基因芯片在畜禽遗传育种中的应用及展望
汪佳豪,赵卿尧,周月玲,史良玉,王楚端,俞英
综 述
基因芯片在畜禽遗传育种中的应用及展望
汪佳豪1,赵卿尧1,周月玲1,史良玉2,王楚端1,俞英1
1. 中国农业大学动物科学技术学院,北京 100193 2. 武汉轻工大学动物科学与营养工程学院,武汉 430023
基因芯片是一种通过DNA双链或DNA-RNA互补杂交检测特定DNA序列的高通量技术,其中SNP基因分型芯片已经广泛用于畜禽的遗传育种工作,在牛()、猪()、羊()、鸡()等畜禽中取得了重大成就。但是在实际生产中使用的基因组选择仅利用了基因组信息,无法完全解释复杂性状的分子遗传基础,限制了基因组选择的准确性。随着表观遗传学研究的不断深入、商用甲基化芯片的推出、表观基因组关联分析(epigenome-wide association study,EWAS)的提出,DNA甲基化已被广泛用于解释遗传与表型的因果关系。未来,有望开发专门针对畜禽的甲基化芯片,通过EWAS探索与畜禽经济性状显著相关的甲基化位点,深化对复杂性状因果变异的理解。结合甲基化芯片与SNP芯片捕获畜禽表观基因组和基因组信息,更准确地解读遗传变异,提高基因组选择的准确性,推动畜禽分子遗传育种工作的精细化发展。本文综述了SNP芯片在畜禽上的应用,并对甲基化芯片在畜禽上的应用进行了展望,以期为基因芯片在动物育种中的进一步应用提供借鉴和参考。
基因芯片;SNPs;DNA甲基化;畜禽遗传育种
基因芯片技术是20世纪90年代发展起来的分子生物学高新技术,经过30多年的快速发展,现已在功能上大致分为3个主要类别:基因表达谱芯片、SNP基因分型芯片和甲基化芯片。随着基因组选择的提出,SNP基因分型芯片被广泛应用于畜禽遗传育种工作,大大提高了畜禽的遗传进展[1]。目前,基因组选择模型主要使用基因组信息[2],不能完全解释复杂性状的分子遗传基础。畜禽的育种值定义为亲代可以稳定遗传给子代的部分[3~5]。随着遗传学的不断发展,科研人员发现不仅DNA序列的信息可以稳定遗传给后代,DNA序列上的表观遗传修饰如DNA甲基化也能遗传给子代并影响子代的表型[6~11]。此外,表观遗传修饰也可以作为环境与基因之间的桥梁,调控基因在特定环境下的表达[12]。未来,有望结合基因组和表观基因组信息,更深入地解释畜禽的遗传变异及环境与基因之间的相互作用,为畜禽遗传育种提供更全面的视角。
本文综述了基因芯片的发展,探讨了其在畜禽遗传育种领域的关键应用。此外,本文对未来如何开发专门针对畜禽的甲基化芯片,以及如何将DNA甲基化信息与基因组信息相结合,从而提高基因组选择的准确性实现更为精细的分子遗传育种进行了深入的探讨。
1 基因芯片
20世纪90年代初,Fodor等[13]组合固相化学、光不稳定保护基团、光刻等技术研发出第一款基因芯片。1995年,Schena等[14]合成了第一款基因表达谱芯片,基因芯片开始进入发展的黄金时期。此后,荧光微球技术[15]、光纤微球技术[16]、靶向捕获测序技术[17]等相继提出并得到实际应用。目前,基因芯片技术主要聚焦于SNP基因分型和DNA甲基化检测。
1.1 SNP芯片
1994年,单核苷酸多态性(single nucleotide polymorphisms,SNPs)的概念首次被提出[18]。1996年,Lander[19]正式提出并认定其为继限制性片段长度多态性(restriction fragment length polymorphisms,RFLP)和简单重复序列(simple sequence repeat,SSR)之后的第三代分子遗传标记。SNPs作为新一代分子遗传标记,不仅遗传稳定性高、在基因组内广泛分布,并且绝大部分是二等位基因(单个位点有两种碱基),便于实现自动化的基因分型[20,21]。Delahunty等[22]于1996年结合PCR与寡核苷酸连接检测(oligonucleotide ligation assay,OLA)技术实现了半自动化的SNPs基因分型。2000年,Affymetrix公司通过单碱基延伸(single base extension,SBE)原理设计出对SNPs进行自动化、高通量基因分型的寡核苷酸阵列[23,24]。2002年,Illumina公司基于微球矩阵技术也实现准确、高性价比的高通量SNPs基因分型[25,26]。此后,随着下一代测序技术的发展,测序速度得到了显著提高。但许多研究和疾病诊断只需测序基因组中的特殊序列就能实现,基于该需求,靶向捕获测序技术被提出并获得了广泛的应用[17]。与具有极高工艺门槛的固相基因芯片不同,基于靶向捕获测序技术的液相芯片只需设计相应的探针即可,使得该技术得到很多公司的青睐。如博瑞迪公司靶向测序基因型检测(genotyping by target sequencing,GBTS)技术[27]、华智公司基于目标区域基因组序列液相捕获的精准定位测序分型技术(genotyping by pinpoint sequencing of captured target,cGPS)(https:// www.higentec.com/)等均是基于靶向捕获测序技术进一步发展而来,此类技术的不断创新和完善使得SNP芯片的种类日益丰富,目前已逐渐形成固相芯片和液相芯片两大类。
1.2 甲基化芯片
