NAT10 is an Acetyltransferase to Lysine and Cytidine Residues and Regulates Various Biological Processes and Diseases
ZHOU Rui, SUI Ya-Qi, ZHAO Wen-Hui
(Department of Physiology, School of Life Sciences, Chongqing University, Chongqing 401331, China)
Abstract The nucleolar protein N-acetyltransferase 10 (NAT10) is responsible for catalyzing the acetylation of both lysine and cytidine residues. At present, plenty of studies have revealed that these acetylation modifications play vital roles in numerous physiological processes, such as telomerase activity, stress response, DNA damage repair, cell cycle regulation, rRNA biosynthesis regulation, mRNA stability, and translation. Additionally, NAT10 has a strong association with the progression and prognosis of human cancers and Hutchinson-Gilford premature aging syndrome (HGPS). However, there are still some limitations. For example, the complete structure of NAT10 and the impact of these structures on functions are still unknown, the cellular functions mediated by NAT10 remain unclear and the exact mechanism by which NAT10 influences human cancers and HGPS progression is needed to be clarified. This review covers the structure, enzymatic activity, biological functions, as well as roles in diseases of NAT10. We also propose the limitations of current research and envision the future research directions, aiming to provide a reference for NAT10 research.
Key words N-acetyltransferase 10 (NAT10); acetylation; rRNA biosynthesis; mRNA stability and translation regulation; Hutchinson-Gilford premature aging syndrome (HGPS)
TheN-acetyltransferase 10 enzyme (NAT10, also known as hALP) belongs to the GNAT family of histone acetyltransferases[1]. In yeasts, Kre33/Rra1p is a homologous protein of NAT10[2]. The enzymatic activity of NAT10 involves the acetylation of lysine and cytidine residues. These acetylation modifications play crucial roles in various physiological processes, including the telomerase activity, stress response, DNA damage repair, cell cycle, rRNA biosynthesis, mRNA stability, and translation[2-4]. Furthermore, NAT10 is strongly associated with the progression and prognosis of human cancers and Hutchinson-Gilford premature aging syndrome (HGPS).
1 Structure of NAT10
TheNAT10 gene in humans is located on chromosome 11 and the NAT10 structural domain is highly conserved from bacteria to human[2], beginning with a domain of unknown function called DUF1726 near theN-terminus, followed by a helicase domain, anN-acetyltransferase (GNAT) domain and a tRNA-binding domain (Fig.1). The GNAT domain is essential for recognizing and bindingNAT10 to acetyl-coenzyme A (Acetyl-CoA)[5]. Additionally, in eukaryotes, the N-terminus and C-terminus of NAT10 contain putative nuclear localization signal (NLS) motifs and nucleolar localization signals (NoLS) motifs, respectively, which are related to localization and functions of NAT10[2].

Fig.1 The structure of NAT10 The NAT10 structure comprises four highly conserved domains, which include the DUF1726 domain located near the N-terminus, followed by the helicase domain, GNAT domain, and tRNA-binding domain[2]
2 Enzymatic activity of NAT10
The NAT10 enzyme exhibits dual activities in modifying proteins and RNA by acetylation. Such acetylation modifications are crucial in various physiological processes in the organism. NAT10 transfers and adds the acetyl group of acetyl-coenzyme A (Acetyl-CoA) to the lysine (K) residues of proteins to acetylate them (Fig.2A), and its catalytic substrates include NAT10 itself, histones, upstream binding factor (UBF), tubulin, apoptosis antagonizing transcription factor (Che-1/AATF), MORC family CW-type zinc finger 2 (MORC2), poly(ADP-ribose) polymerase 1 (PARP1), tumor protein p53 (p53) and the coiled-coil domain-containing 84 protein (CCDC84) (Table 1)[6-7]. Furthermore, NAT10 also catalyzes N4-acetylcytidine (ac4C) modification of tRNA, rRNA and mRNA (Fig.2B)[8-11]. The acetylation of proteins and RNA catalyzed by NAT10 holds significant biological importance and determines most of the functions of NAT10.
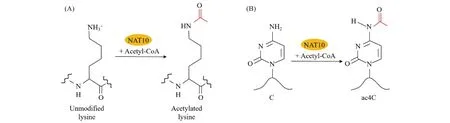
Fig.2 NAT10 acetylates proteins and RNA (A) NAT10 transfers and adds an acetyl group from Acetyl-CoA to lysine residues on proteins[19]; (B) NAT10 catalyzes ac4C modification of RNA[8]

Table 1 Proteins acetylated by NAT10
3 Biological functions of NAT10
3.1 Telomerase activation
Telomerase is an essential nuclear reverse transcriptase consisting of RNA and proteins that is responsible for lengthening telomeres in cells. It plays a crucial role in maintaining telomere stability, genomic integrity, and long-term cellular viability[20]. In 2003, NAT10 was initially shown to activate telomerase by binding and activating hTERT (human telomerase reverse transcriptase)[21]. More recently, Chietal.found that overexpression of NAT10 can impact the assembly and localization of hTERT and enhance the catalytic activity of telomerase[22].
