenging potentiality., 18: 115-125.
Shekhar, T. C., and Anju, G., 2014. Antioxidant activity by DPPH radical scavenging method ofLinn. leaves., 1: 244- 249.
Si, Y., Tang, M., Lin, S., Chen, G., Feng, Q., Wang, Y.,., 2018. Cytotoxic cytochalasans from Aspergillus flavipes PJ03-11 by OSMAC method., 59: 1767- 1771.
Sinurat, A. P., Wina, E., Rakhmani, S. I. W., Wardhani, T., Haryati, T., and Purwadaria, T., 2018. Bioactive substances of some herbals and their effectiveness as antioxidant, antibacteria and antifungi., 23: 18-27.
Sun, J., Shao, J., Sun, C., Song, Y., Li, Q., Lu, L.,., 2018. Borrelidins F-I, cytotoxic and cell migration inhibiting agents from mangrove-derivedSCSIO ZJ89., 26: 1488-1494.
Vandermarken, T., Croes, K., Van Langenhove, K., Boonen, I., Servais, P., Garcia-Armisen, T.,., 2018. Endocrine activity in an urban river system and the biodegradation of estrogen-like endocrine disrupting chemicals through a bio-analy- tical approach using DRE- and ERE-CALUX bioassays., 201: 540-549.
Wang, F. Z., Wei, H. J., Zhu, T. J., Li, D. H., Lin, Z. J., and Gu, Q. Q., 2011. Three new cytochalasins from the marine-de- rived fungusKLA03 by supplementing the cultures with L- and D-Tryptophan., 8: 887-894.
Wei, M. Y., Wang, C. F., Wang, K. L., Qian, P. Y., Wang, C. Y., and Shao, C. L., 2017. Preparation, structure, and potent anti- fouling activity of sclerotioramine derivatives., 19: 372-378.
Yamashita, N., Murata, M., Inoue, S., Burkitt, M. J., Milne, L., and Kawanishi, S., 1998. α-Tocopherol induces oxidative damage to DNA in the presence of copper(II) ions., 11: 855-862.
Yang, L., Lübeck, M., and Lübeck, P. S., 2017.as a versatile cell factory for organic acid production., 31: 33-49.
Yang, L., Xia, J., and Shen, Q., 2016. Establishing target-ori- ented energy consumption quotas for buildings., 41: 57-66.
Zhang, L., Niaz, S. I., Khan, D., Wang, Z., Zhu, Y., Zhou, H.,., 2017. Induction of diverse bioactive secondary metabolites from the mangrove endophytic fungussp. (Strain 307) by co-cultivation with(Strain B2)., 15: 1-14.
Zhou, J., Diao, X., Wang, T., Chen, G., Lin, Q., Yang, X.,., 2018. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove speciesand.in the South China Sea.,13: e0197359-0197359.
(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5096-x
ISSN 1672-5182, 2022 21 (3): 763-772
(June 6, 2021;
September 6, 2021;
October 13, 2021)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
Corresponding authors. E-mail:yaoshuo@ouc.edu.cn E-mail:dahaizhang@ouc.edu.cn
(Edited by Ji Dechun)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Effect of Intertidal Elevation at Tsuyazaki Cove, Fukuoka,Japan on Survival Rate of Horseshoe Crab Tachypleus tridentatusEggs
- Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
- Experimental Investigation on the Interactions Between Dam-Break Flow and a Floating Box
- Variational Solution of Coral Reef Stability Due to Horizontal Wave Loading
- High Microplastic Contamination in Juvenile Tri-Spine Horseshoe Crabs: A Baseline Study of Nursery Habitats in Northern Beibu Gulf, China
- Influence of Autonomous Sailboat Dual-Wing Sail Interaction on Lift Coefficients