DNA甲基化是表观遗传修饰中研究最深入的一种修饰,一般发生在胞嘧啶-磷酸-鸟嘌呤(CpG)中胞嘧啶的第五位碳原子上[28]。DNA甲基化不仅能够调控基因表达,影响细胞分化[29],而且与各种疾病、生理状态有关,被广泛作为生物标志物[30,31]。鉴于其在遗传和分子生物学中的重要性,DNA甲基化也被人们称为“第五碱基”。1992年,Frommer等[32]对DNA进行亚硫酸氢盐处理,将非甲基化的胞嘧啶(C)转化为尿嘧啶(U),PCR扩增中转化为胸腺嘧啶(T),可以实现对DNA甲基化的精确检测。此后,随着对该方法的不断完善,已成为DNA甲基化检测的金标准[33]。DNA甲基化的检测与SNP相似:检测亚硫酸氢盐转化后的CpG中C的位置为C还是T即可。2006年,Illumina在原有SNP基因分型技术上开发出高通量的甲基化检测芯片[34]。此后,在靶向捕获测序技术基础上也开发出靶向亚硫酸氢盐测序(targeted bisulfite sequencing,TBS)技术,用于检测DNA甲基化。其主要分为两种策略:(1)亚硫酸氢盐处理后再对目标序列捕获测序[35];(2)对目标序列进行捕获、亚硫酸氢盐转化、上机测序[36]。经亚硫酸氢盐处理及PCR扩增后使序列的复杂度大大降低,难以在高密度的CpGs区域设计最佳的捕获探针。因此,现行的技术流程主要采用策略(2)。甲基化芯片不仅能对特定区域的甲基化水平进行检测,而且显著降低甲基化检测成本,已成为甲基化检测的主要方法之一[37]。
2 SNP芯片在畜禽上的应用
随着新一代分子遗传标记SNPs的发现及应用、基因芯片的发展,使应用于动物上的商业化SNP芯片成为可能(图1)。Meuwissen等[38]于2001年提出基因组选择:假设覆盖全基因组的高密度标记中有些标记与影响该目标性状的数量性状基因座(quantitative trait locus,QTL)的位置非常接近,处于连锁不平衡,这样使得每个QTL的效应都可以通过标记得到反映,即全基因组范围内的标记辅助选择。基因组选择相比于传统的育种方法具有更高的估计准确性,能大大缩短世代间隔,对传统方法难以实施选择的性状也能有较好选择[39,40]。但是,由于缺乏对动植物进行基因分型的工具而无法有效实施。
2007年,Illumina发布第一款畜禽的商业化SNP基因分型芯片——BovineSNP50 Genotyping BeadChip[41],基因组选择率先应用于奶牛的遗传育种工作,拉开了畜禽遗传育种的新篇章。VanRaden等[42]于2008年使用BovineSNP50 Genotyping BeadChip对5335头公牛进行基因分型,验证基因组选择的可行性。相比于传统的预测方法,基因组预测的可靠性更高。2009年,美国首次公开发布奶牛基因组选择的结果,并完全接纳基因组信息作为官方种公牛遗传评定发布的信息来源,综合传统评定结果与基因组育种值对公牛进行排名[43]。自此,基因组选择被广泛应用于奶牛的遗传育种工作。2014年,Hutchison等[44]对美国荷斯坦牛、娟姗牛的数据研究发现:年轻公牛用于育种的数量越来越多,显著缩短了奶牛的世代间隔。
基因组选择也广泛应用于其他畜禽动物[45]。2009年,首款猪的SNP基因分型芯片Illumina Porcine60 Genotyping BeadChip问世[46],猪的基因组选择育种工作在全球范围内也逐渐开展起来。温氏集团作为我国最大的猪育种与肉猪生产公司,于2011年开始进行基因组选择育种[39],2013年成功选育出一头杜洛克特级种公猪[47]。Genus公司通过基因组选择显著提高了猪的出生仔猪总数、日增重、出生到断奶阶段的死亡率、采食量、眼肌高度等5个性状的估计育种值准确性[48]。2009年,绵羊()SNP基因分型芯片OvineSNP50 BeadChip问世,基因组技术也逐渐应用在绵羊的遗传育种工作[49]。
随着我国对种业工作的不断重视,适用于我国本地品种的SNP芯片陆续被开发出来。自主研发的广泛适用于我国地方鸡种的基因组育种芯片“京芯一号”,不仅摆脱了对国外基因芯片的依赖,而且能更好的适用于本地鸡品种的遗传育种工作[50,51]。此后,在猪[52,53]、牛[54~56]、羊[57,58]上自主研发的SNP芯片的相继问世大大推进了我国畜禽的遗传育种工作(表1)。
3 表观遗传学在畜禽遗传育种上的应用
表观遗传学是指DNA序列不变的情况下,基因的表达却发生了可遗传的变化。造成这些变化的表观遗传修饰包括DNA/RNA甲基化、组蛋白修饰(甲基化、乙酰化、磷酸化等)和非编码RNA (miRNAs、lncRNAs、circRNAs等)。目前,关于表观遗传的研究在DNA甲基化方面最为成熟[69]。
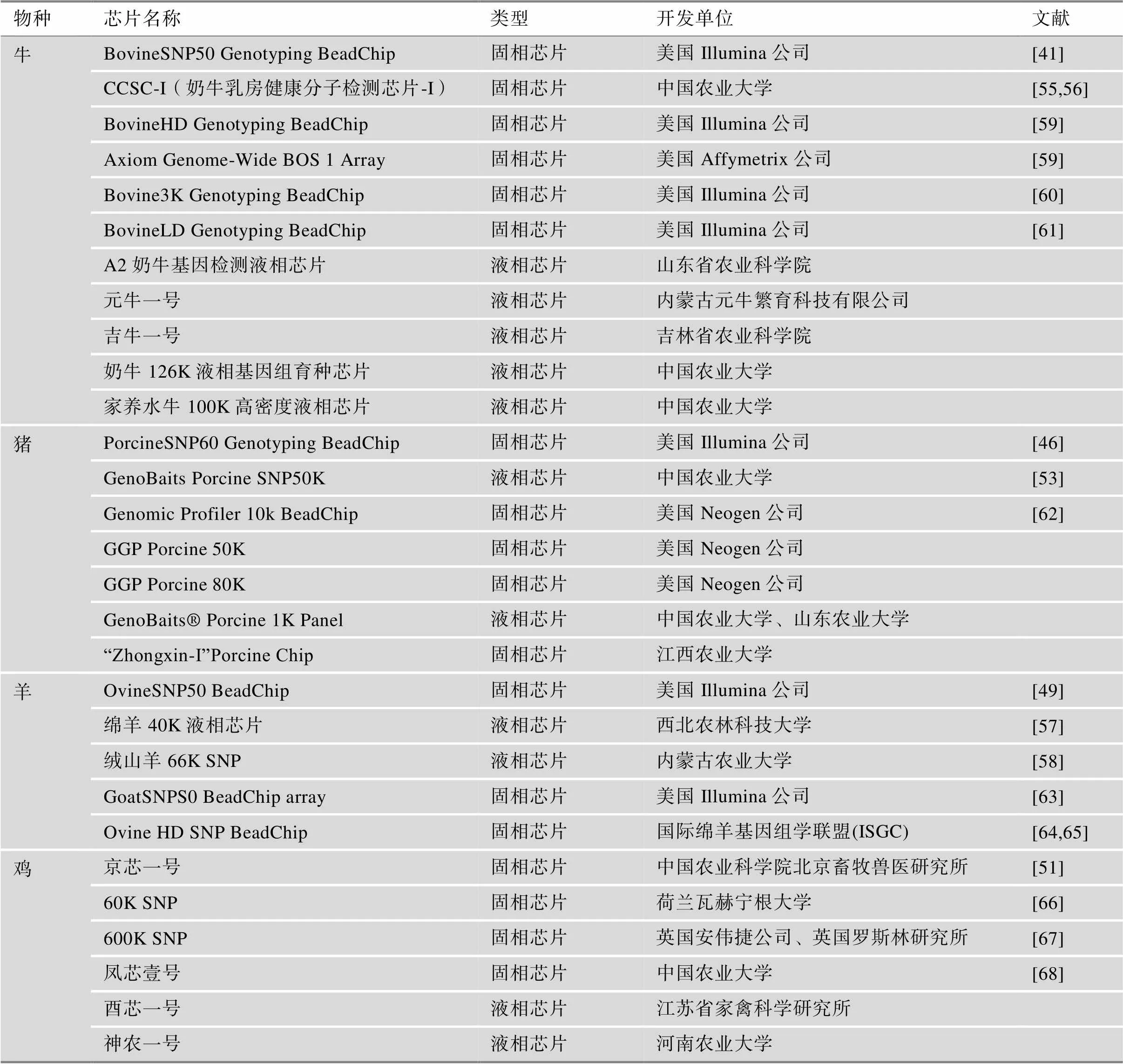
表1 畜禽上常用的SNP芯片
研究表明,DNA甲基化对畜禽繁殖、骨骼肌发育、免疫等性状均有显著影响。例如:差异甲基化基因使同卵双生的两头公牛的表型、繁殖性状的估计育种值均存在差异[70];位于启动子的DNA甲基化能够抑制该基因表达,促进肌细胞增殖、抑制其分化,以调控骨骼肌的生长发育[71];DNA甲基化对T细胞亚群的分化调控等方面具有重要作用,特别是基因的DNA甲基化显著影响CD4+T细胞数量和功能,能够在一定程度上改变家畜的免疫应答能力或抗病性能,以对抗或适应外界环境改变[72]。