3.2 Regulation of basic and core functions of cells
NAT10 is an essential gene that regulates basic and core cellular functions, becauseNAT10 knockout is lethal. Using mutagenesis in the near-haploid chronic myeloid leukemia (CML) cell line KBM7 and its nonhematopoietic derivative HAP1, Blomenetal.found thatNAT10 knockout resulted in lethality in both cell lines, which showed thatNAT10 is a key gene that determines cell survival[3]. In addition, Wangetal.revealedNAT10is cell-indispensable gene by two complementary and concordant approaches, CRISPR and gene trap in KBM7, K562 and two Burkitt’s lymphoma cell lines[4]. These findings suggest that NAT10 plays a critical role in regulating basic and core functions of cells. However, the exact mechanisms are still unknown.
3.3 Cell stress response
Cells respond to diverse stress stimuli by regulating physiological and biochemical processes, and NAT10 is involved in these processes. Liuetal.discovered that NAT10 acts as a novel regulator for p53 activation by acetylating p53 at K120, stabilizing p53 by counteracting MDM2 oncoprotein (Mdm2) action. In addition, NAT10 promotes Mdm2 degradation with its intrinsic E3 ligase activity. After treatment with actinomycin D, NAT10 translocates to the nucleoplasm and activates p53-mediated cell cycle control and apoptosis to respond to stress (Fig.3A)[17]. Furthermore, under normal conditions, NAT10 is acetylated to activate rRNA biogenesis and inhibit autophagy induction. However, under energy stress, NAT10 is deacetylated by sirtuin 1(Sirt1), leading to suppression of NAT10-activated rRNA biogenesis, and deacetylation of NAT10 activates DNA damage inducible transcript 4 (DDIT4/Redd1) and DEP domain containing MTOR interacting protein (Deptor) transcription to reduce energy loss for cells in response to energy stress (Fig.3B)[14]. Therefore, NAT10 is involved in the stress response and plays a crucial role in regulating diverse physiological and biochemical processes.

Fig.3 NAT10 responds to cell stress (A) NAT10 activates p53-mediated cell cycle control and apoptosis to respond to stress[17]; (B) NAT10 binds to and acetylates the autophagy regulator Che-1 at K228 to suppress the Che-1-mediated transcriptional activation of downstream genes Redd1 and Deptor under sufficient energy supply conditions. Under energy stress, NAT10 is deacetylated by Sirt1, leading to suppression of NAT10-activated rRNA biogenesis. In addition, deacetylation of NAT10 abolishes the NAT10-mediated transcriptional repression of Che-1, leading to the release of autophagy inhibition to respond to energy stress[14]
3.4 DNA damage repair
Cells initiate multiple damage responses through various signaling pathways after DNA damage, and NAT10 plays a role in regulating these signaling pathways, such as the MORC2/PARP1 signaling pathway. Zhangetal.demonstrated that MORC2 stabilizes PARP1 against DNA damage by promoting NAT10-mediated acetylation of PARP1 at K949 (Fig.4)[16]. In addition, MORC2 is acetylated by NAT10 at lysine 767 (K767Ac) and acetylated MORC2 binds to histone H3 phosphorylation at threonine 11 (H3pT11) and is crucial for DNA damage-induced reduction of H3pT11 and transcriptional repression of its downstream target genes cyclin-dependent kinase 1 (CDK1) and Cyclin B1, thus contributing to DNA damage-induced G2checkpoint activation (Fig.4)[15]. Furthermore, Liuetal.revealed NAT10 undergoes covalent PARylation modifcation following DNA damage, and PARP1 catalyzes PARylation of NAT10 on three conserved lysine residues (K1016, K1017, and K1020) within its C-terminal NoLS motif (residues 983-1025). Mutation of those three PARylation residues on NAT10, inhibition of PARP1 activity or depletion of PARP1 impair the co-localization and interaction between NAT10 and MORC2, resulting in a decrease in DNA damage-induced MORC2 K767Ac (Fig.4)[23].
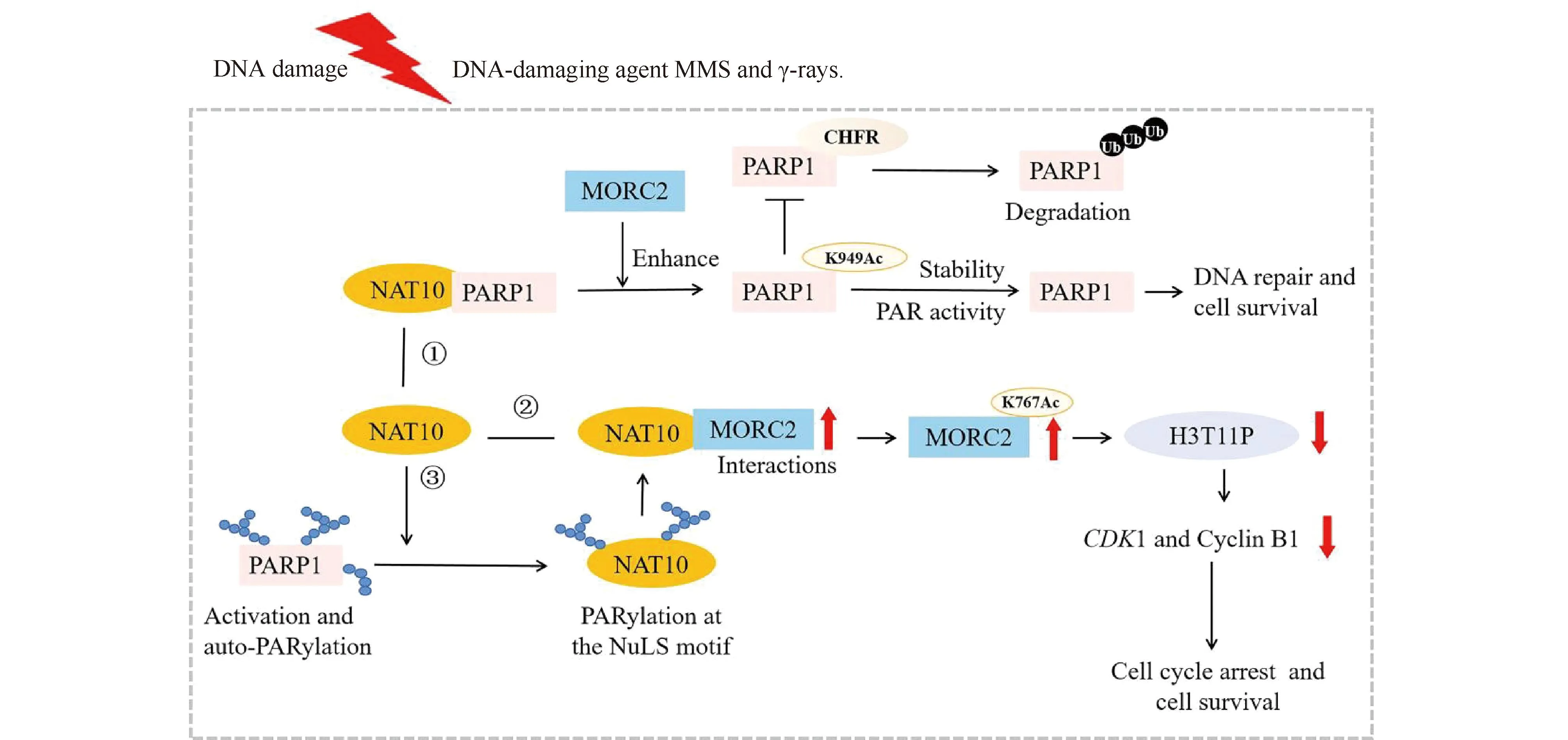
Fig.4 NAT10 repairs DNA damage ① MORC2 enhances NAT10-mediated acetylation of PARP1 at lysine 949 to stabilize PARP1, thus resisting DNA damage[16]; ② Acetylation of MORC2 by NAT10 regulates cell-cycle checkpoint control and resistance to DNA-damage[15]; ③ PARP1-mediated PARylation of NAT10 is key for controlling its nucleoplasmic translocation, and this translocation enhances the interaction between NAT10 and MORC2 to respond to DNA damage[23]
Moreover, the mRNA expression of NAT10 was found to significantly increase in response to hydrogen peroxide (H2O2) or cisplatin treatments in a dose- and time-dependent manner. Additionally, overexpression of NAT10 was shown to enhance the rate of cell survival in the presence of H2O2or cisplatin, indicating that NAT10 could be involved in DNA damage repair processes[24].
3.5 Cell cycle regulation
Regulation of the cell cycle by NAT10 involves a various cellular structures and proteins in mitosis, such as midbody, centrosome, cyclin and cyclin-dependent kinases (CDK). While predominantly distributed in the nucleolus during interphase, NAT10 also concentrates in the mitotic midbody during telophase. Depletion ofNAT10 induces defects in nucleolar assembly, cytokinesis and decreased acetylated α-tubulin, resulting in G2/M cell cycle arrest or delay of mitotic exit. This suggests that NAT10 plays a significant role in cell division by facilitating reformation of the nucleolus and midbody in the late phase of cell mitosis, as well as stabilizing of microtubules[7]. Furthermore, Wangetal.demonstrated that CCDC84 prevents centrosome overduplication by facilitating APC/CCdh1-mediated HsSAS-6 degradation at mitotic exit. SIRT1 and NAT10 regulate the acetylation of CCDC84 at lysine 31, and only acetylated CCDC84 promotes HsSAS-6 binding to APC/CCdh1(Fig.5)[18]. In addition, NAT10 acetylates MORC2 at lysine 767, and acetylated MORC2 binds to histone H3pT11. This process is crucial for DNA damage-induced reduction of H3pT11 and transcriptional repression of its downstream target genesCDK1 and Cyclin B1, thus contributing to DNA damage-induced G2 checkpoint activation (Fig.4)[15]. Studies have also shown that inhibiting NAT10 expression through siRNA in human and mouse melanoma cells leads to S-phase cell cycle arrest via a mechanism related to the regulation of p21/CDK2/Cyclin D1 by NAT10[25]. Additionally, NAT10 induces cell cycle arrest in acute myeloid leukemia (AML) cells by decreasing the expression of CDK2, CDK4, CyclinD1, Cyclin E, while simultaneously increasing the expression of p16 and p21[26].

Fig.5 NAT10 regulates cell cycle by CCDC84 NAT10 catalyzes CCDC84 K31Ac, and only acetylated CCDC84 promotes HsSAS-6 binding to APC/CCdh1 to mediate HsSAS-6 degradation at mitotic exit and prevent centrosome overduplication[18]
3.6 rRNA Biosynthesis regulation
Ribosomal RNA (rRNA) is an essential component of the ribosome, which is the cellular machinery responsible for protein synthesis[27]. The process of rRNA synthesis is highly regulated and involves the coordination of various factors and pathways within the cell. NAT10 has been found to play a role in the regulation of rRNA biosynthesis. Specifically, NAT10 is involved in the processing, modification and transcription of pre-rRNA, which are necessary steps in the maturation of functional rRNA.