目前被广泛应用的GBLUP (genomic best linear unbiased prediction)、ssGBLUP (single-step genomic best linear unbiased prediction)模型仅使用了系谱和DNA序列的信息。虽然将SNPs的功能作为先验信息可以提高模型估计育种值的准确性[2],但是忽略了可以稳定遗传的表观遗传变异对性状的影响。所估计的育种值只捕捉到真实育种值的部分信息,大大限制了基因组选择准确性的提升。
随着表观遗传学研究的深入,数量表观遗传学[12,73]、群体表观遗传学[74,75]不断发展并完善。数量表观遗传学研究表明:DNA甲基化等表观遗传修饰通过影响基因的表达,进而影响等位基因之间的显性效应、非等位基因之间的上位效应,导致遗传方差组分发生变化[12]。群体表观遗传学对经典群体遗传学进行拓展,引入单甲基化多态性(single methylation polymorphism,SMP)的概念,即胞嘧啶位点是否发生甲基化修饰[75]。Hu等[76]根据位点甲基化水平对甲基化信息进行转化,构建与GBLUP中G矩阵类似的epi-G矩阵,结果显示表观遗传变异能够解释65%的表型变异。陈思倩[77]根据SNPs是否位于表观功能基因组区域进行分类,将表观基因组信息引入GFBLUP (genomic feature best linear unbiased prediction)模型,其预测准确性相比于传统GBLUP有所提高。这些都表明将表观基因组信息纳入基因组选择模型中对提高复杂性状预测准确性的重要性。
4 甲基化芯片的应用及展望
畜禽遗传育种工作的核心是选择优秀的个体,而选择的关键是提高选种的准确性。基因组选择已极大的缩短了畜禽的世代间隔,未来提高遗传进展的关键在于提高基因组选择育种值估计的准确性。畜禽的表型并非完全由基因决定,DNA甲基化对畜禽的各种性状都能产生影响。未来通过整合表观遗传学的信息,有望提高基因组选择的准确性,实现更精准的分子遗传育种工作[78]。
4.1 全基因组甲基化芯片
DNA甲基化在遗传调控方面的重要性引起了人们的广泛关注。但是常规的全基因组甲基化测序(whole genome bisulfite sequencing,WGBS)成本极高,不易实现大规模的甲基化分析;简化代表性亚硫酸氢盐测序(reduced representation bisulfite sequencing,RRBS)只能检测CpGs富集的区域[37]。甲基化芯片则可以自主设计检测特定区域甲基化的探针,实现对更多具有生物学意义的区域进行高通量低成本的检测,进行大规模的甲基化研究[79]。
最早的甲基化芯片可以追溯到21世纪初,其主要用于检测癌症[34]。2009年,Illumina公司开发了商业化的人甲基化芯片HumanMethylation27K BeadChip (HM27),该芯片能够检测超过27K个CpG位点的甲基化水平,被广泛用于甲基化研究[80~82]。随着表观基因组关联分析(epigenome-wide association study,EWAS)被人们提出并广泛用于检测与人类疾病相关的CpG位点,解释GWAS中无法解释的因果关系[83],甲基化芯片逐渐成为EWAS的重要工具。此后进一步开发出更高密度的甲基化芯片HumanMethylation450 BeadChip[84]、MethylationEPIC (EPIC) BeadChip[81],并开发出专门进行EWAS的软件[85]和数据平台[86]。在人类研究中,利用甲基化芯片进行EWAS在肥胖[87]、初生重[88]等性状鉴定到显著相关的CpGs位点,进一步解释了遗传与性状的因果关系。目前,EWAS也逐渐应用于动植物上[89,90]。已有研究利用甲基化芯片鉴定到与畜禽重要性状相关的甲基化位点,并进一步开发出生物标志物[90,91]。但针对畜禽的甲基化芯片种类较少,在一定程度上限制了畜禽的甲基化研究。未来,通过开发畜禽的全基因组甲基化芯片,将大大降低甲基化检测的费用,实现更大规模的甲基化分析,进一步解释遗传与表型之间的分子机制,为功能性甲基化芯片的开发提供基础(图2)。
4.2 精子甲基化芯片
在现代畜牧业中,家畜的交配大部分由人工授精完成[92]。一头种公畜每年可以产生成百上千的后代,对群体的贡献远大于母畜[93,94]。因此,种公畜性能的好坏以及精子质量的优劣在很大程度上决定了畜禽遗传改良的进展。而精子中含有丰富的表观基因组信息,更好的利用这些信息有望选择更优秀的种公畜[95]。基因组印记是表观遗传的重要现象之一:基因组上部分基因的表达具有亲本选择特性,即只有一个亲本的等位基因表达,另一个亲本的等位基因不表达或很少表达[96]。畜禽精子中含有大量可以稳定遗传给后代并影响子代生长发育的印记基因[97,98]。这些印记基因通常在染色体上成簇存在,并且大部分由不同亲本来源的等位基因调控区的差异甲基化引起[97~100]。例如最典型的印记基因,印记通过的差异甲基化区域(differentially methylated region,DMR)进行维持,该区域低甲基化将导致的双等位基因表达,引发胎儿过度生长[8]。Nishio等[101]将父母双方的等位基因根据印记效应重新赋予加性效应并创建GBLUP-I模型,相比传统的GBLUP模型,GBLUP-I模型估计遗传方差的准确性更高。