On the one hand, N4-acetylcytidine(ac4C) formation catalyzed by NAT10 plays a critical role in processing of pre-18S rRNA in eukaryotic cells. For instance, Kre33 acetylates 18S rRNA ofSaccharomycescerevisiae(S.cerevisiae) at cytosine 1773 (ac4C1773). Upon depletion of NAT10, the 23S pre-18S rRNA was accumulated significantly, which resulted in a complete loss of 18S rRNA and 40S subunits, suggesting that ac4C1773 formation catalyzed by NAT10 plays a critical role in processing of the 23S precursor to yield 18S rRNA (Fig.6A)[28]. Additionally, NAT10 catalyzes ac4C1842 in the terminal helix of mammalian 18S rRNA, and RNAi mediated knockdown ofNAT10 results in high-level accumulation of the 30S pre-18S rRNA, suggesting that ac4C1842 formation catalyzed by NAT10 is involved in rRNA processing and ribosome biogenesis (Fig.6B)[10].
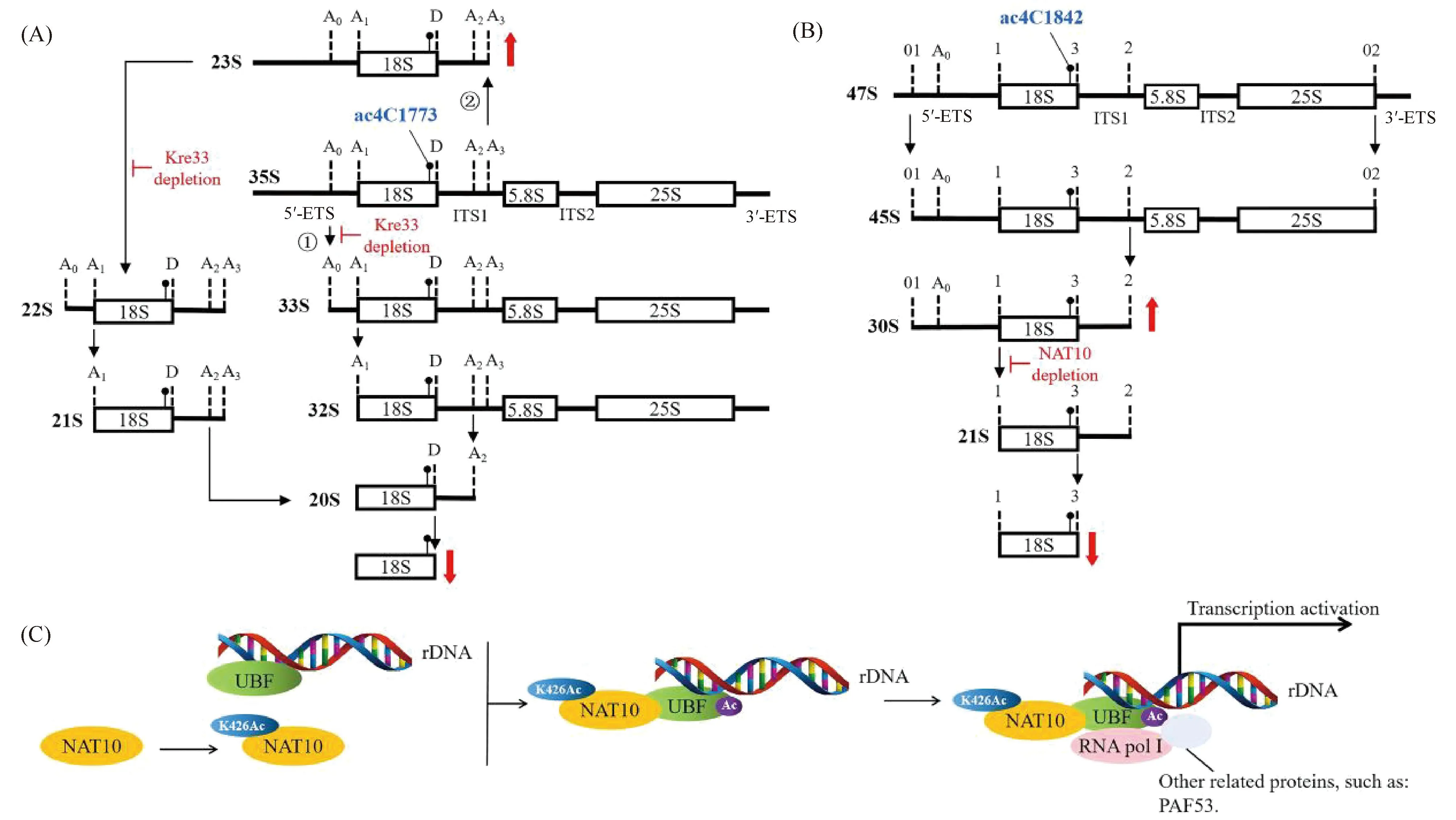
Fig.6 NAT10 regulates rRNA biosynthesis (A) In S. cerevisiae., NAT10(Kre33) acetylates 18S rRNA of ac4C1773. 35S pre-rRNA is processed by a canonical pathway(①) and an alternative pathway(②), and precursors for large subunit formation are not shown. Upon depletion of Kre33, endonucleolytic cleavages at the A0, A1, and A2 sites are inhibited, which significantly accumulates the 23S pre-18S rRNA, resulting in a complete loss of 18S rRNA and 40S subunit[28]; (B) In humans, NAT10 catalyzes ac4C1842 of 18S rRNA. In the canonical pathway (precursors for large subunit formation are not shown), RNAi mediated knockdown of NAT10 results in a high-level accumulation of the 30S pre-18S rRNA, resulting in a complete loss of 18S rRNA[10]. (C) Autoacetylation of NAT10 at K426 activates rRNA transcription by binding and acetylating UBF[12]
On the other hand, NAT10 acetylates proteins involved in rRNA processing to activate pre-rRNA transcription. Kongetal. identified that NAT10 is a novel transcriptional U three proteins (t-UTPs) and NAT10 activates rRNA transcription by binding and acetylating UBF[6,29]. Furthermore, Caietal. reported that NAT10 autoacetylates itself at K426, and the K426R mutant of NAT10 fails to activate rRNA transcription, because NAT10 K426R loses its capability of acetylating UBF though it still binds UBF, which fails to recruit the RNA polymerase I subunit E (POLR1E/PAF53). As a consequence, the assembly of the RNA polymerase I transcription complex is impaired, resulting in decrease of pre-rRNA transcription. Therefore, autoacetylation of NAT10 at K426 is required for its function in activating rRNA transcription (Fig.6C)[12].