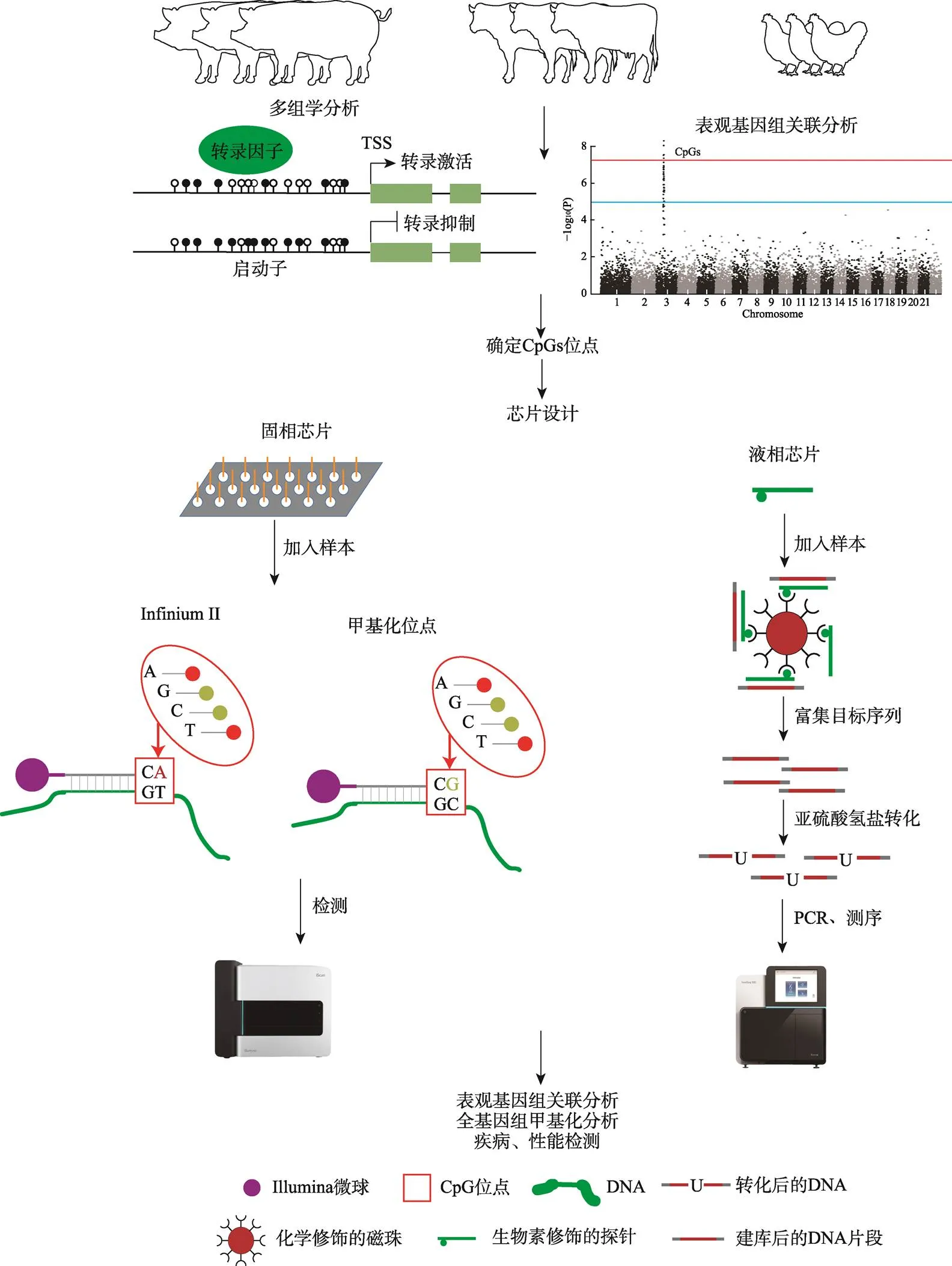
图2 甲基化芯片原理及在畜禽遗传育种上的应用
精子DNA甲基化信息不仅包含印记基因修饰,也包含可以指示精子生育能力以及影响后代表型的甲基化修饰。Costes等[102]对100头生育能力有差异的牛的精子进行甲基化分析,共鉴定到490个与生育力相关的差异甲基化胞嘧啶位点(differentially methylated cytosines,DMCs),使用这些甲基化位点构建模型能够很好的区分低生育力和正常生育力的公牛。Takeda等[91]通过甲基化分析鉴定到多个与公牛繁殖力相关的DMCs和DMRs,通过这些DMRs构建模型能够很好的预测公牛的生育力。Liu等[70]和Shojaei Saadi等[103]均发现:尽管同卵双胞胎牛具有相同的基因组信息,但不同的环境可以导致其DNA甲基化模式的差异,从而影响其精子的表型和公牛的繁殖能力,甚至进一步影响其后代的表型。
随着不同密度SNP芯片的发布,根据目标和需求可以结合不同SNP芯片对畜禽实施更有效的选择策略[104]。未来,开发精子甲基化芯片具有巨大的应用潜力,例如:畜禽出生后首周利用低密度SNP芯片进行初筛,在后备种公畜生产性能测定期,结合中/高密度SNP芯片和精子甲基化芯片数据进行第二次筛选(图3);构建种公畜繁殖力、后代表型与精子DNA甲基化的函数,结合基因组选择指数进一步构建新的综合选择指数以更准确的估计种公畜的繁殖力和育种值,以期选出性能更优异的种公畜。
4.3 游离DNA甲基化芯片
尽管精子甲基化芯片能够检测印记基因、影响公畜繁殖力以及后代表型的甲基化位点,但是精子经历重编程,使得部分在原始生殖细胞阶段被擦除的甲基化信息不能被检测出来[11]。此外,不同细胞、组织均有其特异的启动子甲基化区域集,以决定其功能并维持机体的生理状态,这无疑增加了对整体甲基化信息检测的难度[105]。
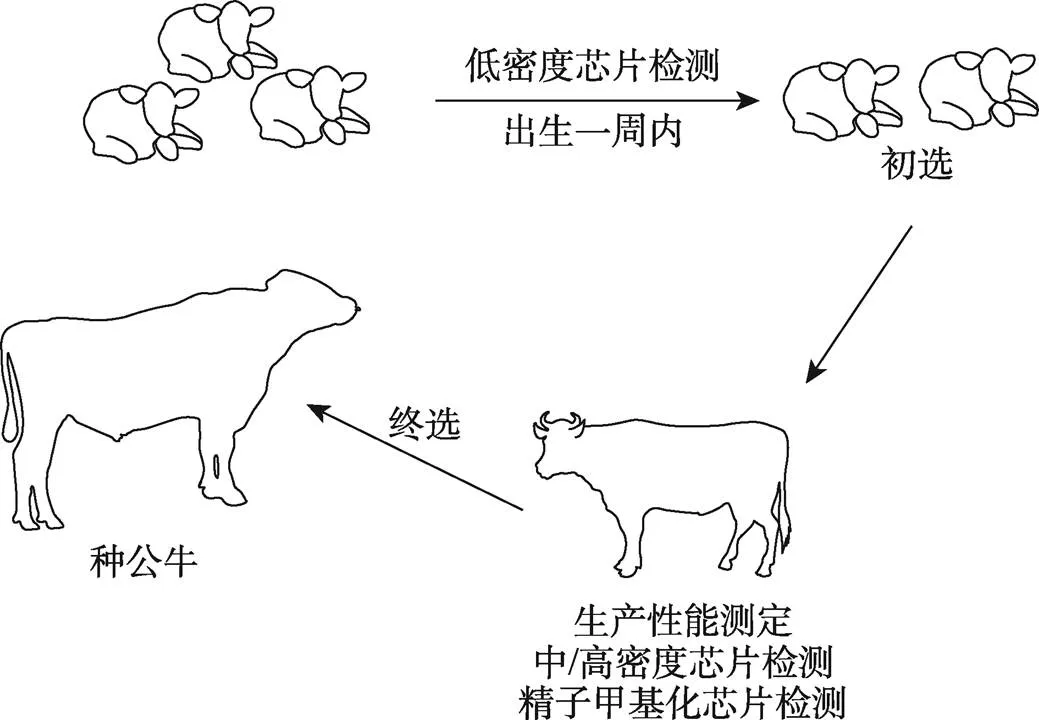
图3 种公畜选择新策略
近年来,游离DNA(cell-free DNA,cfDNA)成为研究热点,使检测畜禽整体的甲基化成为可能。cfDNA是由细胞主动或被动死亡时将胞内的DNA释放到体液中产生的,携带有其起源细胞、组织的遗传、表观遗传信息,可以反映细胞、组织的情况[106~108]。通过反卷积算法能够确定cfDNA的来源、对cfDNA进行甲基化分析可以检测特定组织的甲基化水平是否正常[109]。Lehmann-Werman等[110]基于cfDNA中组织特异性甲基化模式开发出在血液中检测特定组织细胞死亡情况的方法,可通过特定细胞死亡的情况检测疾病。Loyfer等[111]对不同细胞类型的甲基化进行分析,找到了各种细胞内特异的甲基化标记,并基于这些甲基化标记设计的反卷积算法能够在不同来源的cfDNA中很好的检测出其细胞来源。目前,cfDNA的提取、检测流程较为成熟[112],其甲基化模式的研究主要集中在对人类癌症进行无创检测[105,113]。随着对cfDNA甲基化模式研究的不断深入,预期未来可能开发畜禽的cfDNA甲基化芯片,以检测畜禽整体的甲基化状态,进行健康预测。
5 结语与展望
在畜禽分子遗传育种研究中,表观遗传学的重要性日益凸显。开发专门针对畜禽的甲基化基因芯片,将为畜禽甲基化研究提供有力工具,更好的解释复杂性状的分子调控机制。此外,应用精子甲基化芯片可以剖析环境对种公畜繁殖性状以及后代表型的影响,进一步提高种公畜的选择准确性;应用cfDNA甲基化芯片精确探究影响畜禽性状的各个组织甚至各个细胞类型的甲基化模式,揭示复杂性状的深层次分子机制,更全面地了解畜禽的生理生化及健康状态。
因此,全面整合DNA甲基化信息、基因组信息、系谱信息将为畜禽分子遗传育种提供新的视角,实现更精准的分子遗传育种,提高育种选择工作的准确性,选择性能更优良的种畜,推动畜禽遗传育种技术的发展。
[1] Tan C, Bian C, Yang D, Li N, Wu ZF, Hu XX. Application of genomic selection in farm animal breeding., 2017, 39(11): 1033–1045. 谈成, 边成, 杨达, 李宁, 吴珍芳, 胡晓湘. 基因组选择技术在农业动物育种中的应用. 遗传, 2017, 39(11): 1033–1045.
[2] Yin LL, Ma YL, Xiang T, Zhu MJ, Yu M, Li XY, Liu XL, Zhao SH. The progress and prospect of genomic selection models., 2019, 50(2): 233–242. 尹立林, 马云龙, 项韬, 朱猛进, 余梅, 李新云, 刘小磊, 赵书红. 全基因组选择模型研究进展及展望. 畜牧兽医学报, 2019, 50(2): 233–242.
[3] Wu CX. Animal genetics. Beijing: Higher Education Press, 2009. 吴常信. 动物遗传学. 北京: 高等教育出版社, 2009.
[4] Zhang Y. Animal breeding. Beijing: China Agriculture Press, 2001. 张沅. 家畜育种学. 北京: 中国农业出版社, 2001.