3.7 mRNA stability and translation regulation
The regulation of gene expression is crucially reliant on post-transcriptional mechanisms, including mRNA stability, translation initiation and efficiency.In mammalian cells, NAT10 plays a significant role in regulating these mechanisms. By catalyzing mRNA ac4C modification, NAT10 can inhibit mRNA degradation and consequently enhance mRNA stability. Furthermore, NAT10 can also catalyze ac4C in the wobble sites of mRNA codons, affecting the codon-anticodon pairing, thereby improving the efficiency of translation (Fig.7)[8,30].
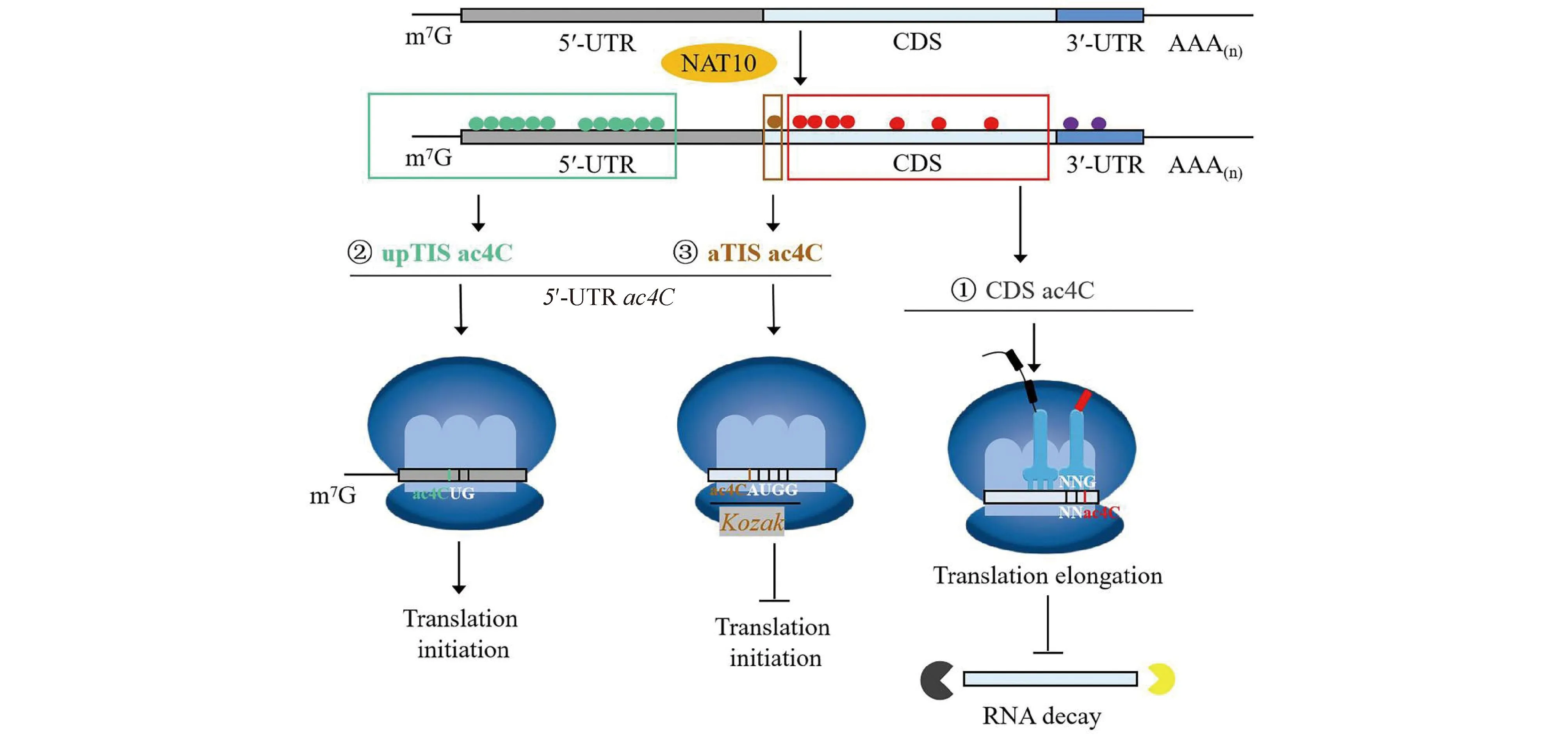
Fig.7 NAT10 regulates the mRNA stability, translation initiation and efficiency by ac4C modification in a position-dependent manner ① NAT10-catalyzed CDS ac4C improves mRNA stability and translation efficiency[8]; ② NAT10-catalyzed 5′-UTR ac4C (Up TIS ac4C) promotes upstream initiation and inhibits canonical start codons[31]; ③ NAT10-catalyzed ac4C within Kozak sequences structurally alters the interaction with tRNAi-Met, thus repressing translation initiation[31]. (The small green circle represents the NAT10-catalyted ac4C modification of mRNA 5′-UTR, the small brown circle represents the NAT10-catalyted ac4C modification of mRNA Kozak sequence, the small red circle represents the NAT10-catalyted ac4C modification of mRNA CDS, and the small purple circle represents the NAT10-catalyted ac4C modification of mRNA 3′-UTR)
NAT10 modifies a wide range of mRNAs through ac4C modification, which is enriched in 5'-UTR and CDS regions but depleted in 3′-UTR (Fig.7)[8]. Notably, the effects of NAT10-catalyzed mRNA ac4C on translation are position-dependent.Specifically, ac4C in the CDS region enhances translation (Fig.7), while Up TIS ac4C in the 5′-UTR region promotes upstream initiation and inhibits canonical start codons (Fig.7). Furthermore, ac4C withinKozaksequences alters the interaction with tRNAi-Met, thereby repressing translation initiation (Fig.7) since it structurally alters the pairing between the start codon AUG and the anticodon tRNAi-Met[31].
Weietal.demonstrated that NAT10 enhances centrosomal protein170 (CEP170) translation efficiency by acetylatingCEP170 mRNA in multiple myeloma (MM) cells[32]. NAT10 also maintains the stability of theO-GlcNAcase(OGA) transcript by ac4C modification, which positively regulatesinvitromaturation (IVM) in oocyte maturation[33]. Additionally, Tsaietal.reported that cellular NAT10 acetylates multiple cytidines on HIV-1 RNAs to ac4C, which boostsHIV-1 gene expression by increasingHIV-1 RNA stability[34]. Furthermore, small RNAs can regulate mRNA stability and translation, and NAT10 plays an important role in small RNA-mediated transcriptional activation[35]. For instance, the heart-apoptosis-associated piRNA (HAAPIR) directly interacts with NAT10 and enhances ac4C acetylation of transcription factor EC (Tfec) mRNA transcripts, thereby increasingTfecexpression[36].
4 NAT10 and diseases
4.1 The role of NAT10 in carcinogenesis
Currently, numerous studies have shown that NAT10 is associated with the carcinogenesis of humans. NAT10 has been found to be highly expressed in approximately 92% of human cancers, and its overexpression is linked to cancer cell survival. While NAT10 mainly localizes in the nucleoli of normal tissues, it is redistributed to the membrane in cancer cells, particularly at the invasive “leading edge” of the tumors[37-38]. These aberrant expressions of NAT10 have been shown to accelerate the proliferation, migration, and invasion of tumor cells, and are significantly correlated with epithelial-mesenchymal transition(EMT), chemoresistance, and a poor prognosis in various types of human cancers (Table 2).
NAT10 promotes cancer cell proliferation, migration, and EMT through ac4C modification of oncogenic mRNA, which enhances mRNA stability and translation efficiency. In bladder urothelial carcinoma (BLCA) cells, NAT10 modifiesBCL9 like (BCL9L), SRY-box transcription factor 4 (SOX4), and AKT serine/threonine kinase 1 (AKT1) mRNA ac4C to promote BLCA progression[43]. In multiple myeloma (MM), NAT10 acetylatesCEP170 andBCL-XLmRNA to enhance their translation efficiency, accelerating cell proliferation[32,44]. In gastric cancer (GC), NAT10 regulates collagen type V alpha 1 chain (COL5A1) mRNA ac4C to promote GC metastasis and EMT[45]. Furthermore, NAT10 binds to long intergenic non-protein coding RNA 623 (LINC00623) and blocks its degradation by recruiting the ubiquitin specific peptidase 39(USP39), which promotes the tumorigenicity and migratory capacity of pancreatic ductal adenocarcinoma (PDAC) cells[46].