[5] Falconer DS, Mackay TFC. Introduction to quantitative genetics. Harlow, UK: Longman Group Limited, 1996.
[6] Elmhiri G, Gloaguen C, Grison S, Kereselidze D, Elie C, Tack K, Benderitter M, Lestaevel P, Legendre A, Souidi M. DNA methylation and potential multigenerational epigenetic effects linked to uranium chronic low-dose exposure in gonads of males and females rats., 2018, 282: 64–70.
[7] Guo XJ, Chen XS, Wang J, Liu ZY, Gaile D, Wu HM, Yu G, Mao GY, Yang ZP, Di Z, Guo XQ, Cao L, Chang PY, Kang BX, Chen JY, Gao W, Ren XF. Multi-generational impacts of arsenic exposure on genome-wide DNA methylation and the implications for arsenic-induced skin lesions., 2018, 119: 250–263.
[8] Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans., 2008, 105(44): 17046–17049.
[9] Liu L, Yang N, Xu GY, Liu SL, Wang D, Song JZ, Duan ZY, Yang S, Yu Y. Transgenerational transmission of maternal stimulatory experience in domesticated birds., 2018, 32(12): 7002–7017.
[10] Sakai K, Hara K, Tanemura K. Testicular histone hyperacetylation in mice by valproic acid administration affects the next generation by changes in sperm DNA methylation., 2023, 18(3): e0282898.
[11] Takahashi Y, Morales Valencia M, Yu Y, Ouchi Y, Takahashi K, Shokhirev MN, Lande K, Williams AE, Fresia C, Kurita M, Hishida T, Shojima K, Hatanaka F, Nuñez-Delicado E, Esteban CR, Izpisua Belmonte JC. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice., 2023, 186(4): 715–731. e719.
[12] Banta JA, Richards CL. Quantitative epigenetics and evolution., 2018, 121(3): 210–224.
[13] Fodor SP, Read JL, Pirrung MC, Stryer L, Lu AT, Solas D. Light-directed, spatially addressable parallel chemical synthesis., 1991, 251(4995): 767–773.
[14] Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray., 1995, 270(5235): 467–470.
[15] McDade RL, Spain MD. Rapid, economical testing in the clinical laboratory: a new flow cytometry-based multiplex system., 1997, 17(10): 154–158.
[16] Yeakley JM, Fan JB, Doucet D, Luo L, Wickham E, Ye Z, Chee MS, Fu XD. Profiling alternative splicing on fiber-optic arrays., 2002, 20(4): 353– 358.
[17] Gnirke A, Melnikov A, Maguire J, Rogov P, LeProust EM, Brockman W, Fennell T, Giannoukos G, Fisher S, Russ C, Gabriel S, Jaffe DB, Lander ES, Nusbaum C. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing., 2009, 27(2): 182–189.
[18] Jordan SA, Humphries P. Single nucleotide polymerphism in exon 2 of the BCP gene on 7q31-q35., 1994, 3(10): 1915–1915.
[19] Lander ES. The new genomics: global views of biology., 1996, 274(5287): 536–539.
[20] 霍艳军, 陈宠霞, 金一. SNPs标记技术及其在牛遗传育种中的应用. 现代畜牧兽医, 2006, (6): 57–60.
[21] Zou YP, Ge S. A novel molecular marker—SNPs and its application., 2003, 11(5): 370–382. 邹喻苹, 葛颂. 新一代分子标记——SNPs及其应用. 生物多样性, 2003, 11(5): 370–382.
[22] Delahunty C, Ankener W, Deng Q, Eng J, Nickerson DA. Testing the feasibility of DNA typing for human identification by PCR and an oligonucleotide ligation assay., 1996, 58(6): 1239–1246.
[23] Fan JB, Chen X, Halushka MK, Berno A, Huang X, Ryder T, Lipshutz RJ, Lockhart DJ, Chakravarti A. Parallel genotyping of human SNPs using generic high-density oligonucleotide tag arrays., 2000, 10(6): 853–860.
[24] Hirschhorn JN, Sklar P, Lindblad-Toh K, Lim YM, Ruiz-Gutierrez M, Bolk S, Langhorst B, Schaffner S, Winchester E, Lander ES. SBE-TAGS: an array-based method for efficient single-nucleotide polymorphism genotyping., 2000, 97(22): 12164–12169.
[25] Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL, Hansen M, Steemers F, Butler SL, Deloukas P, Galver L, Hunt S, McBride C, Bibikova M, Rubano T, Chen J, Wickham E, Doucet D, Chang W, Campbell D, Zhang B, Kruglyak S, Bentley D, Haas J, Rigault P, Zhou L, Stuelpnagel J, Chee MS. Highly parallel SNP genotyping., 2003, 68: 69–78.
[26] Oliphant A, Barker DL, Stuelpnagel JR, Chee MS. BeadArray technology: enabling an accurate, cost- effective approach to high-throughput genotyping., 2002, Suppl: 56–58, 60–61.
[27] Xu YB, Yang QN, Zhen HJ, Xu YF, Sang ZQ, Guo ZF, Peng H, Zhang C, Lan HF, Wang YB, Wu KS, Tao JJ, Zhang JN. Genotyping by target sequencing (GBTS) and its applications ., 2020, 53(15): 2983–3004. 徐云碧, 杨泉女, 郑洪建, 许彦芬, 桑志勤, 郭子锋, 彭海, 张丛, 蓝昊发, 王蕴波, 吴坤生, 陶家军, 张嘉楠. 靶向测序基因型检测(GBTS)技术及其应用. 中国农业科学, 2020, 53(15): 2983–3004.
[28] Zhang M, Yang LL, Jia YL, Wang TY. Research progress in the roles of DNA and histone methylations in epigenetic regulation., 2022, 38(7): 23–30. 张淼, 杨露露, 贾岩龙, 王天云. DNA甲基化和组蛋白甲基化修饰的表观遗传调控作用研究进展. 生物技术通报2022, 38(7): 23–30.
[29] Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells., 2008, 454(7205): 766–770.
[30] Jylhävä J, Pedersen NL, Hägg S. Biological age predictors., 2017, 21: 29–36.
[31] Widschwendter M. 5-methylcytosine--the fifth base of DNA: the fifth wheel on a car or a highly promising diagnostic and therapeutic target in cancer?, 2007, 23(1–2): 1–3.
[32] Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands., 1992, 89(5): 1827–1831.
[33] Grunau C, Clark SJ, Rosenthal A. Bisulfite genomic sequencing: systematic investigation of critical experimental parameters., 2001, 29(13): E65–e65.
[34] Bibikova M, Lin Z, Zhou L, Chudin E, Garcia EW, Wu B, Doucet D, Thomas NJ, Wang Y, Vollmer E, Goldmann T, Seifart C, Jiang W, Barker DL, Chee MS, Floros J, Fan JB. High-throughput DNA methylation profiling using universal bead arrays., 2006, 16(3): 383–393.
[35] Deng J, Shoemaker R, Xie B, Gore A, LeProust EM, Antosiewicz-Bourget J, Egli D, Maherali N, Park IH, Yu JY, Daley GQ, Eggan K, Hochedlinger K, Thomson J, Wang W, Gao Y, Zhang K. Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming., 2009, 27(4): 353–360.
[36] Lee EJ, Pei L, Srivastava G, Joshi T, Kushwaha G, Choi JH, Robertson KD, Wang X, Colbourne JK, Zhang L, Schroth GP, Xu D, Zhang K, Shi H. Targeted bisulfite sequencing by solution hybrid selection and massively parallel sequencing., 2011, 39(19): e127.
[37] Stirzaker C, Taberlay PC, Statham AL, Clark SJ. Mining cancer methylomes: prospects and challenges., 2014, 30(2): 75–84.
[38] Meuwissen TH, Hayes BJ, Goddard ME. Prediction of total genetic value using genome-wide dense marker maps., 2001, 157(4): 1819–1829.
[39] 董航言, 黄生强. 全基因组选择在猪育种上的研究进展. 猪业科学, 2015, (4): 104–105.