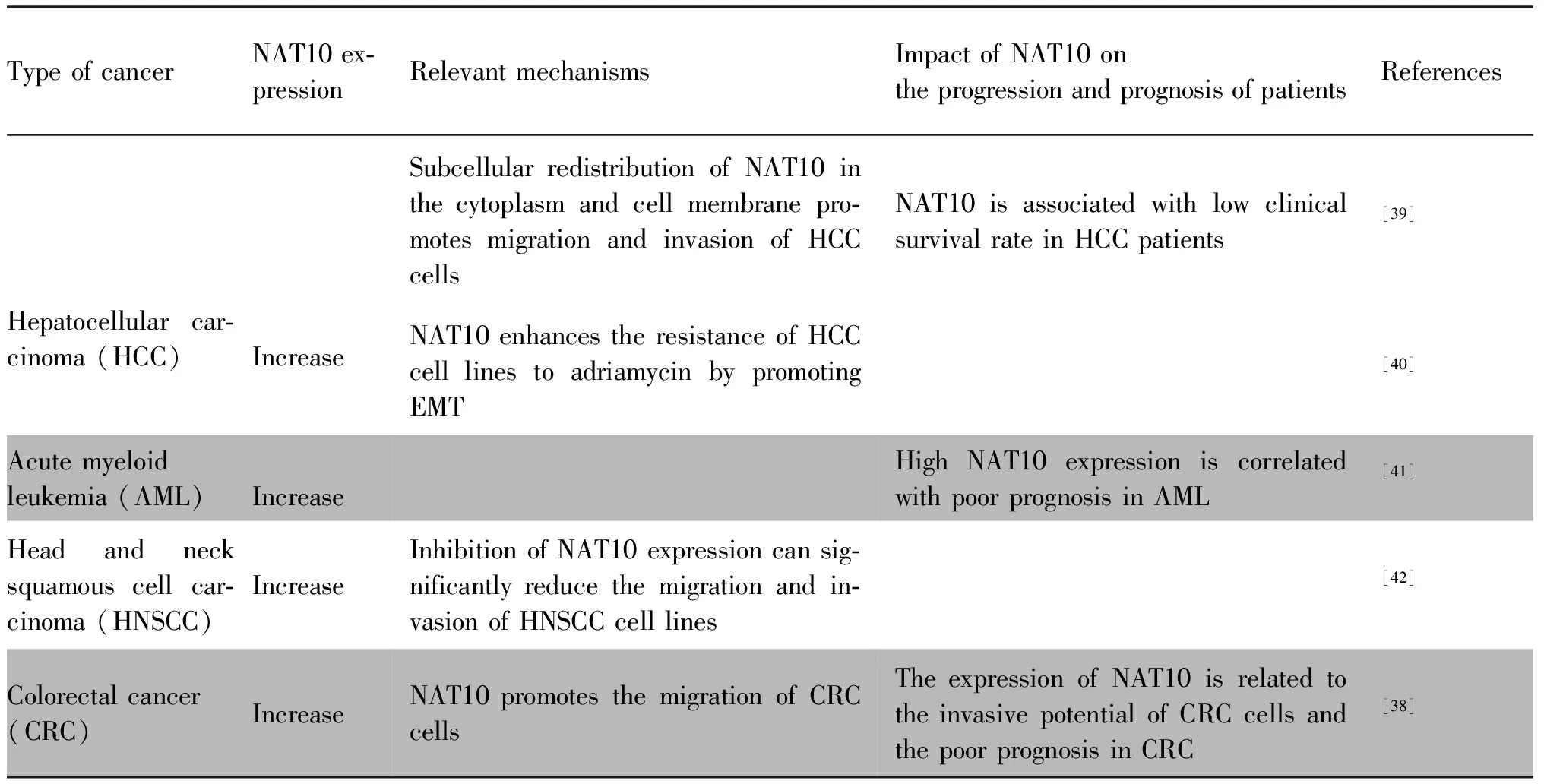
Table 2 The role of NAT10 in carcinogenesis
Beyond its post-transcriptional acetylation of oncogenic mRNA, NAT10 also acetylates metabolism-related mRNA to regulate metabolic pathways in cancer cells. In particular, NAT10 stabilizes ELOVL fatty acid elongase 6 (ELOVL6), acyl-CoA synthetase long chain family member 1, 3 and 4 (ACSL1, ACSL3 and ACSL4), acyl-CoA dehydrogenase short/branched chain (ACADSB),and acetyl-CoA acetyltransferase 1 (ACAT1) mRNA through ac4C modification, thereby regulating fatty acid metabolism. Depletion of NAT10 leads to reduced overall lipid contents, triglycerides, and total cholesterol levels in cancer cells[37].
Furthermore, NAT10 contributes to carcinogenesis by participating in various signaling pathways. The p53-Mdm2 loop is critical in multiple tumorigenic signaling pathways[47]. NAT10 acetylates p53 at K120 and stabilizes p53 by counteracting Mdm2, moreover, NAT10 promotes Mdm2 degradation with its intrinsic E3 ligase activity[17]. NAT10 protein levels are significantly correlated with p53 levels in human hepatocellular carcinoma (HCC)tissues. Furthermore, NAT10 increases mutant p53 levels by counteracting Mdm2 in HCC cells and promotes proliferation in cells withp53 mutation[48]. In colorectal cancer, NAT10 promotes cancer progression by increasing micronuclei (MN) formation and activating the senescence-associated secretory phenotype (SASP) pathway[49]. In AML cells, targeting NAT10 promotes ER stress, triggers the unfolded protein response (UPR) pathway, and activates the Bax/Bcl-2 axis[26]. NAT10 also promotes MM cell proliferation by activating the PI3K-AKT signal pathway and up-regulating the expression of CDK4/CDK6[44]. Furthermore,the CREB/MYC target 1 (MYCT1)/NAT10 axis is involved in laryngeal cancer cell migration. Specifically, CREB expression is significantly upregulated in laryngeal cancer tissues. It promotes NAT10 expression by downregulating MYCT1 gene expression, thus accelerating laryngeal cancer cell migration[50].
4.2 NAT10 and HGPS(Hutchinson-Gilford premature aging syndrome)
Hutchinson-Gilford premature aging syndrome (HGPS) is an extremely rare, fatal premature aging syndrome caused by lamin A/C(LMNA) gene mutation. TheLMNAgene encodes for nuclear lamins, which are scaffold proteins that specify nuclear architecture and provide mechanical strengths to the nucleus and the cell[51]. HGPS arises from adenovoheterozygous G608 G point mutation of theLMNAexon 11, leading to cryptic mRNA splicing and expression of a shorter, dysfunctional form of Lamin A, called progerin[52], which results in abnormal nucleus shape and abnormal chromatin structure.
Recent studies have demonstrated the potential therapeutic role of NAT10 in mediating nuclear architecture and chromatin structure in HGPS. The chemical inhibitor “Remodelin” specifically inhibits NAT10 and has been shown to improve nuclear architecture, chromatin organization and fitness of both human Lamin A/C-depleted cells and HGPS-derived patient cells, and decrease markers of DNA damage in these cells, which suggests NAT10 mediates nuclear shape, and rescues laminopathic cells via microtubule reorganization[13]. Targeting NAT10invivo, either through chemical inhibition or genetic depletion, also significantly enhances the lifespan in anLmnaG609 GHGPS mouse model[53]. Similarly, inhibiting NAT10 could rescue aberrant nuclear shape and chromatin structure in HGPS cells and cells from healthy aged individuals[54].
5 Research future
The structure of NAT10 is highly conserved across different organisms, ranging from bacteria to human[2]. In eukaryotes, the N-terminus and C-terminus of NAT10/Kre33 encompass putative NLS motifs and NoLS motifs[2]. However, the function of DUF1726 domain remains unknown, and the evidence for the presence of NLS and NoLS in the N-terminus and C-terminus of NAT10/Kre33 is still limited. Complete structure of NAT10 and the impact of these structures on functions both are needed to be clarified in the future.
NAT10 exhibits enzymatic activity in two aspects: acetylating lysine residues of proteins and cytosine residues of RNA (ac4C). These modifications are involved in various biological functions. In particular, the acetylation of 18 S rRNA by NAT10 is highly conserved among eukaryotes, but the exact function of this modification remains elusive. Interestingly, NAT10/Kre33 also acetylates tRNASerand tRNALeuwith the THUMPD1/Tan adaptor[11], which creates a crosstalk between rRNAs and tRNAs. However, the regulation of this crosstalk is poorly understood[11]. Additionally, it is unclear whether NAT10/Kre33 requires interaction with other adaptor proteins before or during acetylation of 18S rRNA and mRNA. These unanswered questions remain important areas for future research in this field.
NAT10 is widely expressed at high levels and undergoes subcellular redistribution in many human cancers, which has been linked to the carcinogenesis and poor prognosis in cancers. The acetylation of oncogenic mRNA (ac4C) by NAT10 enhances oncogenic mRNA translation efficiency and promotes cancer cell proliferation, migration, and epithelial-mesenchymal transition (EMT). Moreover, NAT10 participates in various signal pathways that are involved in carcinogenesis. Additionally, NAT10 can acetylate mRNA related to fatty acid metabolism, but the exact mechanism of how it regulates fatty acid metabolism and whether it regulates other metabolic pathways in cancer cells remains unclear[55]. As metabolic reprogramming is a hallmark of malignancy and targeted metabolism has emerged as a new approach to cancer therapy[56], further research is required to elucidate the role of NAT10 in the regulation of metabolic pathways in cancer cells and to aid in the development of targeted drugs for cancer therapy.
NAT10 has been shown to play a role in restoring nuclear structure and chromosome organization by mediating microtubule formation, which makes it a possible therapeutic strategy for HGPS. The use of Remodelin, a specific chemical inhibitor of NAT10, may be an effective approach. However, the exact mechanism by which NAT10 influences HGPS progression is still unclear. HGPS is caused by LaminA mutation, and targeting NAT10 via chemical inhibition can partially restore nuclear structure and chromosome organization.NAT10 has also been found to mediate the formation of MN through DNA replication, and inhibiting NAT10 has been shown to reduce MN formation[49]. This suggests that NAT10 may be associated with chromatin three-dimensional (3D) structure. Therefore, future research could focus on this aspect to gain a better understanding of the regulation mechanism of NAT10 on the chromatin 3D structure and its potential therapeutic application for HGPS.
In conclusion, NAT10 regulates numerous physiological processes by acetylating of lysine and cytidine residues, but the exact mechanism by which NAT10 influences human cancers and HGPS progression is needed to be clarified. Further research should focus on these aspects, especially elucidating the role of NAT10 in the regulation of metabolic pathways in cancer cells to aid in the development of targeted drugs for cancer therapy and demonstrating the regulation mechanism of NAT10 on the chromatin 3D structure and its potential therapeutic application for HGPS.