[40] Schaeffer LR. Strategy for applying genome-wide selection in dairy cattle., 2006, 123(4): 218–223.
[41] Matukumalli LK, Lawley CT, Schnabel RD, Taylor JF, Allan MF, Heaton MP, O'Connell J, Moore SS, Smith TP, Sonstegard TS, Van Tassell CP. Development and characterization of a high density SNP genotyping assay for cattle., 2009, 4(4): e5350.
[42] VanRaden PM, Van Tassell CP, Wiggans GR, Sonstegard TS, Schnabel RD, Taylor JF, Schenkel FS. Invited review: reliability of genomic predictions for North American Holstein bulls., 2009, 92(1): 16–24.
[43] Pan LN, Qin B. Summary on the development of genomic evaluation system in the United States., 2015, 307(21): 9–14. 潘丽娜, 秦波. 美国基因组评估系统的发展概述. 中国奶牛, 2015, 307(21): 9–14.
[44] Hutchison JL, Cole JB, Bickhart DM. Short communication: use of young bulls in the United States., 2014, 97(5): 3213–3220.
[45] Panigrahi M, Kumar H, Saravanan KA, Rajawat D, Sonejita Nayak S, Ghildiyal K, Kaisa K, Parida S, Bhushan B, Dutt T. Trajectory of livestock genomics in South Asia: a comprehensive review., 2022, 843: 146808.
[46] Ramos AM, Crooijmans RPMA, Affara NA, Amaral AJ, Archibald AL, Beever JE, Bendixen C, Churcher C, Clark R, Dehais P, Hansen MS, Hedegaard J, Hu ZL, Kerstens HH, Law AS, Megens HJ, Milan D, Nonneman DJ, Rohrer GA, Rothschild MF, Smith TPL, Schnabel RD, Van Tassell CP, Taylor JF, Wiedmann RT, Schook LB, Groenen MAM. Design of a high density SNP genotyping assay in the pig using SNPs identified and characterized by next generation sequencing technology., 2009, 4(8): e6524.
[47] 王青来, 李亚兰. 全国首例全基因组选育特级种猪在粤诞生. 农业知识(科学养殖), 2014, (2): 25–25.
[48] Ibáñez-Escriche N, Forni S, Noguera JL, Varona L. Genomic information in pig breeding: science meets industry needs., 2014, 166: 94–100.
[49] Kijas JW, Townley D, Dalrymple BP, Heaton MP, Maddox JF, McGrath A, Wilson P, Ingersoll RG, McCulloch R, McWilliam S, Tang D, McEwan J, Cockett N, Oddy VH, Nicholas FW, Raadsma H, International Sheep Genomics Consortium. A genome wide survey of SNP variation reveals the genetic structure of sheep breeds., 2009, 4(3): e4668.
[50] Liu RR, Zhao GP, Wen J. Development of genome-wide SNP genotyping arrays for chicken breeding and conservation., 2018, 40(15): 1–6. 刘冉冉, 赵桂苹, 文杰. 鸡基因组育种和保种用SNP芯片研发及应用. 中国家禽, 2018, 40(15): 1–6.
[51] Liu RR, Xing SY, Wang J, Zheng MQ, Cui HX, Crooijmans RPMA, Li QH, Zhao GP, Wen J. A new chicken 55K SNP genotyping array., 2019, 20(1): 410.
[52] 邱奥, 张梓鹏, 王雪, 罗文学, 王贵江, 丁向东. 猪50K液相芯片基因组选择效果分析. 中国畜牧杂志, 2022, 58(8): 82–86.
[53] Zhang ZP, Xing SY, Qiu A, Zhang N, Wang WW, Qian CS, Zhang JN, Wang CD, Zhang Q, Ding XD. The development of a porcine 50K SNP panel using genotyping by target sequencing and its application1., 2023.
[54] Xu L. The design of low-density SNP array for genomic selection in simmental beef cattle[Dissertation]. Chinese Academy of Agricultural Sciences, 2019. 徐玲. 肉用西门塔尔牛全基因组选择的低密度SNP芯片设计研究[学位论文]. 中国农业科学院, 2019.
[55] Khan MZ, Dari G, Khan A, Yu Y. Genetic polymorphisms ofandgenes and their association with milk production and mastitis resistance phenotypic traits in Chinese Holstein., 2022, 9: 1008497.
[56] Khan MZ, Wang D, Liu L, Usman T, Wen H, Zhang R, Liu S, Shi L, Mi S, Xiao W, Yu Y. Significant genetic effects ofandmutations on milk fat content and mastitis resistance in Holsteins., 2019, 86(4): 388–393.
[57] Guo YW. Development and application of 40K liquid gene chip for sheep[Dissertation]. Northwest A&F University, 2022. 郭应威. 绵羊40K液相基因芯片的开发与应用[学位论文]. 西北农林科技大学, 2022.
[58] Qiao X, Su R, Wang Y, Wang RJ, Yang T, Li XK, Chen W, He SY, Jiang Y, Xu QW, Wan WT, Zhang YL, Zhang WG, Chen J, Liu B, Liu X, Fan YX, Chen DY, Jiang HZ, Fang DM, Liu ZH, Wang XW, Zhang YJ, Mao DQ, Wang ZY, Di R, Zhao QJ, Zhong T, Yang HM, Wang J, Wang W, Dong Y, Chen XL, Xu X, Li JQ. Genome-wide target enrichment-aided chip design: a 66 K SNP chip for Cashmere goat., 2017, 7(1): 8621.
[59] Rincon G, Weber KL, Van Eenennaam AL, Golden BL, Medrano JF. Hot topic: performance of bovine high- density genotyping platforms in Holsteins and Jerseys., 2011, 94(12): 6116–6121.
[60] Wiggans GR, Cooper TA, Vanraden PM, Olson KM, Tooker ME. Use of the Illumina Bovine3K BeadChip in dairy genomic evaluation., 2012, 95(3): 1552–1558.
[61] Boichard D, Chung H, Dassonneville R, David X, Eggen A, Fritz S, Gietzen KJ, Hayes BJ, Lawley CT, Sonstegard TS, Van Tassell CP, VanRaden PM, Viaud-Martinez KA, Wiggans GR, Bovine LD Consortium. Design of a bovine low-density SNP array optimized for imputation., 2012, 7(3): e34130.
[62] Derks MFL, Megens HJ, Bosse M, Lopes MS, Harlizius B, Groenen MAM. A systematic survey to identify lethal recessive variation in highly managed pig populations., 2017, 18(1): 858.
[63] Tosser-Klopp G, Bardou P, Bouchez O, Cabau C, Crooijmans R, Dong Y, Donnadieu-Tonon C, Eggen A, Heuven HCM, Jamli S, Jiken AJ, Klopp C, Lawley CT, McEwan J, Martin P, Moreno CR, Mulsant P, Nabihoudine I, Pailhoux E, Palhière I, Rupp R, Sarry J, Sayre BL, Tircazes A, Jun W, Wang W, Zhang WG, International Goat Genome Consortium. Design and characterization of a 52K SNP chip for goats., 2014, 9(1): e86227.
[64] Anderson, R. Development of a high density (600 K) Illumina ovine SNP chip and its use to fine map the yellow fat locus. In: Plant and Animal Genome XXII Conference. 2014.
[65] Ma Q, Liu XX, Pan JF, Ma LN, Ma YH, He XH, Zhao QJ, Pu YB, Li YK, Jiang L. Genome-wide detection of copy number variation in Chinese indigenous sheep using an ovine high-density 600 K SNP array., 2017, 7(1): 912.
[66] Groenen MAM, Megens HJ, Zare Y, Warren WC, Hillier LW, Crooijmans RPMA, Vereijken A, Okimoto R, Muir WM, Cheng HH. The development and characterization of a 60K SNP chip for chicken., 2011, 12(1): 274.
[67] Kranis A, Gheyas AA, Boschiero C, Turner F, Yu L, Smith S, Talbot R, Pirani A, Brew F, Kaiser P, Hocking PM, Fife M, Salmon N, Fulton J, Strom TM, Haberer G, Weigend S, Preisinger R, Gholami M, Qanbari S, Simianer H, Watson KA, Woolliams JA, Burt DW. Development of a high density 600K SNP genotyping array for chicken., 2013, 14: 59.
[68] Liu J, Shen QM, Bao HG. Comparison of seven SNP calling pipelines for the next-generation sequencing data of chickens., 2022, 17(1): e0262574.
[69] Mattei AL, Bailly N, Meissner A. DNA methylation: a historical perspective., 2022, 38(7): 676–707.
[70] Liu SL, Chen SQ, Cai WT, Yin HW, Liu AX, Li YH, Liu GE, Wang YC, Yu Y, Zhang SL. Divergence analyses of sperm DNA methylomes between monozygotic twin AI bulls., 2019, 3(4): 21.
[71] Yang YL, Fan XH, Yan JY, Chen MY, Zhu M, Tang YJ, Liu SY, Tang ZL. A comprehensive epigenome atlas reveals DNA methylation regulating skeletal muscle development., 2021, 49(3): 1313–1329.
[72] Wang XS, Zhao HM, Tang T, Li K, Qian X, Zhang X, Tu Y, Zheng YS, Wang HD, Yu Y. DNA methylation modification ofgene and its application in livestock diseases-resistant breeding., 2017, 48(10): 1796–1806. 王晓铄, 赵会敏, 唐天, 李凯, 钱旭, 张雪, 图雅, 郑云胜, 王怀栋, 俞英.基因的DNA甲基化修饰在家畜抗病育种中的应用. 畜牧兽医学报, 2017, 48(10): 1796–1806.
[73] Ibeagha-Awemu EM, Yu Y. Consequence of epigenetic processes on animal health and productivity: is additional level of regulation of relevance?, 2021, 11(6): 7–18.
[74] Richards EJ. Population epigenetics., 2008, 18(2): 221–226.
[75] Zhao LN, Liu D, Xu J, Wang ZY, Chen Y, Lei CG, Li Y, Liu GY, Jiang YS. The framework for population epigenetic study., 2018, 19(1): 89–100.
[76] Hu YD, Morota G, Rosa GJM, Gianola D. Prediction of plant height in arabidopsis thaliana using DNA methylation data., 2015, 201(2): 779–793.
[77] Chen SQ. Identification of ruminant epigenomic evolutionary features and their application in Holstein genomic selection[Dissertation]., 2022. 陈思倩. 反刍动物表观基因组进化特征鉴定及其在荷斯坦牛基因组选择中应用效果的研究[学位论文]. 中国农业大学, 2022.
[78] Yang YL, Zhou R, Li K. Future livestock breeding: precision breeding based on multi-omics information and population personalization., 2017, 16(12): 2784–2791.
[79] Christiansen SN, Andersen JD, Kampmann M-L, Liu J, Andersen MM, Tfelt-Hansen J, Morling N. Reproducibility of the Infinium methylationEPIC BeadChip assay using low DNA amounts., 2022, 17(12): 1636–1645.
[80] Bibikova M, Le J, Barnes B, Saedinia-Melnyk S, Zhou LX, Shen R, Gunderson KL. Genome-wide DNA methylation profiling using Infinium® assay., 2009, 1(1): 177–200.
[81] Pidsley R, Zotenko E, Peters TJ, Lawrence MG, Risbridger GP, Molloy P, Van Djik S, Muhlhausler B, Stirzaker C, Clark SJ. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole- genome DNA methylation profiling., 2016, 17(1): 208.
[82] Wan ES, Qiu WL, Baccarelli A, Carey VJ, Bacherman H, Rennard SI, Agusti A, Anderson W, Lomas DA, Demeo DL. Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome., 2012, 21(13): 3073–3082.
[83] Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases., 2011, 12(8): 529–541.
[84] Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, Delano D, Zhang L, Schroth GP, Gunderson KL, Fan JB, Shen R. High density DNA methylation array with single CpG site resolution., 2011, 98(4): 288–295.
[85] Xu J, Zhao LN, Liu D, Hu SM, Song XL, Li J, Lv HC, Duan L, Zhang MM, Jiang QH, Liu GY, Jin SL, Liao MZ, Zhang M, Feng RN, Kong FW, Xu LD, Jiang YS. EWAS: epigenome-wide association study software 2. 0., 2018, 34(15): 2657– 2658.
[86] Xiong Z, Yang F, Li MW, Ma YK, Zhao W, Wang GL, Li ZH, Zheng XC, Zou D, Zong WT, Kang HG, Jia YK, Li RJ, Zhang Z, Bao YM. EWAS open platform: integrated data, knowledge and toolkit for epigenome-wide association study., 2022, 50(D1): D1004–D1009.
[87] Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, Tsai PC, Ried JS, Zhang WH, Yang YW, Tan SL, Fiorito G, Franke L, Guarrera S, Kasela S, Kriebel J, Richmond RC, Adamo M, Afzal U, Ala-Korpela M, Albetti B, Ammerpohl O, Apperley JF, Beekman M, Bertazzi PA, Black SL, Blancher C, Bonder MJ, Brosch M, Carstensen-Kirberg M, de Craen AJM, de Lusignan S, Dehghan A, Elkalaawy M, Fischer K, Franco OH, Gaunt TR, Hampe J, Hashemi M, Isaacs A, Jenkinson A, Jha S, Kato N, Krogh V, Laffan M, Meisinger C, Meitinger T, Mok ZY, Motta V, Ng HK, Nikolakopoulou Z, Nteliopoulos G, Panico S, Pervjakova N, Prokisch H, Rathmann W, Roden M, Rota F, Rozario MA, Sandling JK, Schafmayer C, Schramm K, Siebert R, Slagboom PE, Soininen P, Stolk L, Strauch K, Tai ES, Tarantini L, Thorand B, Tigchelaar EF, Tumino R, Uitterlinden AG, van Duijn C, van Meurs JBJ, Vineis P, Wickremasinghe AR, Wijmenga C, Yang TP, Yuan W, Zhernakova A, Batterham RL, Smith GD, Deloukas P, Heijmans BT, Herder C, Hofman A, Lindgren CM, Milani L, van der Harst P, Peters A, Illig T, Relton CL, Waldenberger M, Järvelin MR, Bollati V, Soong R, Spector TD, Scott J, McCarthy MI, Elliott P, Bell JT, Matullo G, Gieger C, Kooner JS, Grallert H, Chambers JC. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity., 2017, 541(7635): 81–86.
[88] Li SB, Mancuso N, Metayer C, Ma XM, de Smith AJ, Wiemels JL. Incorporation of DNA methylation quantitative trait loci (mQTLs) in epigenome-wide association analysis: application to birthweight effects in neonatal whole blood., 2022, 14(1): 158.
[89] Can SN, Nunn A, Galanti D, Langenberger D, Becker C, Volmer K, Heer K, Opgenoorth L, Fernandez-Pozo N, Rensing SA. The EpiDiverse plant epigenome-wide association studies (EWAS) pipeline., 2021, 5(2): 12.
[90] Ratan P, Rubbi L, Thompson M, Naresh K, Waddell J, Jones B, Pellegrini M. Epigenetic aging in cows is accelerated by milk production., 2023, 18(1): 2240188.
[91] Takeda K, Kobayashi E, Ogata K, Imai A, Sato S, Adachi H, Hoshino Y, Nishino K, Inoue M, Kaneda M, Watanabe S. Differentially methylated CpG sites related to fertility in Japanese Black bull spermatozoa: epigenetic biomarker candidates to predict sire conception rate., 2021, 67(2): 99–107.
[92] Yang XN, Zhao MM, Zhang L. Application process of artificial insemination in animal husbandry., 2023, 31(1): 43–49, 60杨轩宁, 赵萌萌, 张鲁. 人工授精技术在畜牧生产中的应用历程. 黑龙江动物繁殖, 2023, 31(1): 43–49, 60.
[93] 寇海军, 幸福. 猪的人工授精技术. 甘肃畜牧兽医, 2013, 43(4): 37–38.
[94] Knox RV. Artificial insemination in pigs today., 2016, 85(1): 83–93.
[95] Wang X, Li WL, Feng X, Li JB, Liu GE, Fang LZ, Yu Y. Harnessing male germline epigenomics for the genetic improvement in cattle., 2023, 14(1): 76.
[96] Reik W, Walter J. Genomic imprinting: parental influence on the genome., 2001, 2(1): 21–32.
[97] Wang DX, Zhao HB. Progress of cattle imprinted genes., 2022, 48(6): 80–84. 王丁香, 赵红波. 牛印记基因的研究进展. 中国牛业科学, 2022, 48(6): 80–84.
[98] Zhao RL, Jia CQ, Li AC, Zhang Y, Su JM. Mechanism of erasure, establishment and maintenance of mammalian genomic imprinting., 2020, 42(3): 512–518. 赵若琳, 贾晨琪, 李艾聪, 张涌, 苏建民. 哺乳动物基因组印记的擦除、建立和维持机理. 中国细胞生物学学报, 2020, 42(3): 512–518.
[99] Tian JH, Chen YG, Hu JH, Lei AM. Imprinted genes and cell reprogramming., 2022, 41(3): 30–33, 36. 田佳卉, 陈昱光, 胡建宏, 雷安民. 印记基因与细胞重编程. 畜牧兽医杂志, 2022, 41(3): 30–33, 36.
[100] Gigante S, Gouil Q, Lucattini A, Keniry A, Beck T, Tinning M, Gordon L, Woodruff C, Speed TP, Blewitt ME, Ritchie ME. Using long-read sequencing to detect imprinted DNA methylation., 2019, 47(8): e46.
[101] Nishio M, Satoh M. Genomic best linear unbiased prediction method including imprinting effects for genomic evaluation., 2015, 47(1): 32.
[102] Costes V, Chaulot-Talmon A, Sellem E, Perrier JP, Aubert-Frambourg A, Jouneau L, Pontlevoy C, Hozé C, Fritz S, Boussaha M, Le Danvic C, Sanchez MP, Boichard D, Schibler L, Jammes H, Jaffrézic F, Kiefer H. Predicting male fertility from the sperm methylome: application to 120 bulls with hundreds of artificial insemination records., 2022, 14(1): 54.
[103] Shojaei Saadi HA, Fournier É, Vigneault C, Blondin P, Bailey J, Robert C. Genome-wide analysis of sperm DNA methylation from monozygotic twin bulls., 2017, 29(4): 838–843.
[104] 丁向东, 王楚端, 张勤. 基于液相芯片的猪基因组选择实施新策略. 中国畜牧杂志, 2022, 58(4): 65–69.
[105] Spector BL, Harrell L, Sante D, Wyckoff GJ, Willig L. The methylome and cell-free DNA: current applications in medicine and pediatric disease., 2023, 94(1): 89–95.
[106] Hu Y, Ni H. Advance in human free circulating DNA., 2008, 30(7): 815–820. 胡影, 倪虹. 人体游离循环DNA的研究进展. 遗传, 2008, 30(7): 815–820.
[107] He WS, Bishop KS. The potential use of cell-free- circulating-tumor DNA as a biomarker for prostate cancer., 2016, 16(8): 839–852.
[108] Qi T, Pan M, Shi HJ, Wang LY, Bai YF, Ge QY. Cell-free DNA fragmentomics: the novel promising biomarker., 2023, 24(2): 1503.
[109] Sharma M, Verma RK, Kumar S, Kumar V. Computational challenges in detection of cancer using cell-free DNA methylation., 2022, 20: 26–39.
[110] Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, Rubertsson S, Nellgård B, Blennow K, Zetterberg H, Spalding K, Haller MJ, Wasserfall CH, Schatz DA, Greenbaum CJ, Dorrell C, Grompe M, Zick A, Hubert A, Maoz M, Fendrich V, Bartsch DK, Golan T, Ben Sasson SA, Zamir G, Razin A, Cedar H, James Shapiro AM, Glaser B, Shemer R, Dor Y. Identification of tissue-specific cell death using methylation patterns of circulating DNA., 2016, 113(13): E1826–E1834.
[111] Loyfer N, Magenheim J, Peretz A, Cann G, Bredno J, Klochendler A, Fox-Fisher I, Shabi-Porat S, Hecht M, Pelet T, Moss J, Drawshy Z, Amini H, Moradi P, Nagaraju S, Bauman D, Shveiky D, Porat S, Dior U, Rivkin G, Or O, Hirshoren N, Carmon E, Pikarsky A, Khalaileh A, Zamir G, Grinbaum R, Abu Gazala M, Mizrahi I, Shussman N, Korach A, Wald O, Izhar U, Erez E, Yutkin V, Samet Y, Rotnemer Golinkin D, Spalding KL, Druid H, Arner P, Shapiro AMJ, Grompe M, Aravanis A, Venn O, Jamshidi A, Shemer R, Dor Y, Glaser B, Kaplan T. A DNA methylation atlas of normal human cell types., 2023, 613(7943): 355–364.
[112] Pös Z, Pös O, Styk J, Mocova A, Strieskova L, Budis J, Kadasi L, Radvanszky J, Szemes T. Technical and methodological aspects of cell-free nucleic acids analyzes., 2020, 21(22): 8634.
[113] Rapado-González Ó, Rodríguez-Ces AM, López-López R, Suárez-Cunqueiro MM. Liquid biopsies based on cell-free DNA as a potential biomarker in head and neck cancer., 2023, 59: 289–302.
Application and prospect of gene chip in genetic breeding of livestock and poultry
Jiahao Wang1, Qingyao Zhao1, Yueling Zhou1, Liangyu Shi2, Chuduan Wang1, Ying Yu1
Gene chip is a high-throughput technique for detecting specific DNA sequences by DNA or DNA-RNA complementary hybridization, among which SNP genotyping chips have been widely employed in the animal genetics and breeding, and have made great achievements in cattle, pigs, sheep, chickensand other livestock.However, genomic selection applied in production merely uses genomic information and cannot fully explain the molecular mechanism of complex traits genetics, which limits the accuracy of genomic selection.With the continuous progresses in epigenetic research, the development of commercial methylation chips and the application of the epigenome-wide association study (EWAS), DNA methylation has been extensively used to draw the causal connections between genetics and phenotypes.In the future, it is hopefully expected to develop methylation chips customized for livestock and poultry and explore methylation sites significantly related to economic traits of livestock and poultry through EWAS thereby extending the understanding of causal variation of complex traits.Combining methylation chips and SNP chips, we can capture the epigenomic and genomic information of livestock and poultry, interpret genetic variation more precisely, improve the accuracy of genome selection, and promote the fine evolution of molecular genetic breeding of livestock and poultry.In this review, we summarize the application of SNP chips and depict the prospects of the application of methylation chips in livestock and poultry. This review will provide valuable insights for further application of gene chips in farm animal breeding.
gene chip; SNPs; DNA methylation; genetic breeding
2023-09-06;
2023-10-05;
2023-11-08
国家重点研发计划项目(编号:2021YFD1200903,2021YFD1200900),国家奶牛产业技术体系项目(编号:CARS-36)和国家自然科学基金国际(地区)合作项目(编号:31961143009)资助[Supported by the National Key R&D Program of China (Nos.2021YFD1200903, 2021YFD1200900), the National Dairy Industry Technology System Project (No.CARS-36) and the National Natural Science Foundation of China-Pakistan Science Foundation Join Project (No.31961143009)]
汪佳豪,硕士研究生,专业方向:动物遗传育种与繁殖。E-mail: 2423280404@qq.com
俞英,教授,博士生导师,研究方向:表观遗传抗病育种。E-mail: yuying@cau.edu.cn
王楚端,教授,博士生导师,研究方向:猪遗传育种与规模化生产。E-mail: cdwang@cau.edu.cn
10.16288/j.yczz.23-233
(责任编委: 李明洲)